Flavanol-Rich Cocoa Powder Interacts with Lactobacillus rhamnossus LGG to Alter the Antibody Response to Infection with the Parasitic Nematode Ascaris suum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dietary Supplements
2.2. Animals and Diets
2.3. Blood Collection and Processing
2.4. Tissue and Intestinal Sample Processing
2.5. Lactobacillus Rhamnosus Abundance
2.6. Isolation of Alveolar Macrophage
2.7. RNA Extraction, cDNA Synthesis, and Real-Time PCR Analysis (RT-PCR)
2.8. Statistical Analysis
3. Results
3.1. Cocoa Powder (CP) Flavanols
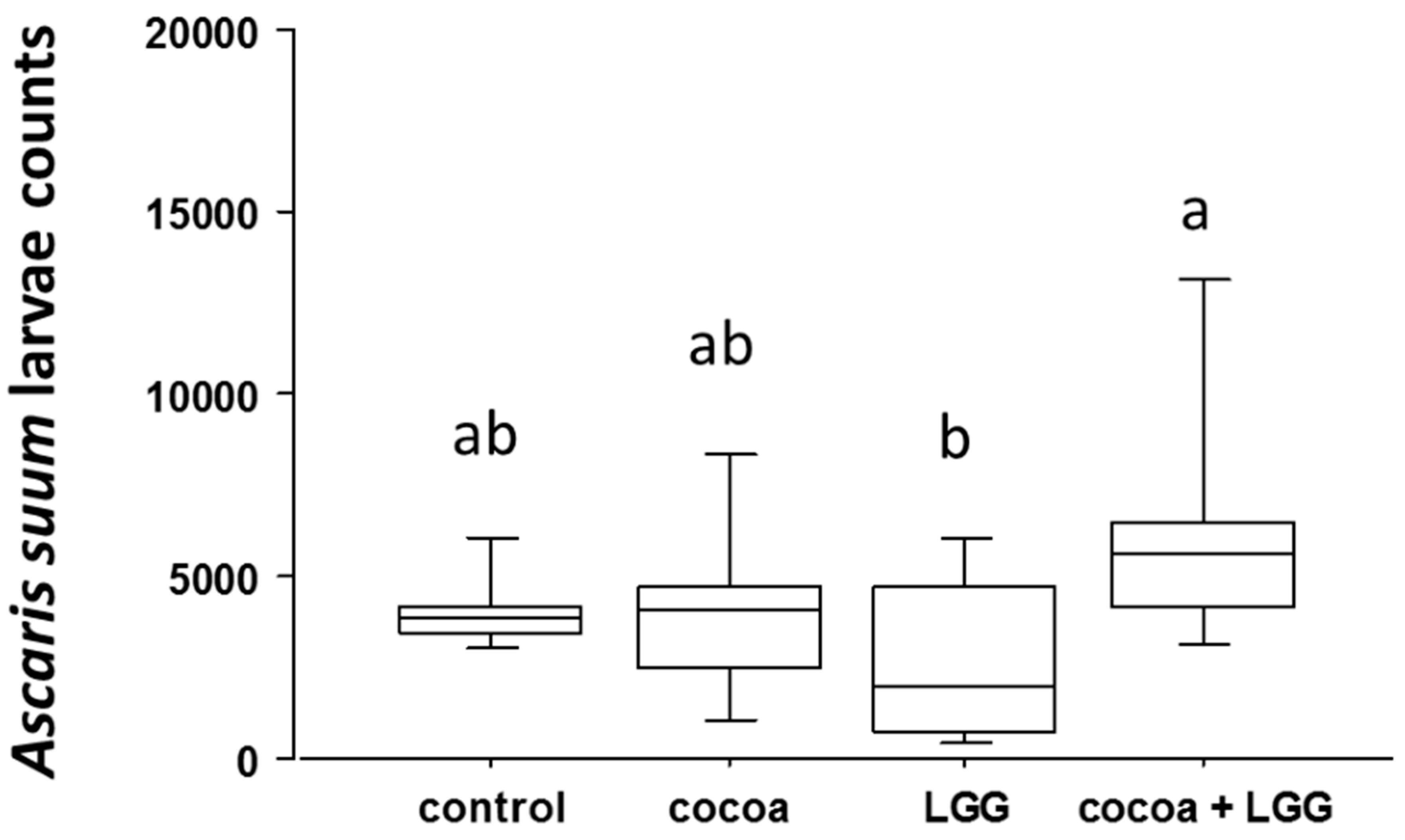
3.2. Clinical Signs
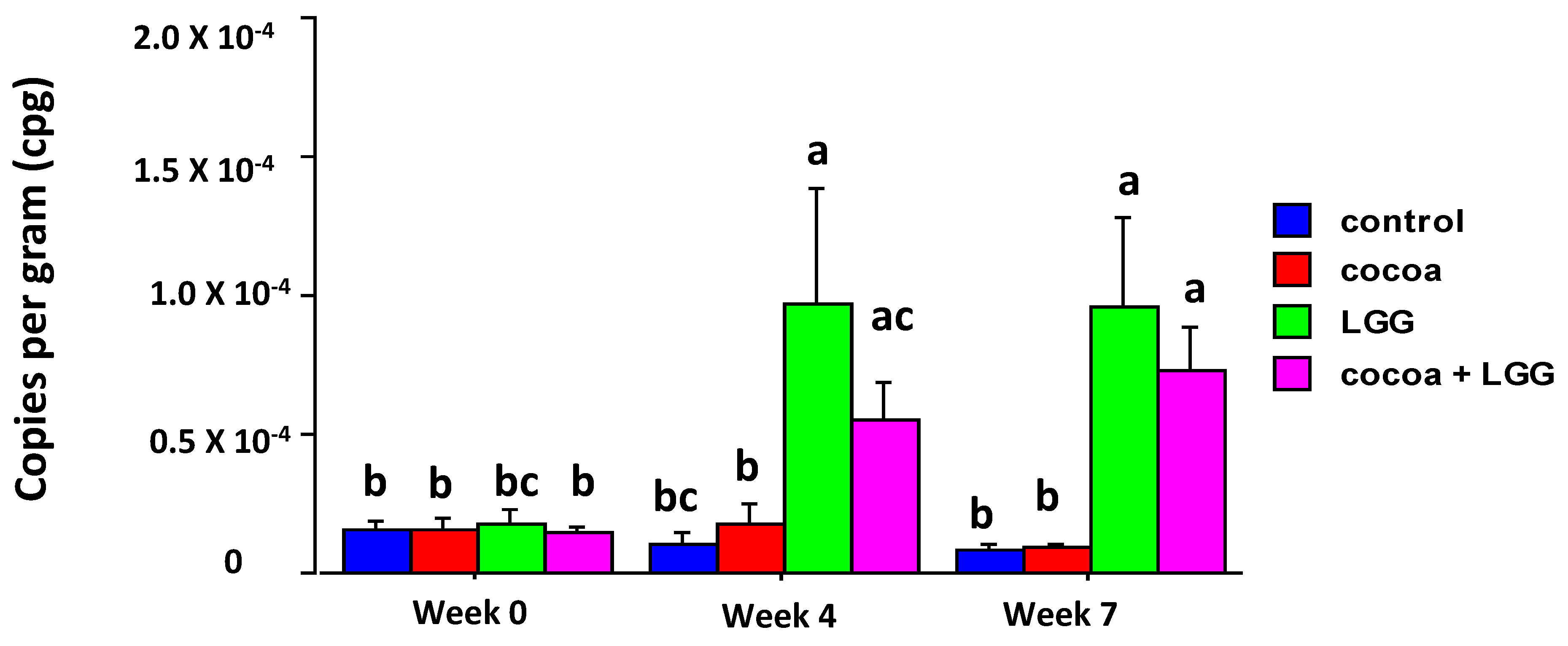
3.3. Abundance of Lactobacillus Rhamnosus in Feces
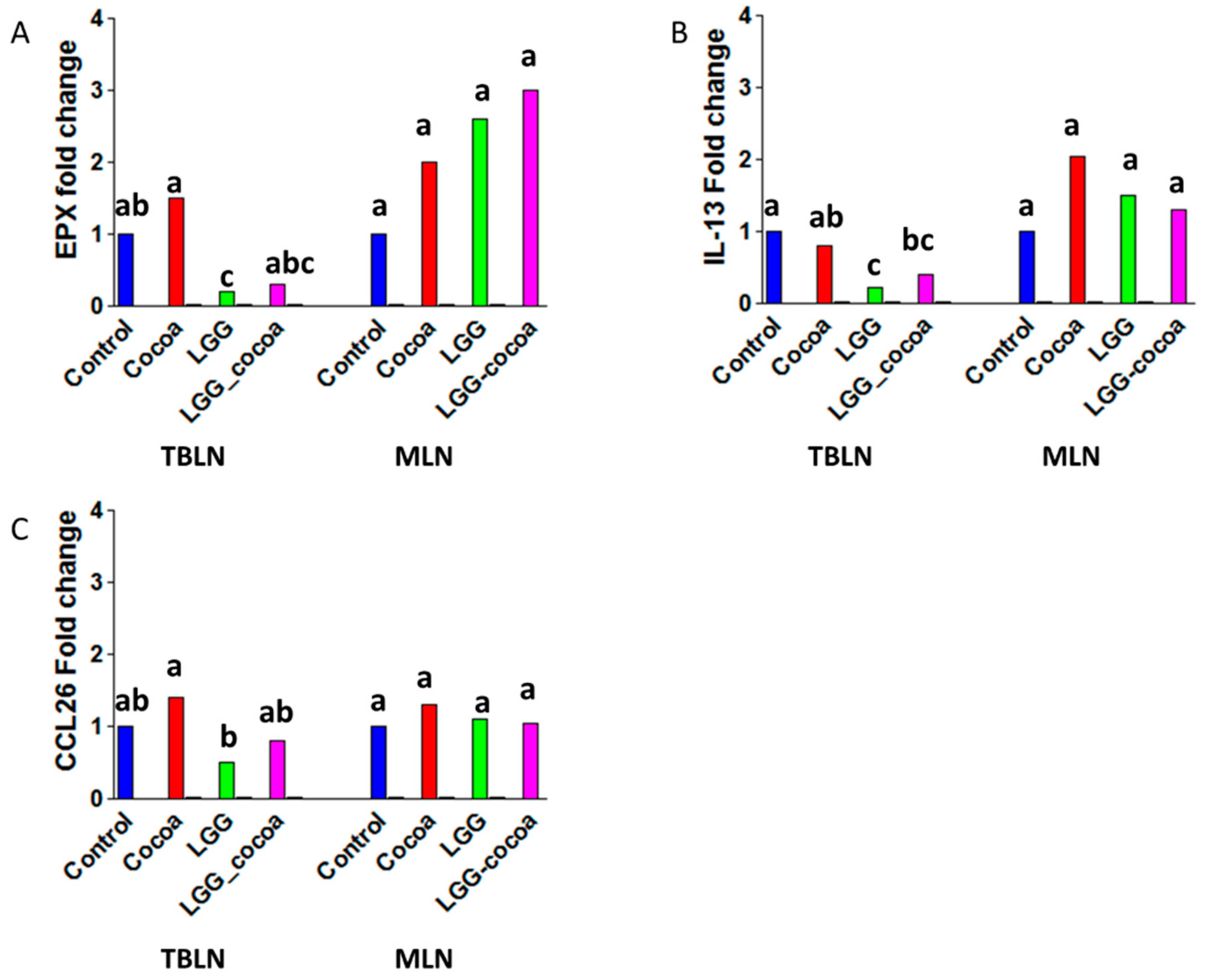
3.4. Type 2 Immune Response-Related Gene Expression in the Draining Lymph Nodes
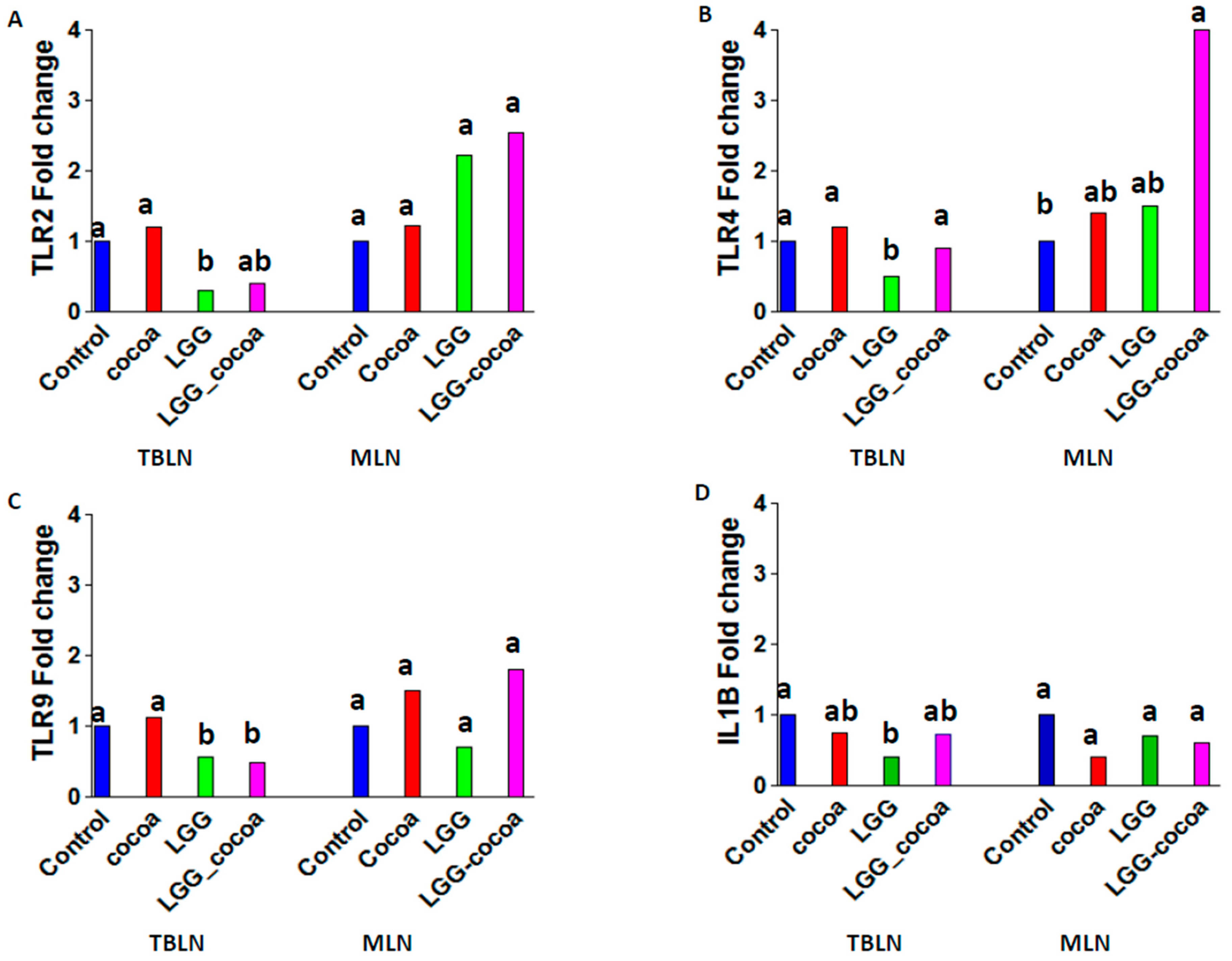
3.5. Toll-Like Receptor Gene Expression in the Draining Lymph Nodes
3.6. LPS-Induced Alveolar Macrophage (AM) Gene Expression In Vitro
3.7. Antibody Response to Ascaris suum
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cifuentes-Gomez, T.; Rodriguez-Mateos, A.; Gonzalez-Salvador, I.; Alanon, M.E.; Spencer, J.P. Factors affecting the absorption, metabolism, and excretion of cocoa flavanols in humans. J. Agric. Food Chem. 2015, 63, 7615–7623. [Google Scholar] [CrossRef] [PubMed]
- Goya, L.; Martin, M.A.; Sarria, B.; Ramos, S.; Mateos, R.; Bravo, L. Effect of cocoa and its flavonoids on biomarkers of inflammation: Studies of cell culture, animals and humans. Nutrients 2016, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Lamuela-Raventos, R.M.; Santos-Buelga, C.; Sacanella, E.; Castell, M.; Permanyer, J.; Andres-Lacueva, C. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal. Bioanal. Chem. 2009, 394, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban, J.F., Jr.; Davis, C.D.; Solano-Aguilar, G. Flavanol-enriched cocoa powder alters the intestinal microbiota, tissue and fluid metabolite profiles, and intestinal gene expression in pigs. J. Nutr. 2016, 146, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellinger, S.; Stehle, P. Impact of cocoa consumption on inflammation processes-a critical review of randomized controlled trials. Nutrients 2016, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and dark chocolate polyphenols: From biology to clinical applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Massot-Cladera, M.; Abril-Gil, M.; Franch, A.; Perez-Cano, F.J.; Castell, M. Cocoa diet and antibody immune response in preclinical studies. Front. Nutr. 2017, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Leles, D.; Gardner, S.L.; Reinhard, K.; Iniguez, A.; Araujo, A. Are ascaris lumbricoides and ascaris suum a single species? Parasit Vectors 2012, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.D.; Beshah, E.; Nishi, S.; Solano-Aguilar, G.; Morimoto, M.; Zhao, A.; Madden, K.B.; Ledbetter, T.K.; Dubey, J.P.; Shea-Donohue, T.; et al. Localized multigene expression patterns support an evolving th1/th2-like paradigm in response to infections with toxoplasma gondii and ascaris suum. Infect. Immun. 2005, 73, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Sylvin, H.; Weitzberg, E.; Alving, K. Endothelin-induced vascular and bronchial effects in pig airways: Role in acute allergic responses. J. Appl. Physiol. (1985) 2002, 93, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Sylvin, H.; Dahlback, M.; Van Der Ploeg, I.; Alving, K. The tryptase inhibitor apc-366 reduces the acute airway response to allergen in pigs sensitized to ascaris suum. Clin. Exp. Allergy 2002, 32, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J.; Husmann, R.J.; Villamar, M.; Winship, T.R.; Buck, R.H.; Zuckermann, F.A. Lactobacillus rhamnosus hn001 attenuates allergy development in a pig model. PLoS ONE 2011, 6, e16577. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. Quantitation of flavanols, proanthocyanidins, isoflavones, flavanones, dihydrochalcones, stilbenes, benzoic acid derivatives using ultraviolet absorbance after identification by liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2012, 60, 5832–5840. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.F., Jr.; Romanowski, R.D. Ascaris suum: Protective immunity in pigs immunized with products from eggs and larvae. Exp. Parasitol. 1985, 60, 245–254. [Google Scholar] [CrossRef]
- Solano-Aguilar, G.; Molokin, A.; Botelho, C.; Fiorino, A.M.; Vinyard, B.; Li, R.; Chen, C.; Urban, J., Jr.; Dawson, H.; Andreyeva, I.; et al. Transcriptomic profile of whole blood cells from elderly subjects fed probiotic bacteria lactobacillus rhamnosus gg atcc 53103 (lgg) in a phase i open label study. PLoS ONE 2016, 11, e0147426. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.S.; Abraham, W.M.; Brogden, K.A.; Engelhardt, J.F.; Fisher, J.T.; McCray, P.B., Jr.; McLennan, G.; Meyerholz, D.K.; Namati, E.; Ostedgaard, L.S.; et al. The porcine lung as a potential model for cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L240–L263. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.; Solano-Aguilar, G.; Beal, M.; Beshah, E.; Vangimalla, V.; Jones, E.; Botero, S.; Urban, J.F., Jr. Localized th1-, th2-, t regulatory cell-, and inflammation-associated hepatic and pulmonary immune responses in ascaris suum-infected swine are increased by retinoic acid. Infect. Immun. 2009, 77, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.D.; Chen, C.; Gaynor, B.; Shao, J.; Urban, J.F., Jr. The porcine translational research database: A manually curated, genomics and proteomics-based research resource. BMC Gen. 2017, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- Khan-Malek, R.; Wang, Y. Statistical analysis of quantitative rt-pcr results. Methods Mol. Biol. 2011, 691, 227–241. [Google Scholar] [PubMed]
- Hurvich, C.M.; Tsai, C.L. Model selection for extended quasi-likelihood models in small samples. Biometrics 1995, 51, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Murrell, K.D.; Eriksen, L.; Nansen, P.; Slotved, H.C.; Rasmussen, T. Ascaris suum: A revision of its early migratory path and implications for human ascariasis. J. Parasitol. 1997, 83, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Ma, C.; Basra, M.A.; Wang, J.; Xu, J. Amelioration of ovalbumin induced allergic symptoms in balb/c mice by potentially probiotic strains of lactobacilli. Benef. Microbes 2015, 6, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Kringel, H.; Thamsborg, S.M.; Petersen, H.H.; Goring, H.H.; Skallerup, P.; Nejsum, P. Serum antibody responses in pigs trickle-infected with ascaris and trichuris: Heritabilities and associations with parasitological findings. Vet. Parasitol. 2015, 211, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Frontera, E.; Carron, A.; Serrano, F.J.; Roepstorff, A.; Reina, D.; Navarrete, I. Specific systemic igg1, igg2 and igm responses in pigs immunized with infective eggs or selected antigens of ascaris suum. Parasitology 2003, 127, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Saliganti, V.; Kapila, R.; Sharma, R.; Kapila, S. Feeding probiotic Lactobacillus rhamnosus (MTCC 5897) fermented milk to suckling mothers alleviates ovalbumin-induced allergic sensitisation in mice offspring. Br. J. Nutr. 2015, 114, 1168–1179. [Google Scholar] [CrossRef]
- Perez-Berezo, T.; Ramiro-Puig, E.; Perez-Cano, F.J.; Castellote, C.; Permanyer, J.; Franch, A.; Castell, M. Influence of a cocoa-enriched diet on specific immune response in ovalbumin-sensitized rats. Mol. Nutr. Food Res. 2009, 53, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Abril-Gil, M.; Massot-Cladera, M.; Perez-Cano, F.J.; Castellote, C.; Franch, A.; Castell, M. A diet enriched with cocoa prevents ige synthesis in a rat allergy model. Pharmacol. Res. 2012, 65, 603–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Zhang, Y.Q.; Hobson, S.A.; Garcia Lloret, M.; Chehoud, C.; Kuczynski, J.; DeSantis, T.; Warrington, J.; et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]

| Measure | Concentration, mg/g |
|---|---|
| Total Flavanols a | 137.19 |
| Flavanols (up to 5P) b | 15.7 |
| Individual flavanol | |
| Catechin | 2.87 |
| Epicatechin | 4.27 |
| Procyanidin B2 | 3.7 |
| Other B-type dimer | 0.95 |
| Procyanidin C1 | 2.72 |
| Other B-type trimer | 0.43 |
| Other B-type tetramer | 0.56 |
| Other B-type pentamer | 0.2 |
| Theobromine | 19.26 |
| Caffeine | 1.45 |
| Caffeoyl asparitic acid | 1.83 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Lakshman, S.; Beshah, E.; Xie, Y.; Molokin, A.; Vinyard, B.T.; Urban, J.F., Jr.; Davis, C.D.; Solano-Aguilar, G.I. Flavanol-Rich Cocoa Powder Interacts with Lactobacillus rhamnossus LGG to Alter the Antibody Response to Infection with the Parasitic Nematode Ascaris suum. Nutrients 2017, 9, 1113. https://doi.org/10.3390/nu9101113
Jang S, Lakshman S, Beshah E, Xie Y, Molokin A, Vinyard BT, Urban JF Jr., Davis CD, Solano-Aguilar GI. Flavanol-Rich Cocoa Powder Interacts with Lactobacillus rhamnossus LGG to Alter the Antibody Response to Infection with the Parasitic Nematode Ascaris suum. Nutrients. 2017; 9(10):1113. https://doi.org/10.3390/nu9101113
Chicago/Turabian StyleJang, Saebyeol, Sukla Lakshman, Ethiopia Beshah, Yue Xie, Aleksey Molokin, Bryan T. Vinyard, Joseph F. Urban, Jr., Cindy D. Davis, and Gloria I. Solano-Aguilar. 2017. "Flavanol-Rich Cocoa Powder Interacts with Lactobacillus rhamnossus LGG to Alter the Antibody Response to Infection with the Parasitic Nematode Ascaris suum" Nutrients 9, no. 10: 1113. https://doi.org/10.3390/nu9101113





