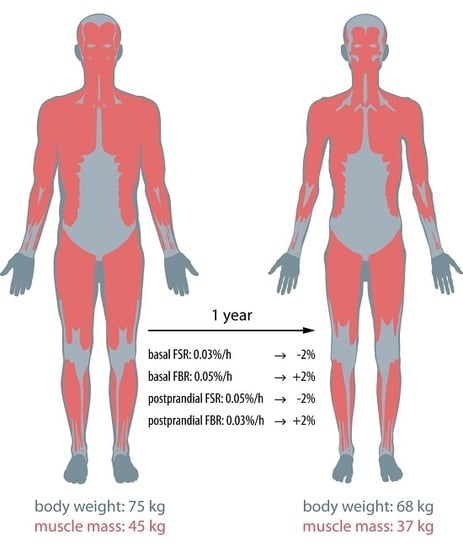
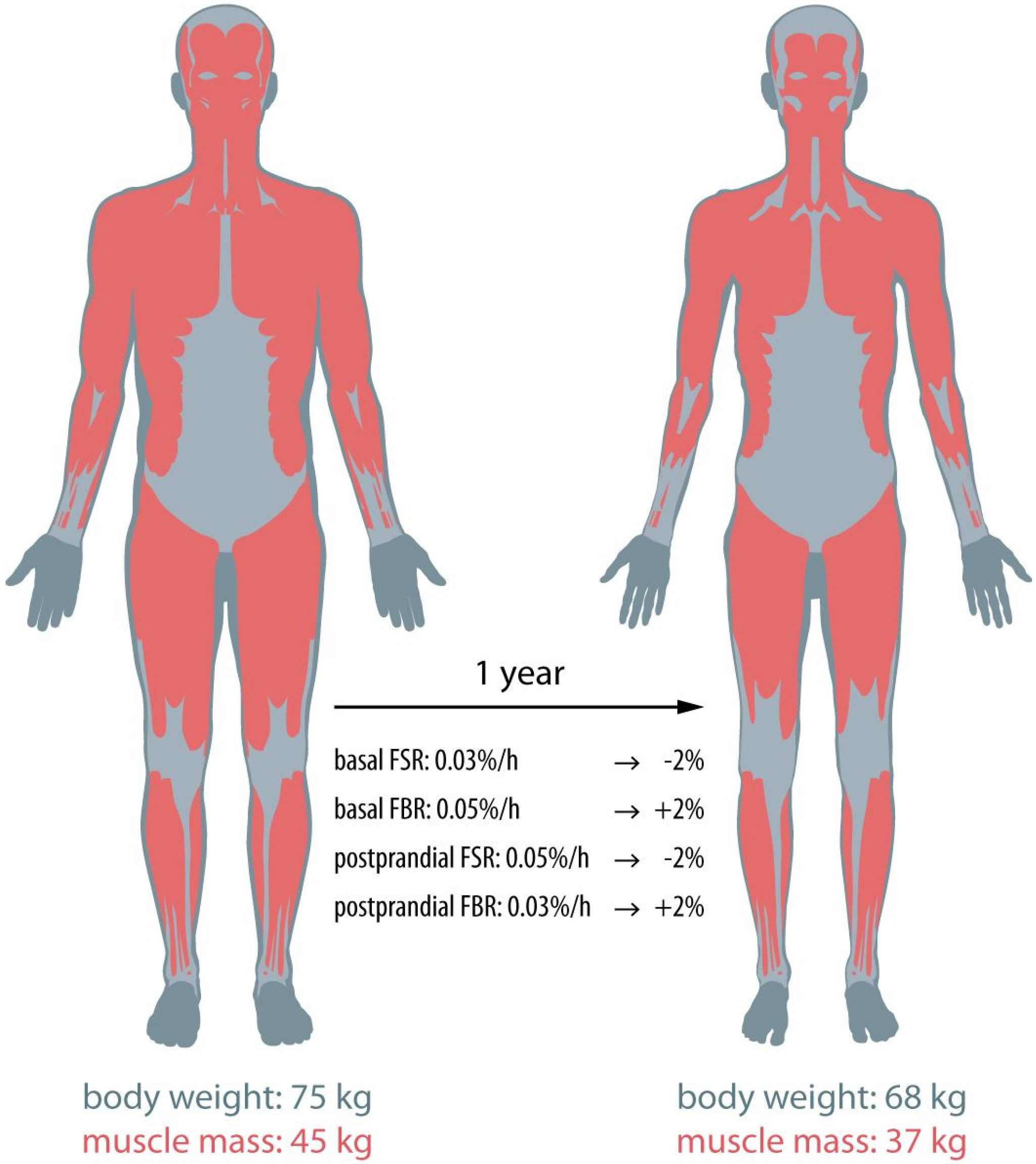
Is Cancer Cachexia Attributed to Impairments in Basal or Postprandial Muscle Protein Metabolism?
Abstract
:1. Introduction
2. Post-Absorptive Muscle Protein Metabolism
3. Postprandial Muscle Metabolism
4. Conclusions and Future Directions
Author Contributions
Conflicts of Interest
References
- Moses, A.W.; Slater, C.; Preston, T.; Barber, M.D.; Fearon, K.C. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br. J. Cancer 2004, 90, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, J.; Heiligensetzer, M.; Krakowski-Roosen, H.; Buchler, M.W.; Friess, H.; Martignoni, M.E. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J. Gastrointest. Surg. 2008, 12, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.; Voss, A.C.; Hustead, D.S.; Cancer Cachexia Study Group. Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–1350. [Google Scholar] [PubMed]
- Muscaritoli, M.; Bossola, M.; Aversa, Z.; Bellantone, R.; Rossi Fanelli, F. Prevention and treatment of cancer cachexia: New insights into an old problem. Eur. J. Cancer 2006, 42, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C. The mechanisms and treatment of weight loss in cancer. Proc. Nutr. Soc. 1992, 51, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Busquets, S.; Lopez-Soriano, F.J. The pivotal role of cytokines in muscle wasting during cancer. Int. J. Biochem. Cell Biol. 2005, 37, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.N.; Green, S.R.; Goggin, P.M. Cancer cachexia. QJM 2005, 98, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Churm, D.; Andrew, I.M.; Holden, K.; Hildreth, A.J.; Hawkins, C. A questionnaire study of the approach to the anorexia-cachexia syndrome in patients with cancer by staff in a district general hospital. Support Care Cancer 2009, 17, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Spiro, A.; Baldwin, C.; Patterson, A.; Thomas, J.; Andreyev, H.J. The views and practice of oncologists towards nutritional support in patients receiving chemotherapy. Br. J. Cancer 2006, 95, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Ebadi, M.; Mazurak, V.C. Evidence and mechanisms of fat depletion in cancer. Nutrients 2014, 6, 5280–5297. [Google Scholar] [CrossRef] [PubMed]
- Tsoli, M.; Swarbrick, M.M.; Robertson, G.R. Lipolytic and thermogenic depletion of adipose tissue in cancer cachexia. Semin. Cell Dev. Biol. 2016, 54, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Safar, A.; Schutzler, S.; Memelink, R.; Ferrando, A.; Spencer, H.; van Helvoort, A.; Wolfe, R.R. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin. Nutr. 2011, 30, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Dillon, E.L.; Basra, G.; Horstman, A.M.; Casperson, S.L.; Randolph, K.M.; Durham, W.J.; Urban, R.J.; Diaz-Arrastia, C.; Levine, L.; Hatch, S.S.; et al. Cancer cachexia and anabolic interventions: A case report. J. Cachexia Sarcopenia Muscle 2012, 3, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Dillon, E.L.; Volpi, E.; Wolfe, R.R.; Sinha, S.; Sanford, A.P.; Arrastia, C.D.; Urban, R.J.; Casperson, S.L.; Paddon-Jones, D.; Sheffield-Moore, M. Amino acid metabolism and inflammatory burden in ovarian cancer patients undergoing intense oncological therapy. Clin. Nutr. 2007, 26, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Phillips, B.E.; Smith, K.; Atherton, P.J.; Rankin, D.; Selby, A.L.; Liptrot, S.; Lund, J.; Larvin, M.; Rennie, M.J. Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am. J. Clin. Nutr. 2012, 96, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Sheffield-Moore, M.; Rasmussen, B.B.; Wolfe, R.R. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 2001, 286, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J.; Edwards, R.H.; Halliday, D.; Matthews, D.E.; Wolman, S.L.; Millward, D.J. Muscle protein synthesis measured by stable isotope techniques in man: The effects of feeding and fasting. Clin. Sci. 1982, 63, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J.C. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.I.; Correia, M.; Camilo, M.; Ravasco, P. Length of stay in surgical patients: Nutritional predictive parameters revisited. Br. J. Nutr. 2013, 109, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Morton, J.P.; van Loon, L.J. Strategies to maintain skeletal muscle mass in the injured athlete: Nutritional considerations and exercise mimetics. Eur. J. Sport Sci. 2015, 15, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Senden, J.M.; Dolmans, J.; van Loon, L.J. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2014, 210, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Cermak, N.M.; van Loon, L.J. Dietary protein considerations to support active aging. Sports Med. 2014, 44, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J. What is sarcopenia? J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.; Kazemi-Bajestani, S.M. Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int. J. Biochem. Cell Biol. 2013, 45, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.M.; Sheffield-Moore, M. Nutritional/metabolic response in older cancer patients. Nutrition 2015, 31, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Groen, B.B.; Horstman, A.M.; Hamer, H.M.; de Haan, M.; van Kranenburg, J.; Bierau, J.; Poeze, M.; Wodzig, W.K.; Rasmussen, B.B.; van Loon, L.J. Post-prandial protein handling: You are what you just ate. PLoS ONE 2015, 10, e0141582. [Google Scholar] [CrossRef] [PubMed]
- Op den Kamp, C.M.; Langen, R.C.; Snepvangers, F.J.; de Theije, C.C.; Schellekens, J.M.; Laugs, F.; Dingemans, A.M.; Schols, A.M. Nuclear transcription factor kappa B activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am. J. Clin. Nutr. 2013, 98, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Chinkes, D.L.; Wolfe, R.R. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E753–E764. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.; Uitdehaag, M.J.; Spaander, M.; van Steenbergen-Langeveld, S.; Vos, P.; Berkhout, M.; Lamers, C.; Rümke, H.; Tilanus, H.; Siersema, P.; et al. Improved body weight and performance status and reduced serum PGE2 levels after nutritional intervention with a specific medical food in newly diagnosed patients with esophageal cancer or adenocarcinoma of the gastro-esophageal junction. J. Cachexia Sarcopenia Muscle 2015, 6, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lara, K.; Turcott, J.G.; Juarez-Hernandez, E.; Nunez-Valencia, C.; Villanueva, G.; Guevara, P.; de la Torre-Vallejo, M.; Mohar, A.; Arrieta, O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: Randomised trial. Clin. Nutr. 2014, 33, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Vashi, P.G.; Dahlk, S.; Popiel, B.; Lammersfeld, C.A.; Ireton-Jones, C.; Gupta, D. A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer 2014, 14, 593. [Google Scholar] [CrossRef] [PubMed]
- Vasson, M.P.; Talvas, J.; Perche, O.; Dillies, A.F.; Bachmann, P.; Pezet, D.; Achim, A.C.; Pommier, P.; Racadot, S.; Weber, A.; et al. Immunonutrition improves functional capacities in head and neck and esophageal cancer patients undergoing radiochemotherapy: A randomized clinical trial. Clin. Nutr. 2014, 33, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Tang, J.E.; Moore, D.R.; Phillips, S.M. Exercise training and protein metabolism: Influences of contraction, protein intake, and sex-based differences. J. Appl. Physiol. 2009, 106, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
| Methods Used | Basal FSR (%/h) | Postprandial FSR (%/h) | Basal FBR (%/h) | Nutritional Intervention | |
|---|---|---|---|---|---|
| Deutz, 2011 [13] | Primed continuous infusion l-[ring-13C6]-Phe | 0.073 ± 0.023 a 0.073 ± 0.022 b | 0.097 ± 0.033 a 0.065 ± 0.028 b | - | a conventional medical food b re-designed medical food |
| Dillon, 2012 (case report) [14] | Pulse bolus injection [30] l-[ring-13C6]-Phe and 15N-Phe | 0.07 | - | 0.03 | - |
| Dillon, 2007 [15] | Primed continuous infusion l-[ring-2H5]-Phe | 0.052 ± 0.009 | 0.120 ± 0.008 | - | amino acid supplement |
| Williams, 2012 [16] | Primed continuous infusion [1,2-13C2]-Leu and ring-D5-Phe | 0.028 ± 0.004 | 0.038 ± 0.004 | - | intravenous mixed amino acids |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horstman, A.M.H.; Olde Damink, S.W.; Schols, A.M.W.J.; Van Loon, L.J.C. Is Cancer Cachexia Attributed to Impairments in Basal or Postprandial Muscle Protein Metabolism? Nutrients 2016, 8, 499. https://doi.org/10.3390/nu8080499
Horstman AMH, Olde Damink SW, Schols AMWJ, Van Loon LJC. Is Cancer Cachexia Attributed to Impairments in Basal or Postprandial Muscle Protein Metabolism? Nutrients. 2016; 8(8):499. https://doi.org/10.3390/nu8080499
Chicago/Turabian StyleHorstman, Astrid M. H., Steven W. Olde Damink, Annemie M. W. J. Schols, and Luc J. C. Van Loon. 2016. "Is Cancer Cachexia Attributed to Impairments in Basal or Postprandial Muscle Protein Metabolism?" Nutrients 8, no. 8: 499. https://doi.org/10.3390/nu8080499