Effect of Carotenoid Supplemented Formula on Carotenoid Bioaccumulation in Tissues of Infant Rhesus Macaques: A Pilot Study Focused on Lutein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Diets
2.3. Plasma, Serum, and Tissue Collection
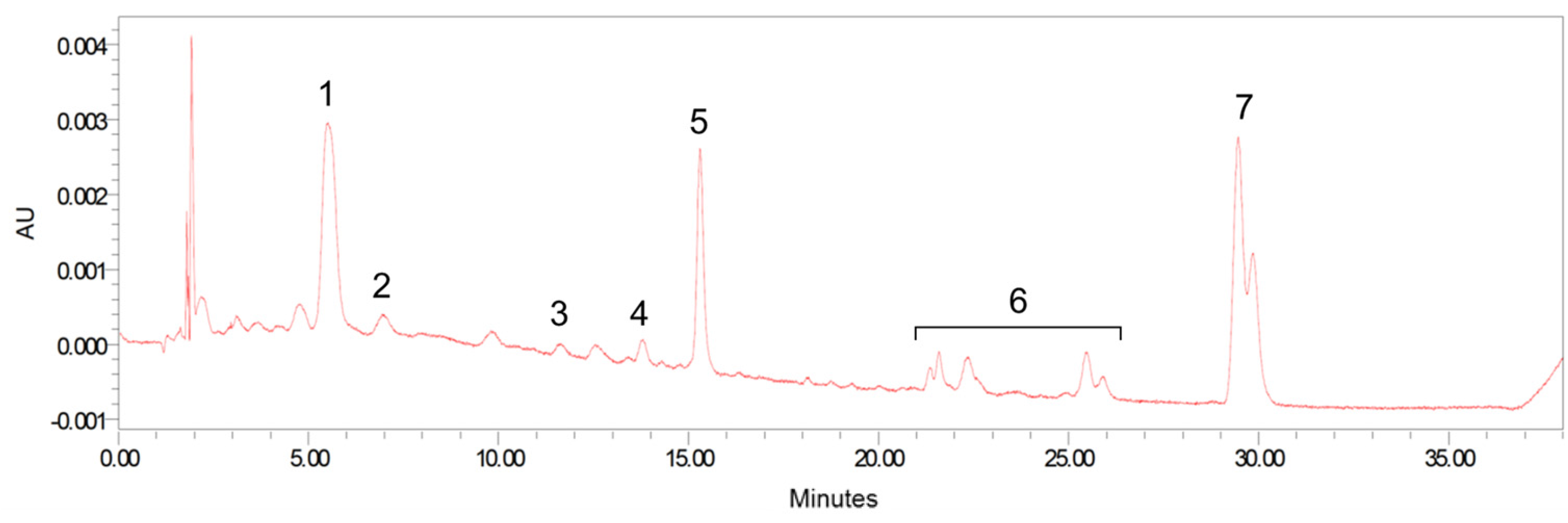
2.4. Carotenoid Analysis
2.4.1. Formula Extraction for Carotenoids
2.4.2. Plasma or Serum Extraction for Carotenoids
2.4.3. Brain Extraction for Carotenoids
2.4.4. Retinal Extraction for Carotenoids
2.4.5. Adipose Tissue Extraction for Carotenoids
2.4.6. Extraction of Other Tissues for Carotenoids
2.4.7. HPLC
2.5. Statistical Analysis
3. Results
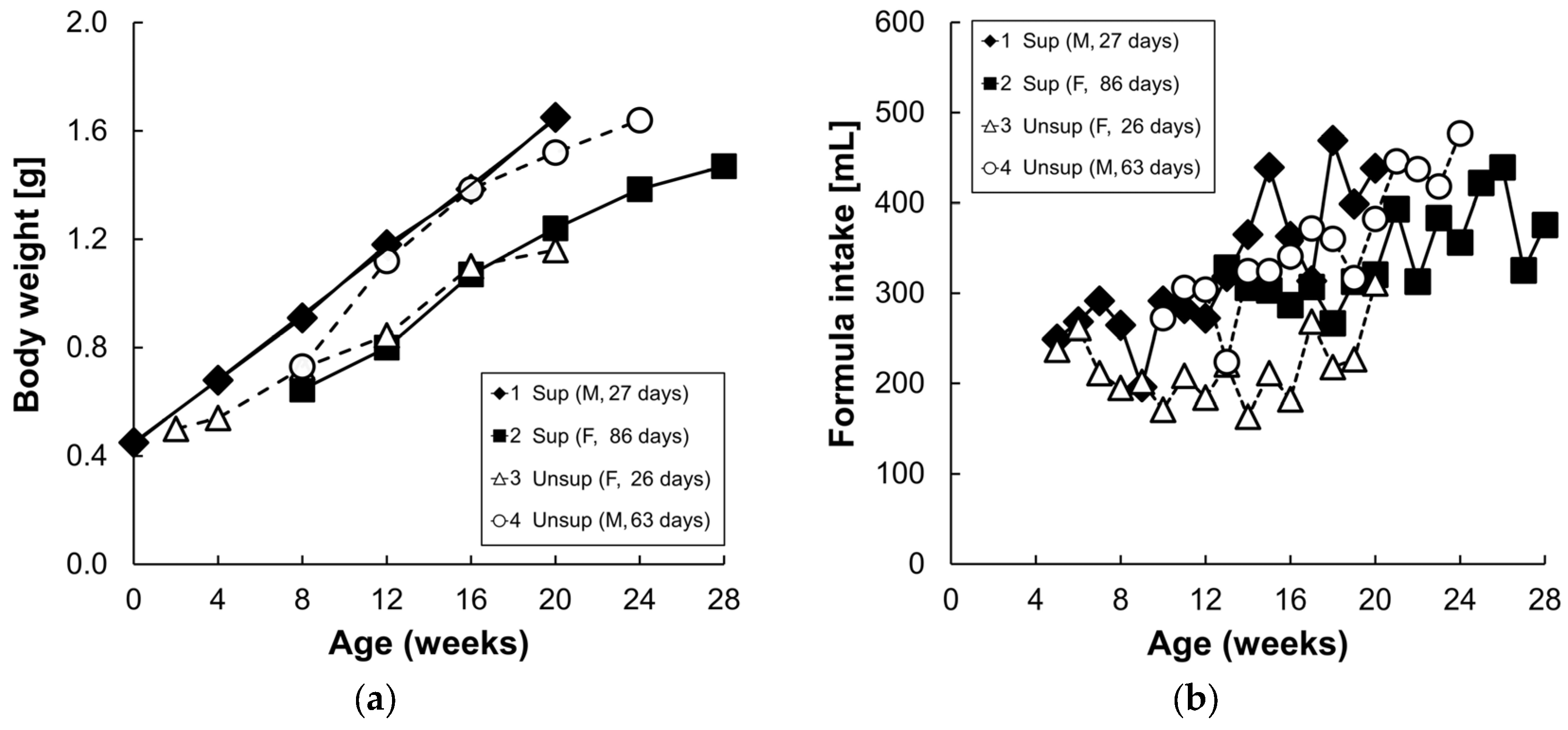
3.1. Formula Carotenoids Profile, Formula Intake, and Body Weight
3.2. Plasma/Serum Carotenoids
3.3. Brain Carotenoids
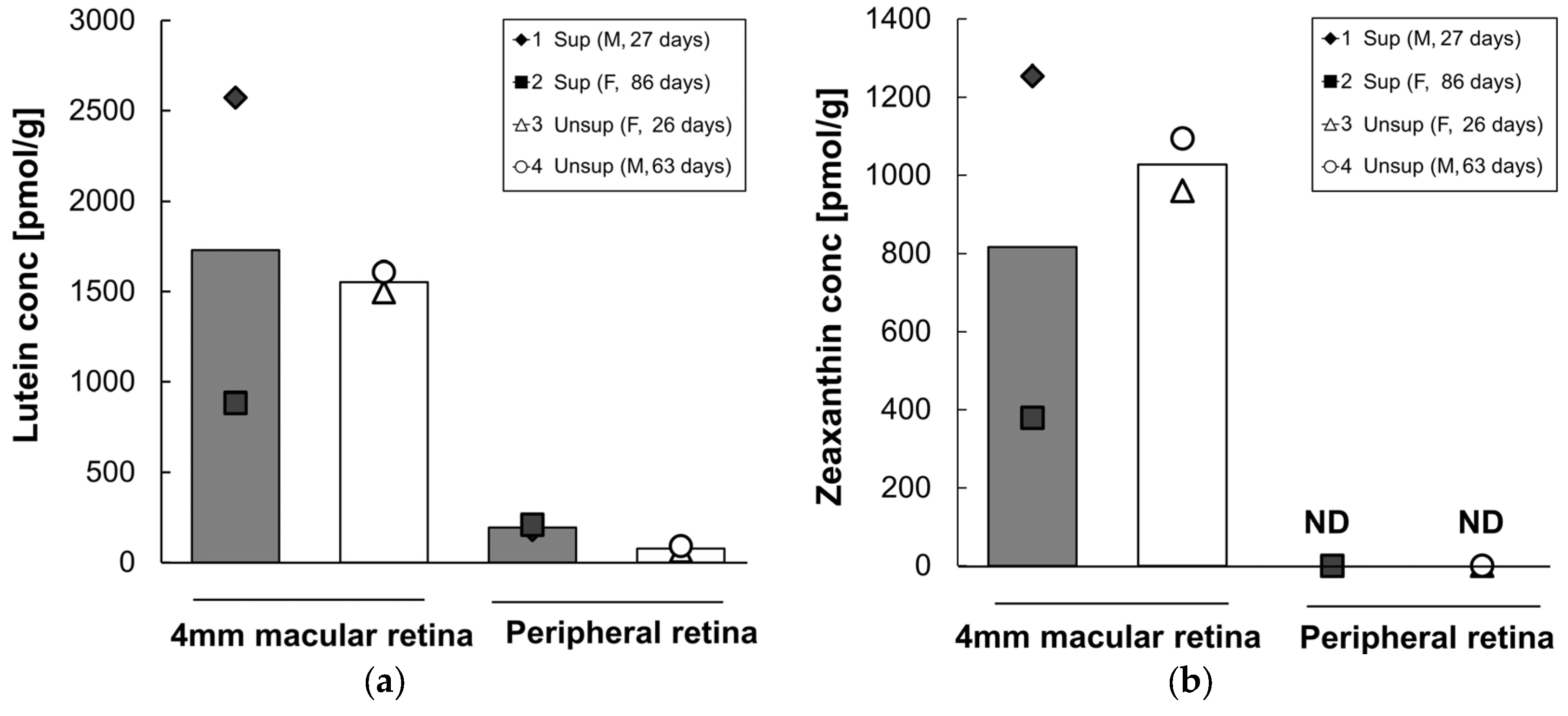
3.4. Retinal Carotenoids
3.5. Adipose Tissue Carotenoids
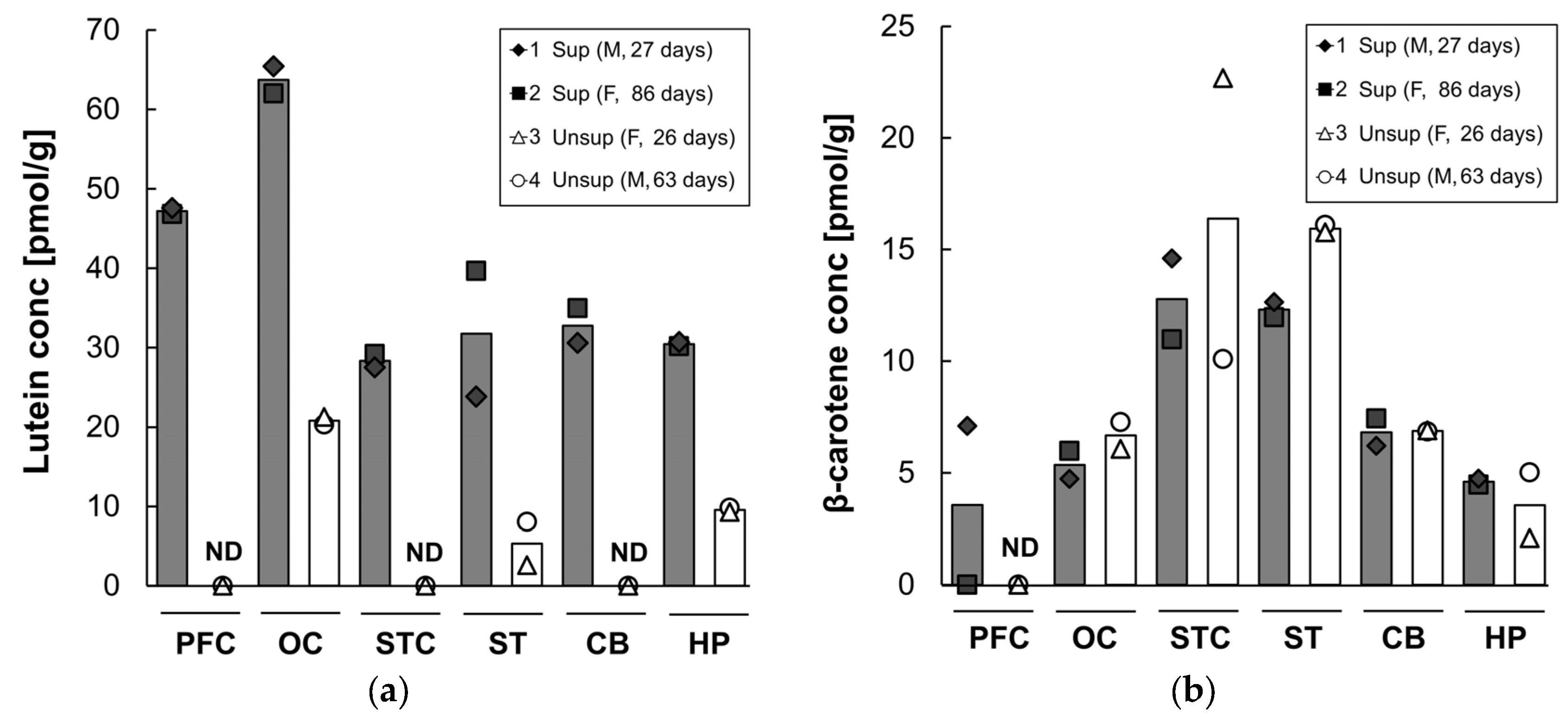
3.6. Carotenoids in Other Tissues
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sommerburg, O.; Keunen, J.E.; Bird, A.C.; van Kuijk, F.J. Fruits and vegetables that are sources for lutein and zeaxanthin: The macular pigment in human eyes. Br. J. Ophthalmol. 1998, 82, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Koushan, K.; Rusovici, R.; Li, W.; Ferguson, L.; Chalam, K. The role of lutein in eye-related disease. Nutrients 2013, 5, 1823–1839. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, E.T.; Darweesh, S.K.; Baena, C.P.; Moreira, E.M.; Melo van Lent, D.; Tielemans, M.J.; Muka, T.; Vitezova, A.; Chowdhury, R.; Bramer, W.M.; et al. The effects of lutein on cardiometabolic health across the life course: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the georgia centenarian study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Kuchan, M.J.; Sen, S.; Johnson, E.J. Lutein and preterm infants with decreased concentrations of brain carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.Y.; Troen, A.M.; Snodderly, D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Feeney, J.; Finucane, C.; Savva, G.M.; Cronin, H.; Beatty, S.; Nolan, J.M.; Kenny, R.A. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol. Aging 2013, 34, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Iannaccone, A.; Scott, T.M.; Kritchevsky, S.B.; Jennings, B.J.; Carboni, G.; Forma, G.; Satterfield, S.; Harris, T.; Johnson, K.C.; et al. Macular pigment optical density is related to cognitive function in older people. Age Ageing 2014, 43, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Renzi, L.M.; Dengler, M.J.; Puente, A.; Miller, L.S.; Hammond, B.R., Jr. Relationships between macular pigment optical density and cognitive function in unimpaired and mildly cognitively impaired older adults. Neurobiol. Aging 2014, 35, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.J.; Ferland, G.; Patry, J.; Russell, R.M. Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J. Am. Coll. Nutr. 1998, 17, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R., Jr. Possible role for dietary lutein and zeaxanthin in visual development. Nutr. Rev. 2008, 66, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Lieblein-Boff, J.C.; Johnson, E.J.; Kennedy, A.D.; Lai, C.S.; Kuchan, M.J. Exploratory metabolomic analyses reveal compounds correlated with lutein concentration in frontal cortex, hippocampus, and occipital cortex of human infant brain. PLoS ONE 2015, 10, e0136904. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Tei, M.; Longini, M.; Santacroce, A.; Turrisi, G.; Proietti, F.; Felici, C.; Picardi, A.; Bazzini, F.; Vasarri, P.; et al. Lipid and protein oxidation in newborn infants after lutein administration. Oxidative Med. Cell. Longev. 2014, 2014, 781454. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W., Jr.; Smith, J.W.; Kuchan, M.J.; Mohn, E.S.; Johnson, E.J.; Rubakhin, S.S.; Wang, L.; Sweedler, J.V.; Neuringer, M. Lutein and brain function. Foods 2015, 4, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Vachali, P.P.; Gorusupudi, A.; Shen, Z.; Sharifzadeh, H.; Besch, B.M.; Nelson, K.; Horvath, M.M.; Frederick, J.M.; Baehr, W.; et al. Inactivity of human beta,beta-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc. Natl. Acad. Sci. USA 2014, 111, 10173–10178. [Google Scholar] [CrossRef] [PubMed]
- Babino, D.; Palczewski, G.; Widjaja-Adhi, M.A.; Kiser, P.D.; Golczak, M.; von Lintig, J. Characterization of the role of beta-carotene 9,10-dioxygenase in macular pigment metabolism. J. Biol. Chem. 2015, 290, 24844–24857. [Google Scholar] [CrossRef] [PubMed]
- Palczewski, G.; Amengual, J.; Hoppel, C.L.; von Lintig, J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J. 2014, 28, 4457–4469. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Qin, J.; Krinsky, N.I.; Russell, R.M. Beta-carotene isomers in human serum, breast milk and buccal mucosa cells after continuous oral doses of all-trans and 9-cis beta-carotene. J. Nutr. 1997, 127, 1993–1999. [Google Scholar] [PubMed]
- Nierenberg, D.W.; Nann, S.L. A method for determining concentrations of retinol, tocopherol, and five carotenoids in human plasma and tissue samples. Am. J. Clin Nutr. 1992, 56, 417–426. [Google Scholar] [PubMed]
- Vishwanathan, R.; Neuringer, M.; Snodderly, D.M.; Schalch, W.; Johnson, E.J. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr. Neurosci. 2013, 16, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sy, C.; Gleize, B.; Dangles, O.; Landrier, J.F.; Veyrat, C.C.; Borel, P. Effects of physicochemical properties of carotenoids on their bioaccessibility, intestinal cell uptake, and blood and tissue concentrations. Mol. Nutr. Food Res. 2012, 56, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Conlon, L.E.; King, R.D.; Moran, N.E.; Erdman, J.W., Jr. Coconut oil enhances tomato carotenoid tissue accumulation compared to safflower oil in the mongolian gerbil (meriones unguiculatus). J. Agric. Food Chem. 2012, 60, 8386–8394. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.J.; Booth, S.L.; Sadowski, J.A.; Liu, C.; Tang, G.; Krinsky, N.I.; Russell, R.M. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am. J. Clin. Nutr. 1996, 64, 594–602. [Google Scholar] [PubMed]
- Bettler, J.; Zimmer, J.P.; Neuringer, M.; DeRusso, P.A. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur. J. Nutr. 2010, 49, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Kobayashi, M.; Terasaki, M.; Nagao, A. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J. Nutr. 2010, 140, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Neuringer, M.; Russell, R.M.; Schalch, W.; Snodderly, D.M. Nutritional manipulation of primate retinas, iii: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investig. Ophthalmol. Vis. Sci. 2005, 46, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T.; Guerra, L.H.; Ruiz, C.A. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J. Nutr. 2003, 133, 992–998. [Google Scholar] [PubMed]
- Landrum, J.T.; Bone, R.A.; Joa, H.; Kilburn, M.D.; Moore, L.L.; Sprague, K.E. A one year study of the macular pigment: The effect of 140 days of a lutein supplement. Exp. Eye Res. 1997, 65, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.H.; Murray, I.J.; Nolan, D.; Carden, D.; Feather, J.; Beatty, S. Plasma and macular responses to lutein supplement in subjects with and without age-related maculopathy: A pilot study. Exp. Eye Res. 2004, 79, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.L.; Aleman, T.S.; Gardner, L.M.; De Castro, E.; Marks, D.A.; Emmons, J.M.; Bieber, M.L.; Steinberg, J.D.; Bennett, J.; Stone, E.M.; et al. Macular pigment and lutein supplementation in choroideremia. Exp. Eye Res. 2002, 74, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Aleman, T.S.; Duncan, J.L.; Bieber, M.L.; de Castro, E.; Marks, D.A.; Gardner, L.M.; Steinberg, J.D.; Cideciyan, A.V.; Maguire, M.G.; Jacobson, S.G. Macular pigment and lutein supplementation in retinitis pigmentosa and usher syndrome. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1873–1881. [Google Scholar]
- Johnson, E.J.; Chung, H.Y.; Caldarella, S.M.; Snodderly, D.M. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am. J. Clin. Nutr. 2008, 87, 1521–1529. [Google Scholar] [PubMed]
- Bartlett, H.; Howells, O.; Eperjesi, F. The role of macular pigment assessment in clinical practice: A review. Clin. Exp. Optom. 2010, 93, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Goulinet, S.; Chapman, M.J. Plasma ldl and hdl subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Relevance to oxidative resistance and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Romanchik, J.E.; Morel, D.W.; Harrison, E.H. Distributions of carotenoids and alpha-tocopherol among lipoproteins do not change when human plasma is incubated in vitro. J. Nutr. 1995, 125, 2610–2617. [Google Scholar] [PubMed]
- Wang, W.; Connor, S.L.; Johnson, E.J.; Klein, M.L.; Hughes, S.; Connor, W.E. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am. J. Clin. Nutr. 2007, 85, 762–769. [Google Scholar] [PubMed]
- During, A.; Doraiswamy, S.; Harrison, E.H. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a srbi-dependent mechanism. J. Lipid Res. 2008, 49, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Vachali, P.; Frederick, J.M.; Bernstein, P.S. Identification of stard3 as a lutein-binding protein in the macula of the primate retina. Biochemistry 2011, 50, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Tanprasertsuk, J.; Li, B.; Bernstein, P.S.; Vishwanathan, R.; Johnson, M.A.; Poon, L.; Johnson, E.J. Relationship between concentrations of lutein and stard3 among pediatric and geriatric human brain tissue. PLoS ONE 2016, 11, e0155488. [Google Scholar]
- Chung, H.Y.; Ferreira, A.L.; Epstein, S.; Paiva, S.A.; Castaneda-Sceppa, C.; Johnson, E.J. Site-specific concentrations of carotenoids in adipose tissue: Relations with dietary and serum carotenoid concentrations in healthy adults. Am. J. Clin. Nutr. 2009, 90, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.P.; Fong, B.S.; Julien, P.; Jimenez, J.; Angel, A. Regional variation in HDL metabolism in human fat cells: Effect of cell size. Am. J. Physiol. 1987, 252, E654–E659. [Google Scholar] [PubMed]
- Benoist, F.; Lau, P.; McDonnell, M.; Doelle, H.; Milne, R.; McPherson, R. Cholesteryl ester transfer protein mediates selective uptake of high density lipoprotein cholesteryl esters by human adipose tissue. J. Biol. Chem. 1997, 272, 23572–23577. [Google Scholar] [CrossRef] [PubMed]
- Dusserre, E.; Moulin, P.; Vidal, H. Differences in mrna expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim. Biophys. Acta 2000, 1500, 88–96. [Google Scholar] [CrossRef]
- Kim, J.H.; Na, H.J.; Kim, C.K.; Kim, J.Y.; Ha, K.S.; Lee, H.; Chung, H.T.; Kwon, H.J.; Kwon, Y.G.; Kim, Y.M. The non-provitamin A carotenoid, lutein, inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: Role of H(2)O(2) in NF-kappaB activation. Free Radic. Biol. Med. 2008, 45, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Izumi-Nagai, K.; Nagai, N.; Ohgami, K.; Satofuka, S.; Ozawa, Y.; Tsubota, K.; Umezawa, K.; Ohno, S.; Oike, Y.; Ishida, S. Macular pigment lutein is antiinflammatory in preventing choroidal neovascularization. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Leite, J.O.; DeOgburn, R.; Smyth, J.A.; Clark, R.M.; Fernandez, M.L. A lutein-enriched diet prevents cholesterol accumulation and decreases oxidized LDL and inflammatory cytokines in the aorta of guinea pigs. J. Nutr. 2011, 141, 1458–1463. [Google Scholar] [CrossRef] [PubMed]

| Formula Type | Lutein | Zeaxanthin | β-Carotene | Lycopene | β-Cryptoxanthin | α-Carotene |
|---|---|---|---|---|---|---|
| Supplemented formula (Similac) | 248 | 23 | 88 | 362 | ND 2 | ND |
| Unsupplemented formula | 16 | ND | 32 | ND | ND | ND |
| Group, Animal ID | Gender | Age (Days) at Enrollment |
|---|---|---|
| Supplemented formula | ||
| 1 | Male | 27 |
| 2 | Female | 86 |
| Unsupplemented formula | ||
| 3 | Female | 26 |
| 4 | Male | 63 |
| Carotenoid | Group, Animal ID | MAT | ASAT | TSAT | BAT |
|---|---|---|---|---|---|
| Mean (Individual Values), pmol/g | |||||
| β-carotene | Supplemented | 77 | 125 | 56 | 105 |
| (1, 2) | (49, 105) | (91, 159) | (68, 45) | (107, 102) | |
| Unsupplemented | 82 | 83 | 100 | 59 | |
| (3, 4) | (61, 103) | (32, 133) | (131, 68) | (43, 75) | |
| Lutein | Supplemented | 39 | 112 | 88 | 63 |
| (1, 2) | (25, 53) | (71, 154) | (69, 107) | (78, 47) | |
| Unsupplemented | 17 | 43 | 39 | 5 | |
| (3, 4) | (13, 21) | (35, 52) | (34, 43) | (ND, 10) | |
| Total lycopene | Supplemented | 227 | 391 | 350 | 333 |
| (1, 2) | (162, 291) | (280, 502) | (220, 481) | (295, 371) | |
| Unsupplemented | ND 1 | ND | ND | ND | |
| (3, 4) | |||||
| Carotenoid | Group (Animal ID) | Liver | Lung | Kidney | Heart | Quadriceps | Spleen |
|---|---|---|---|---|---|---|---|
| Mean (Individual Values), pmol/g | |||||||
| β-carotene | Supplemented | 56 | 134 | 118 | 122 | 116 | 105 |
| (1, 2) | (51, 61) | (125, 142) | (121, 115) | (124, 120) | (117, 115) | (86, 125) | |
| Unsupplemented | 29 | 128 | 113 | 57 | 111 | 59 | |
| (3, 4) | (29, 30) | (131, 125) | (103, 122) | (0, 114) | (117, 104) | (56, 63) | |
| Lutein | Supplemented | 505 | 120 | 67 | 60 | 54 | 227 |
| (1, 2) | (453, 557) | (129, 111) | (66, 67) | (63, 56) | (52, 56) | (219, 236) | |
| Unsupplemented | 59 | 69 | 35 | 35 | 34 | 67 | |
| (3, 4) | (54, 63) | (93, 44) | (30, 39) | (34, 35) | (37, 31) | (71, 63) | |
| Zeaxanthin | Supplemented | 34 | ND1 | ND | ND | ND | ND |
| (1, 2) | (33, 35) | ||||||
| Unsupplemented | ND | ND | ND | ND | ND | ND | |
| (3, 4) | |||||||
| Total lycopene | Supplemented | 376 | 209 | 183 | 148 | 150 | 278 |
| (1, 2) | (253, 500) | (174, 243) | (167, 198) | (134, 161) | (105, 194) | (139, 418) | |
| Unsupplemented | ND | ND | ND | ND | ND | ND | |
| (3, 4) | |||||||
| α-carotene | Supplemented | 18 | ND | ND | ND | ND | ND |
| (1, 2) | (ND, 35) | ||||||
| Unsupplemented | 34 | ND | ND | ND | ND | ND | |
| (3, 4) | (33, 35) | ||||||
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.; Neuringer, M.; Johnson, E.E.; Kuchan, M.J.; Pereira, S.L.; Johnson, E.J.; Erdman, J.W. Effect of Carotenoid Supplemented Formula on Carotenoid Bioaccumulation in Tissues of Infant Rhesus Macaques: A Pilot Study Focused on Lutein. Nutrients 2017, 9, 51. https://doi.org/10.3390/nu9010051
Jeon S, Neuringer M, Johnson EE, Kuchan MJ, Pereira SL, Johnson EJ, Erdman JW. Effect of Carotenoid Supplemented Formula on Carotenoid Bioaccumulation in Tissues of Infant Rhesus Macaques: A Pilot Study Focused on Lutein. Nutrients. 2017; 9(1):51. https://doi.org/10.3390/nu9010051
Chicago/Turabian StyleJeon, Sookyoung, Martha Neuringer, Emily E. Johnson, Matthew J. Kuchan, Suzette L. Pereira, Elizabeth J. Johnson, and John W. Erdman. 2017. "Effect of Carotenoid Supplemented Formula on Carotenoid Bioaccumulation in Tissues of Infant Rhesus Macaques: A Pilot Study Focused on Lutein" Nutrients 9, no. 1: 51. https://doi.org/10.3390/nu9010051




