Preventive Effects of Drinking Hydrogen-Rich Water on Gingival Oxidative Stress and Alveolar Bone Resorption in Rats Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Measurements of Serum Parameters
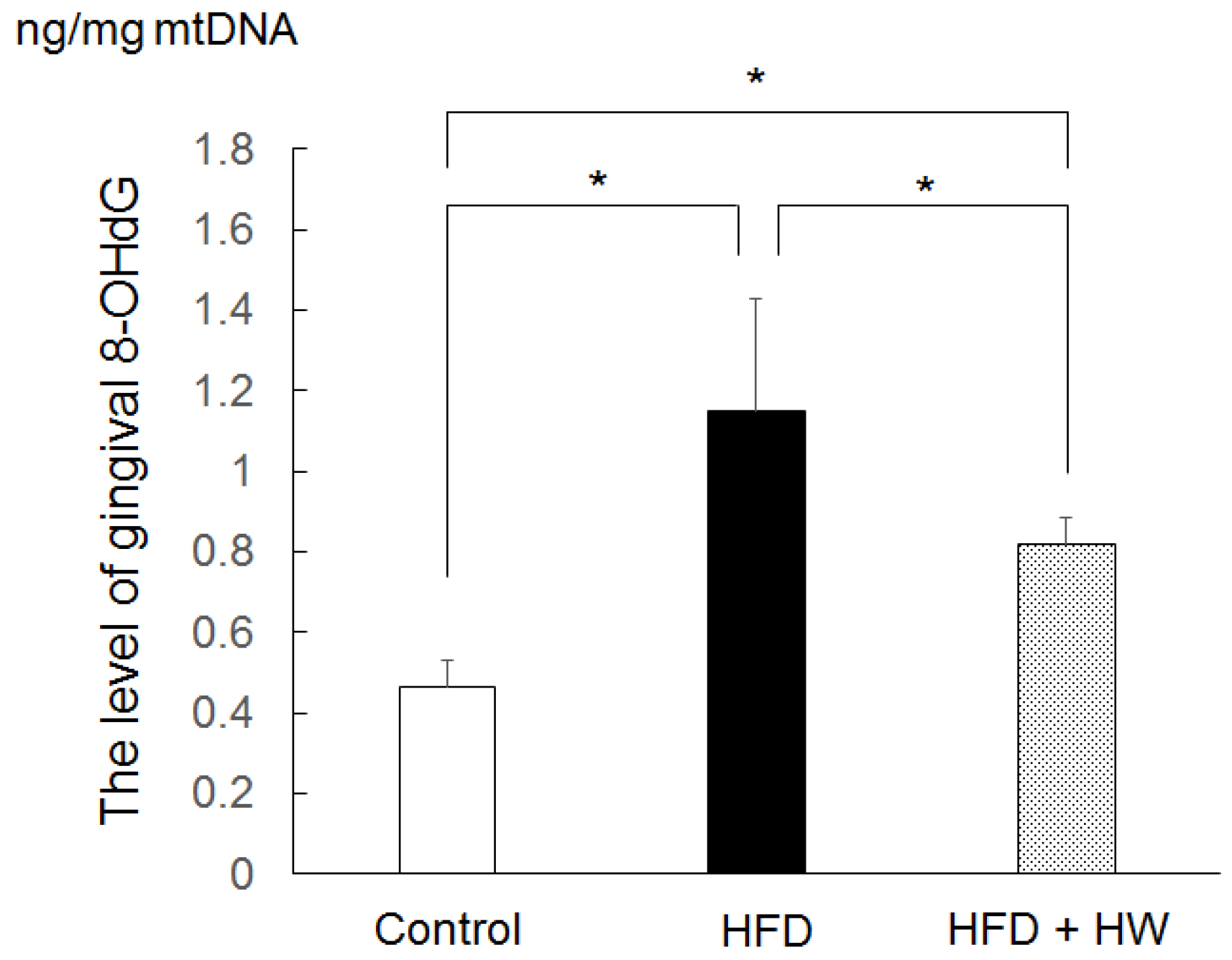
2.4. Measurements of Gingival Level of 8-OHdG
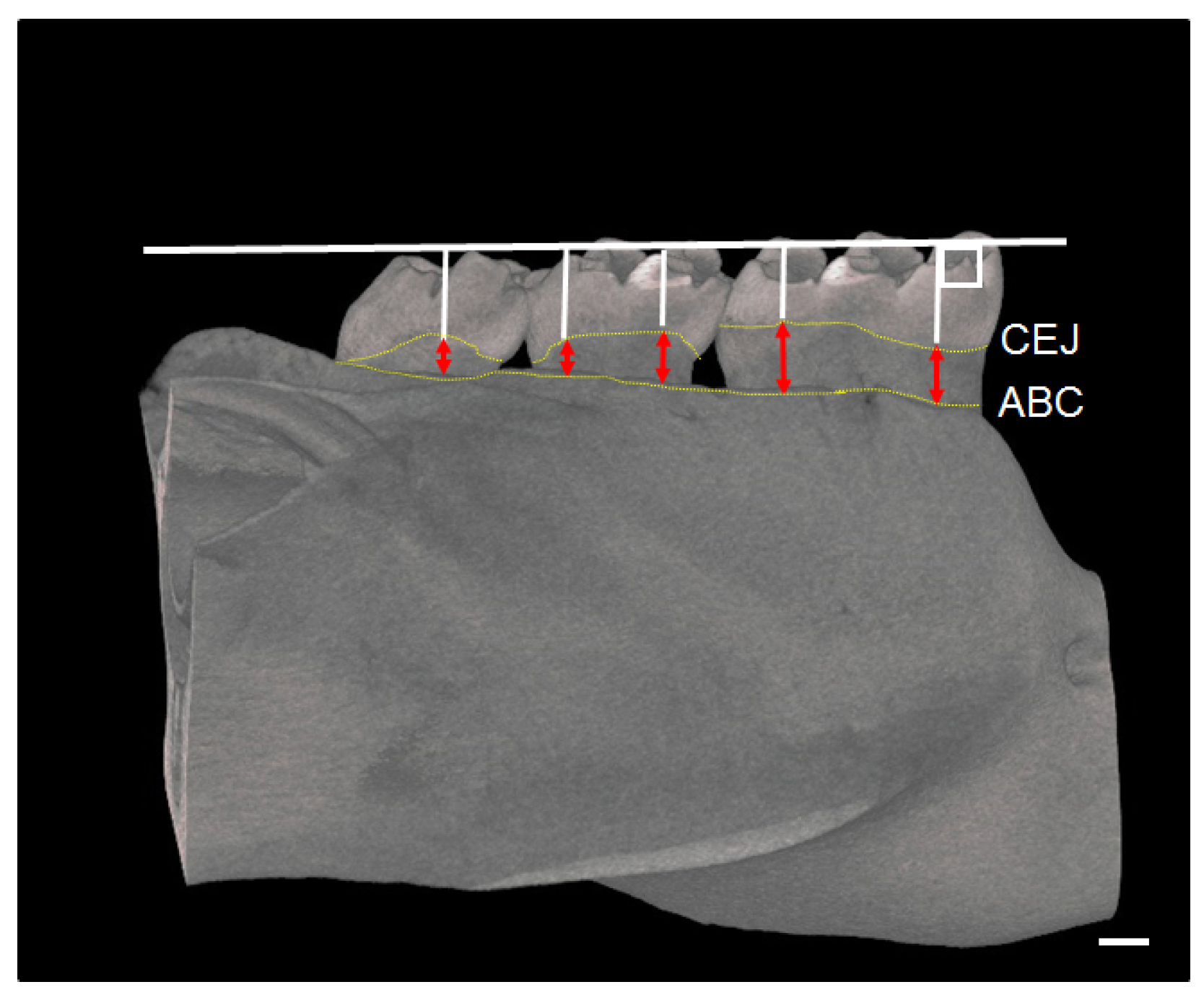
2.5. Micro-Computed Tomography (CT) Assessment of Mandible
2.6. RNA Isolation and PCR Array Analysis
2.7. Statistical Analysis
3. Results
3.1. Results of Body Weight and Gain of Body Weight
3.2. Results of Serum Levels of Cholesterols and 8-OHdG
3.3. Results of Gingival Level of 8-OHdG
3.4. Results of Micro-CT Analyses of Mandibular Bone
3.5. Results of Changes in Oxidative Stress-Related Gene Expression
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nascimento, G.G.; Leite, F.R.; Do, L.G.; Peres, K.G.; Correa, M.B.; Demarco, F.F.; Peres, M.A. Is weight gain associated with the incidence of periodontitis? A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Gaio, E.J.; Haas, A.N.; Rosing, C.K.; Oppermann, R.V.; Albandar, J.M.; Susin, C. Effect of obesity on periodontal attachment loss progression: A 5-year population-based prospective study. J. Clin. Periodontol. 2016, 43, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sanchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Chapple, I.L. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, S.; Lee, H.; Yang, Y.; Jeong, W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic. Biol. Med. 2013, 65, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Bartell, S.M.; Kim, H.N.; Ambrogini, E.; Han, L.; Iyer, S.; Serra Ucer, S.; Rabinovitch, P.; Jilka, R.L.; Weinstein, R.S.; Zhao, H.; et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat. Commun. 2014, 5, 3773. [Google Scholar] [CrossRef] [PubMed]
- Sanbe, T.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Tamaki, N.; Yamamoto, T. Oral administration of vitamin C prevents alveolar bone resorption induced by high dietary cholesterol in rats. J. Periodontol. 2007, 78, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, F.; Shinohara, F.; Kajiya, M.; Kodama, T. The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J. Biol. Chem. 2013, 288, 23009–23020. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Bandow, K.; Kakimoto, K.; Machigashira, M.; Matsuyama, T.; Matsuguchi, T. Oxidative stress causes alveolar bone loss in metabolic syndrome model mice with type 2 diabetes. J. Periodontal Res. 2009, 44, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bosca, A.B.; Miclaus, V.; Ilea, A.; Campian, R.S.; Rus, V.; Ruxanda, F.; Ratiu, C.; Uifalean, A.; Parvu, A.E. Role of nitro-oxidative stress in the pathogenesis of experimental rat periodontitis. Clujul Med. 2016, 89, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Newman, H.N.; Battino, M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: A shared pathology via oxidative stress and mitochondrial dysfunction? Periodontology 2000 2014, 64, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Sobue, S.; Yamai, K.; Ito, M.; Ohno, K.; Ito, M.; Iwamoto, T.; Qiao, S.; Ohkuwa, T.; Ichihara, M. Simultaneous oral and inhalational intake of molecular hydrogen additively suppresses signaling pathways in rodents. Mol. Cell Biochem. 2015, 403, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Tomofuji, T.; Kawabata, Y.; Kasuyama, K.; Endo, Y.; Yoneda, T.; Yamane, M.; Azuma, T.; Ekuni, D.; Morita, M. Effects of hydrogen-rich water on aging periodontal tissues in rats. Sci. Rep. 2014, 4, 5534. [Google Scholar] [CrossRef] [PubMed]
- Kasuyama, K.; Tomofuji, T.; Ekuni, D.; Tamaki, N.; Azuma, T.; Irie, K.; Endo, Y.; Morita, M. Hydrogen-rich water attenuates experimental periodontitis in a rat model. J. Clin. Periodontol. 2011, 38, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Orihuela-Campos, R.C.; Fukui, M.; Ito, H.O. Hydrogen-rich water intake accelerates oral palatal wound healing via activation of the Nrf2/antioxidant defense pathways in a rat model. Oxid. Med. Cell. Longev. 2016, 2016, 5679040. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H. Chemistry-based studies on oxidative DNA damage: Formation, repair, and mutagenesis. Free Radic. Biol. Med. 2002, 33, 450–456. [Google Scholar] [CrossRef]
- Fujita, Y.; Maki, K. High-fat diet-induced obesity triggers alveolar bone loss and spontaneous periodontal disease in growing mice. BMC Obes. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Muluke, M.; Gold, T.; Kiefhaber, K.; Al-Sahli, A.; Celenti, R.; Jiang, H.; Cremers, S.; van Dyke, T.; Schulze-Spate, U. Diet-induced obesity and its differential impact on periodontal bone loss. J. Dent. Res. 2016, 95, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Tomofuji, T.; Endo, Y.; Tamaki, N.; Ekuni, D.; Irie, K.; Kasuyama, K.; Kato, T.; Morita, M. Effects of exercise training on gingival oxidative stress in obese rats. Arch. Oral Biol. 2011, 56, 768–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usui, S.; Yasuda, H.; Koketsu, Y. Lipoprotein cholesterol and triglyceride concentrations associated with dog body condition score; effect of recommended fasting duration on sample concentrations in Japanese private clinics. J. Vet. Med. Sci. 2015, 77, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Ekuni, D.; Tomofuji, T.; Tamaki, N.; Sanbe, T.; Azuma, T.; Yamanaka, R.; Yamamoto, T.; Watanabe, T. Mechanical stimulation of gingiva reduces plasma 8-OHdG level in rat periodontitis. Arch. Oral Biol. 2008, 53, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Koide, M.; Kobayashi, Y.; Ninomiya, T.; Nakamura, M.; Yasuda, H.; Arai, Y.; Okahashi, N.; Yoshinari, N.; Takahashi, N.; Udagawa, N. Osteoprotegerin-deficient male mice as a model for severe alveolar bone loss: Comparison with RANKL-overexpressing transgenic male mice. Endocrinology 2013, 154, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity 2011, 19, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Landis, G.N.; Tower, J. Superoxide dismutase evolution and life span regulation. Mech. Ageing Dev. 2005, 126, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Youssoufian, H. Fanconi anaemia proteins: Major roles in cell protection against oxidative damage. Bioessays 2003, 25, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Tanko, L.B.; Bagger, Y.Z.; Nielsen, S.B.; Christiansen, C. Does serum cholesterol contribute to vertebral bone loss in postmenopausal women? Bone 2003, 32, 8–14. [Google Scholar] [CrossRef]
- Donaldson, J.; Pillay, K.; Madziva, M.T.; Erlwanger, K.H. The effect of different high-fat diets on erythrocyte osmotic fragility, growth performance and serum lipid concentrations in male, Japanese quail (Coturnix coturnix japonica). J. Anim. Physiol. Anim. Nutr. 2014, 99, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Peebles, E.D.; Cheaney, J.D.; Brake, J.D.; Boyle, C.R.; Latour, M.A.; McDaniel, C.D. Effects of added lard fed to broiler chickens during the starter phase. 2. Serum lipids. Poult. Sci. 1997, 76, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Atabay, V.E.; Lutfioglu, M.; Avci, B.; Sakallioglu, E.E.; Aydogdu, A. Obesity and oxidative stress in patients with different periodontal status: A case-control study. J. Periodontal Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dursun, E.; Akalin, F.A.; Genc, T.; Cinar, N.; Erel, O.; Yildiz, B.O. Oxidative stress and periodontal disease in obesity. Medicine 2016, 95, e3136. [Google Scholar] [CrossRef] [PubMed]
- Tomofuji, T.; Ekuni, D.; Azuma, T.; Irie, K.; Endo, Y.; Yamamoto, T.; Ishikado, A.; Sato, T.; Harada, K.; Suido, H.; et al. Supplementation of broccoli or Bifidobacterium longum-fermented broccoli suppresses serum lipid peroxidation and osteoclast differentiation on alveolar bone surface in rats fed a high-cholesterol diet. Nutr. Res. 2012, 31, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Varela-Lopez, A.; Bullon, P.; Battino, M.; Ramirez-Tortosa, M.C.; Ochoa, J.J.; Cordero, M.D.; Ramirez-Tortosa, C.L.; Rubini, C.; Zizzi, A.; Quiles, J.L. Coenzyme Q protects against age-related alveolar bone loss associated to n-6 polyunsaturated fatty acid rich-diets by modulating mitochondrial mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 593–600. [Google Scholar] [CrossRef] [PubMed]

| Control | HFD | HFD + HW | |
|---|---|---|---|
| body weight (baseline) (g) | 269 ± 12 | 278 ± 14 | 276 ± 9 |
| body weight (20 weeks old) (g) | 338 ± 14 | 360 ± 16 * | 342 ± 12 |
| body weight gain (20 weeks old—base line) (g) | 69 ± 8 | 82 ± 10 * | 66 ± 7 † |
| Control | HFD | HFD + HW | |
|---|---|---|---|
| total cholesterol (mg/dL) | 43.2 ± 24.8 | 101.1 ± 53.7 * | 94.3 ± 18.0 |
| VLDL cholesterol (mg/dL) | 5.2 ± 3.3 | 34.6 ± 20.3 * | 34.1 ± 6.4 * |
| LDL cholesterol (mg/dL) | 14.9 ± 9.6 | 15.6 ± 7.7 | 16.8 ± 4.2 |
| HDL cholesterol (mg/dL) | 21.7 ± 11.5 | 27.3 ± 11.9 | 27.9 ± 5.2 |
| total triglycerides (mg/dL) | 65.3 ± 46.8 | 27.6 ± 14.3 | 19.1 ± 5.3 * |
| VLDL triglycerides (mg/dL) | 43.3 ± 29.5 | 14.2 ± 8.0 * | 10.1 ± 3.4 * |
| LDL triglycerides (mg/dL) | 4.88 ± 3.4 | 2.86 ± 1.3 | 1.98 ± 0.4 |
| HDL triglycerides (mg/dL) | 3.6 ± 2.1 | 3.0 ± 1.1 | 2.6 ± 0.5 |
| 8-OHdG (ng/mL) | 0.12 ± 0.03 | 0.17 ± 0.05 * | 0.12 ± 0.03 † |
| Gene Symbol | Description | Fold Up- or Down Regulation | p-Value |
|---|---|---|---|
| (HFD+HW Group/HFD Group) | |||
| Idh1 | isocitrate dehydrogenase (NADP(+)) 1, cytosolic | 4.04 | 0.014 |
| Sod2 | superoxide dismutase 2 | 2.25 | 0.032 |
| Sod3 | superoxide dismutase 3 | 3.35 | 0.034 |
| Fancc | Fanconi anemia, complementation group C | −2.88 | 0.046 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneda, T.; Tomofuji, T.; Kunitomo, M.; Ekuni, D.; Irie, K.; Azuma, T.; Machida, T.; Miyai, H.; Fujimori, K.; Morita, M. Preventive Effects of Drinking Hydrogen-Rich Water on Gingival Oxidative Stress and Alveolar Bone Resorption in Rats Fed a High-Fat Diet. Nutrients 2017, 9, 64. https://doi.org/10.3390/nu9010064
Yoneda T, Tomofuji T, Kunitomo M, Ekuni D, Irie K, Azuma T, Machida T, Miyai H, Fujimori K, Morita M. Preventive Effects of Drinking Hydrogen-Rich Water on Gingival Oxidative Stress and Alveolar Bone Resorption in Rats Fed a High-Fat Diet. Nutrients. 2017; 9(1):64. https://doi.org/10.3390/nu9010064
Chicago/Turabian StyleYoneda, Toshiki, Takaaki Tomofuji, Muneyoshi Kunitomo, Daisuke Ekuni, Koichiro Irie, Tetsuji Azuma, Tatsuya Machida, Hisataka Miyai, Kouhei Fujimori, and Manabu Morita. 2017. "Preventive Effects of Drinking Hydrogen-Rich Water on Gingival Oxidative Stress and Alveolar Bone Resorption in Rats Fed a High-Fat Diet" Nutrients 9, no. 1: 64. https://doi.org/10.3390/nu9010064





