Relationships of Dietary Histidine and Obesity in Northern Chinese Adults, an Internet-Based Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development and Validation of the IDQC
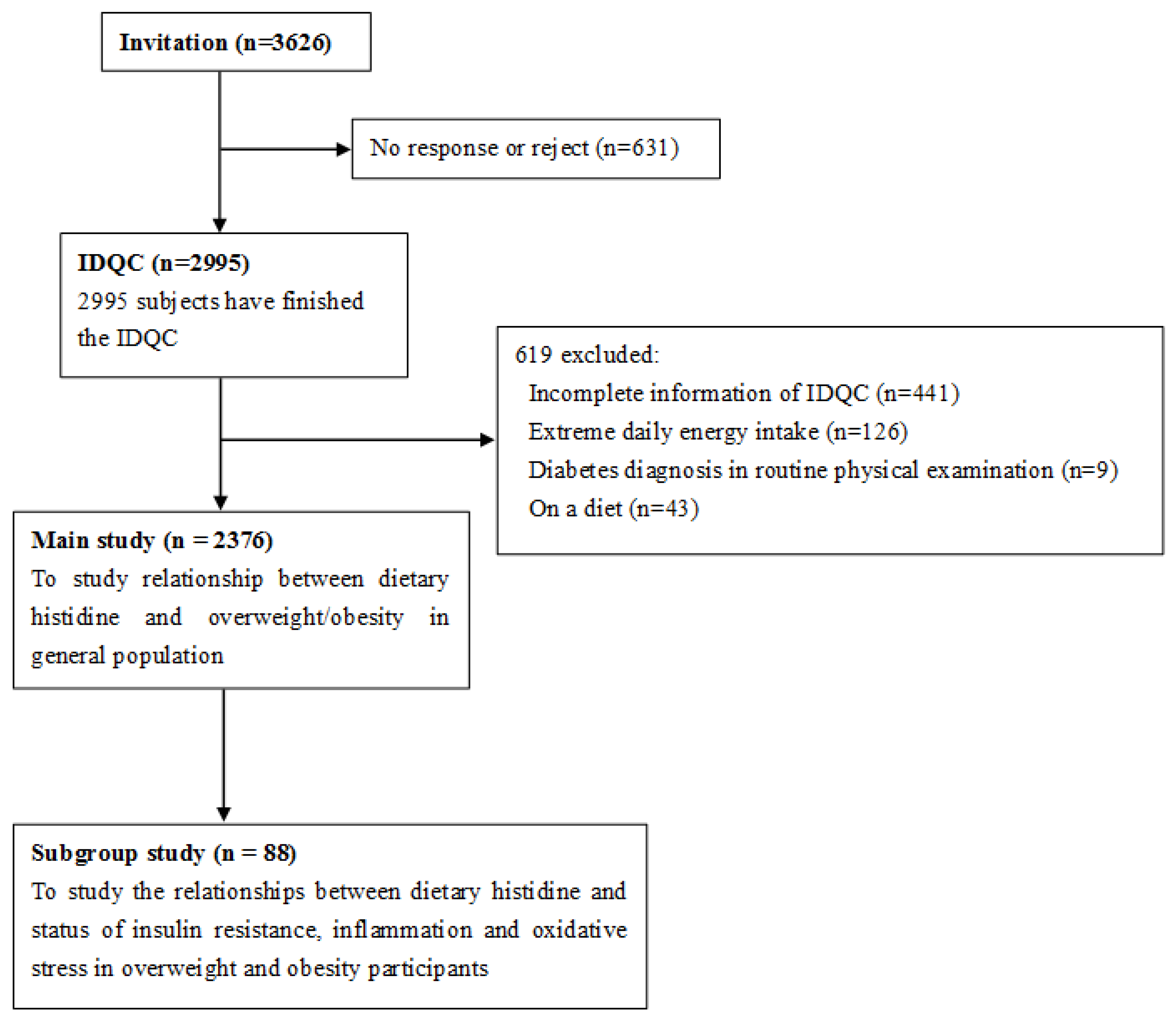
2.2. Participants, Exclusive Criteria, Power Calculation and Study Design
2.3. Estimation of Dietary Nutrients Intake
2.4. Anthropometric Measurements
2.5. Definition of Overweight/Obesity and Abdominal Obesity
2.6. Collection and Laboratory Analysis of Serum
2.7. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Dietary Histidine of Overweight and Obesity Participants Was Lower than Healthy Controls
3.3. Correlations between Dietary Histidine and Body Weight, BMI, WC, SBP and DBP
3.4. Higher Dietary Histidine Intake Is Associated with Lower Prevalence of Overweight/Obesity and Abdominal Obesity in Northern Chinese
3.5. Correlations between Dietary Histidine and Status of Insulin Resistance, Inflammation and Oxidative Stress in Overweight/Obese Participants
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, H.J.; Hur, K.Y.; Choi, S.H.; Ahn, C.W.; Lim, S.K.; Kim, K.R.; Lee, H.C.; Huh, K.B.; Cha, B.S. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am. J. Clin. Nutr. 2004, 79, 593–599. [Google Scholar] [PubMed]
- Wolongevicz, D.M.; Zhu, L.; Pencina, M.J.; Kimokoti, R.W.; Newby, P.K.; D’Agostino, R.B.; Millen, B.E. Diet quality and obesity in women: The framingham nutrition studies. Br. J. Nutr. 2010, 103, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Devereux, R.B.; Chinali, M.; Roman, M.J.; Best, L.G.; Welty, T.K.; Lee, E.T.; Howard, B.V. Strong Heart Study Investigators. Risk factors for arterial hypertension in adults with initial optimal blood pressure: The strong heart study. Hypertension 2006, 47, 162–167. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Liu, Y.; Willett, W.C. Preventing chronic diseases by promoting healthy diet and lifestyle: Public policy implications for China. Obes. Rev. 2011, 12, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Martinez-Gonzalez, M.A. Mediterranean diet for primary prevention of cardiovascular disease. N. Engl. J. Med. 2013, 369, 676–677. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Woo, H.D.; Lee, J.H.; Kim, J. Dietary patterns and risk for metabolic syndrome in Korean women: A cross-sectional study. Medicine (Baltimore) 2015, 94, e1424. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Kiazand, A.; Alhassan, S.; Kim, S.; Stafford, R.S.; Balise, R.R.; Kraemer, H.C.; King, A.C. Comparison of the atkins, zone, ornish, and learn diets for change in weight and related risk factors among overweight premenopausal women: The a to z weight loss study: A randomized trial. JAMA 2007, 297, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Suliman, M.E.; Qureshi, A.R.; Garcia-Lopez, E.; Barany, P.; Heimburger, O.; Stenvinkel, P.; Lindholm, B. Consequences of low plasma histidine in chronic kidney disease patients: Associations with inflammation, oxidative stress, and mortality. Am. J. Clin. Nutr. 2008, 87, 1860–1866. [Google Scholar] [PubMed]
- Sun, X.; Feng, R.; Li, Y.; Lin, S.; Zhang, W.; Li, Y.; Sun, C.; Li, S. Histidine supplementation alleviates inflammation in the adipose tissue of high-fat diet-induced obese rats via the nf-kappab- and ppargamma-involved pathways. Br. J. Nutr. 2014, 112, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Kasaoka, S.; Tsuboyama-Kasaoka, N.; Kawahara, Y.; Inoue, S.; Tsuji, M.; Ezaki, O.; Kato, H.; Tsuchiya, T.; Okuda, H.; Nakajima, S. Histidine supplementation suppresses food intake and fat accumulation in rats. Nutrition 2004, 20, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.C.; Feng, R.N.; Hou, Y.; Li, K.; Kang, Z.; Wang, J.; Sun, C.H.; Li, Y. Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br. J. Nutr. 2012, 108, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.N.; Niu, Y.C.; Sun, X.W.; Li, Q.; Zhao, C.; Wang, C.; Guo, F.C.; Sun, C.H.; Li, Y. Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: A randomised controlled trial. Diabetologia 2013, 56, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Sasaki, S. Histidine intake may negatively correlate with energy intake in human: A cross-sectional study in Japanese female students aged 18 years. J. Nutr. Sci. Vitaminol. (Tokyo) 2005, 51, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Sublette, M.E.; Segal-Isaacson, C.J.; Cooper, T.B.; Fekri, S.; Vanegas, N.; Galfalvy, H.C.; Oquendo, M.A.; Mann, J.J. Validation of a food frequency questionnaire to assess intake of n-3 polyunsaturated fatty acids in subjects with and without major depressive disorder. J. Am. Diet. Assoc. 2011, 111, 117–123.e1-2. [Google Scholar] [CrossRef] [PubMed]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Du, S.S.; Jiang, Y.S.; Chen, Y.; Li, Z.; Zhang, Y.F.; Sun, C.H.; Feng, R.N. Development and applicability of an internet-based diet and lifestyle questionnaire for college students in china: A cross-sectional study. Medicine (Baltimore) 2015, 94, e2130. [Google Scholar] [CrossRef] [PubMed]
- Yingyangjiayuan. Available online: http://www.yyjy365.org/diet (accessed on 23 March 2012).
- Li, Y.C.; Li, Y.; Liu, L.Y.; Chen, Y.; Zi, T.Q.; Du, S.S.; Jiang, Y.S.; Feng, R.N.; Sun, C.H. The ratio of dietary branched-chain amino acids is associated with a lower prevalence of obesity in young northern Chinese adults: An internet-based cross-sectional study. Nutrients 2015, 7, 9573–9589. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Guo, X. China Food Composition Tables; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Zhou, B.F. Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases—Report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed. Environ. Sci. 2002, 15, 245–252. [Google Scholar] [PubMed]
- Hu, J.; Wallace, D.C.; Jones, E.; Liu, H. Cardiometabolic health of Chinese older adults with diabetes living in Beijing, China. Public Health Nurs. 2009, 26, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, A.C.; Bel-Serrat, S.; Manios, Y.; Molnar, D.; Kafatos, A.; Cuenca-Garcia, M.; Huybrechts, I.; Sette, S.; Widhalm, K.; Stehle, P.; et al. Dietary protein and amino acids intake and its relationship with blood pressure in adolescents: The Helena study. Eur. J. Public Health 2015, 25, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Kotsis, V.; Stabouli, S.; Papakatsika, S.; Rizos, Z.; Parati, G. Mechanisms of obesity-induced hypertension. Hypertens. Res. 2010, 33, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [PubMed]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [PubMed]
- Gonzalez, A.S.; Guerrero, D.B.; Soto, M.B.; Diaz, S.P.; Martinez-Olmos, M.; Vidal, O. Metabolic syndrome, insulin resistance and the inflammation markers c-reactive protein and ferritin. Eur. J. Clin. Nutr. 2006, 60, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Hsu, C.C.; Lin, M.H.; Liu, K.S.; Yin, M.C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Son, D.O.; Satsu, H.; Shimizu, M. Histidine inhibits oxidative stress- and tnf-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005, 579, 4671–4677. [Google Scholar] [CrossRef] [PubMed]
- Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Adiponectin: Action, regulation and association to insulin sensitivity. Obes. Rev. 2005, 6, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Funahashi, T.; Bodkin, N.L.; Ortmeyer, H.K.; Arita, Y.; Hansen, B.C.; Matsuzawa, Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 2001, 50, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef] [PubMed]
- Youn, B.S.; Kloting, N.; Kratzsch, J.; Lee, N.; Park, J.W.; Song, E.S.; Ruschke, K.; Oberbach, A.; Fasshauer, M.; Stumvoll, M.; et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008, 57, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Li, Y.; Wang, C.; Luo, C.; Liu, L.; Chuo, F.; Li, Q.; Sun, C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Vannacci, A.; Rastogi, S. Oxidative stress and inflammation in obesity, diabetes, hypertension, and related cardiometabolic complications. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kasaoka, S.; Kawahara, Y.; Inoue, S.; Tsuji, M.; Kato, H.; Tsuchiya, T.; Okuda, H.; Nakajima, S. Gender effects in dietary histidine-induced anorexia. Nutrition 2005, 21, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Tanida, M.; Niijima, A.; Tsuruoka, N.; Kiso, Y.; Horii, Y.; Shen, J.; Okumura, N. Role of L-carnosine in the control of blood glucose, blood pressure, thermogenesis, and lipolysis by autonomic nerves in rats: Involvement of the circadian clock and histamine. Amino Acids 2012, 43, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Courten, B.; Jakubova, M.; de Courten, M.P.; Kukurova, I.J.; Vallova, S.; Krumpolec, P.; Valkovic, L.; Kurdiova, T.; Garzon, D.; Barbaresi, S.; et al. Effects of carnosine supplementation on glucose metabolism: Pilot clinical trial. Obesity (Silver Spring) 2016, 24, 1027–1034. [Google Scholar] [CrossRef] [PubMed]

| Quartiles of Histidine (% Total Protein Intake) | p | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Histidine, % total protein intake | <1.38 | 1.38–1.59 | 1.59–1.77 | >1.77 | |
| Participants, n | 594 | 594 | 594 | 594 | |
| BMI categories | <0.001 | ||||
| <24.0, n (%) | 327 (55.1) | 352 (59.3) | 384 (64.6) | 424 (71.4) | |
| 24.0–28.0, n (%) | 191 (32.2) | 182 (30.6) | 165 (27.8) | 141 (23.7) | |
| ≥28.0, n (%) | 76 (12.8) | 60 (10.1) | 45 (7.6) | 29 (4.9) | |
| Abdominal obesity | 0.020 | ||||
| Yes, n (%) | 191 (32.1) | 174 (29.3) | 168 (28.3) | 143 (24.1) | |
| No, n (%) | 403 (67.9) | 420 (70.7) | 426 (71.7) | 451 (75.9) | |
| Age, year | 34.7 ± 14.9 | 34.6 ± 16.3 | 33.3 ± 15.5 | 29.9 ± 14.5 | <0.001 |
| Gender | 0.018 | ||||
| Men, n (%) | 319 (53.7) | 296 (49.8) | 266 (44.8) | 283 (47.6) | |
| Women, n (%) | 275 (46.3) | 298 (50.2) | 328 (55.2) | 311 (52.4) | |
| Body weight, kg | 67.6 ± 12.5 | 66.1 ± 11.6 | 65.4 ± 11.3 | 64.2 ± 11.4 | <0.001 |
| BMI, kg/m2 | 23.8 ± 3.7 | 23.3 ± 3.3 | 23.2 ± 3.3 | 22.8 ± 3.1 | <0.001 |
| WC, cm | 80.6 ± 10.3 | 79.2 ± 9.7 | 79.0 ± 9.5 | 78.2 ± 9.8 | <0.001 |
| Income per month | <0.001 | ||||
| <2000 yuan, n (%) | 355 (59.8) | 366 (61.6) | 374 (63.0) | 442 (74.4) | |
| 2000–5000 yuan, n (%) | 223 (37.5) | 207 (34.8) | 203 (34.2) | 123 (20.7) | |
| ≥5000 yuan, n (%) | 16 (2.7) | 21 (3.5) | 17 (2.9) | 29 (4.9) | |
| Education | 0.021 | ||||
| Under college, n (%) | 175 (29.5) | 145 (24.4) | 132 (22.2) | 122 (20.5) | |
| Bachelor, n (%) | 398 (67.0) | 424 (71.4) | 437 (73.6) | 449 (75.6) | |
| Master or doctor, n (%) | 21 (3.5) | 25 (4.2) | 25 (4.2) | 23 (3.9) | |
| Labor | <0.001 | ||||
| Light, n (%) | 164 (27.6) | 179 (30.1) | 149 (25.1) | 135 (22.7) | |
| Medium, n (%) | 391 (65.8) | 397 (66.8) | 430 (72.4) | 453 (76.3) | |
| Heavy, n (%) | 39 (6.6) | 18 (3.0) | 15 (2.5) | 6 (1.0) | |
| Exercise | <0.001 | ||||
| <10 h/week, n (%) | 281 (47.3) | 223 (3.8) | 182 (30.6) | 137 (23.1) | |
| 10–20 h/week, n (%) | 300 (50.5) | 315 (53.0) | 317 (53.4) | 334 (56.2) | |
| ≥20 h/week, n (%) | 13 (2.2) | 56 (9.4) | 95 (16.0) | 123 (20.7) | |
| Smoking | 0.046 | ||||
| Non-smoker, n (%) | 493 (83.0) | 494 (83.2) | 530 (89.2) | 507 (85.4) | |
| Current smoker, n (%) | 73 (12.3) | 75 (12.6) | 49 (8.2) | 61 (10.3) | |
| Quit smoking, n (%) | 28 (4.7) | 25 (4.2) | 15 (2.5) | 26 (4.4) | |
| Drinking | 0.44 | ||||
| Non-drinker, n (%) | 475 (80.0) | 489 (82.3) | 495 (83.7) | 480 (80.8) | |
| Current drinker, n (%) | 119 (20.0) | 105 (17.7) | 99 (16.7) | 114 (19.2) | |
| SBP (mmHg) | 119.1 ± 12.9 | 119.0 ± 14.1 | 117.7 ± 12.4 | 115.1 ± 11.4 | <0.001 |
| DBP (mmHg) | 78.9 ± 8.7 | 78.7 ± 9.1 | 77.9 ± 8.9 | 76.2 ± 8.4 | <0.001 |
| Dietary intakes | |||||
| Energy, kcal/day | 2448.4 ± 824.5 | 2534.2 ± 867.6 | 2430.5 ± 865.5 | 2399.9 ± 896.0 | 0.048 |
| Total protein, g/day | 79.7 ± 30.5 | 92.7 ± 35.2 | 93.8 ± 37.9 | 95.0 ± 41.7 | <0.001 |
| Total amino acids, g/day | 36.9 ± 16.8 | 54.2 ± 20.7 | 61.3 ± 24.7 | 69.8 ± 30.5 | <0.001 |
| Histidine, g/day | 0.9 ± 0.4 | 1.4 ± 0.5 | 1.6 ± 0.6 | 1.8 ± 0.8 | <0.001 |
| Total fat, g/day | 60.8 ± 28.2 | 78.5 ± 35.3 | 83.4 ± 37.6 | 81.2 ± 43.0 | <0.001 |
| Total carbohydrate, g/day | 406.9 ± 146.3 | 382.5 ± 137.9 | 345.2 ± 133.4 | 341.9 ± 141.9 | <0.001 |
| Cholesterol, mg/day | 405.7 ± 309.9 | 495.8 ± 338.9 | 489.6 ± 309.6 | 435.8 ± 349.0 | <0.001 |
| Fiber, g/day | 15.7 ± 7.6 | 20.9 ± 10.6 | 21.7 ± 11.1 | 22.6 ± 13.0 | <0.001 |
| Unadjusted | Adjusted * | Unadjusted | Adjusted * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | ||
| Overall | Normal BMI | ||||||||
| Energy intake | −0.031 | NS | −0.053 | <0.05 | Energy intake | −0.032 | NS | −0.027 | NS |
| Body weight | −0.093 | <0.001 | −0.076 | <0.01 | Body weight | −0.042 | NS | −0.029 | NS |
| BMI | −0.126 | <0.001 | −0.129 | <0.001 | BMI | −0.046 | NS | −0.037 | NS |
| WC | −0.127 | <0.001 | −0.132 | <0.001 | WC | −0.061 | NS | −0.071 | NS |
| SBP | −0.119 | <0.001 | −0.106 | <0.001 | SBP | −0.109 | <0.001 | −0.056 | <0.05 |
| DBP | −0.104 | <0.001 | −0.092 | <0.001 | DBP | −0.079 | <0.01 | −0.052 | NS |
| Men | Overweight | ||||||||
| Energy intake | −0.022 | NS | −0.034 | NS | Energy intake | −0.055 | NS | −0.058 | NS |
| Body weight | −0.061 | <0.05 | −0.064 | <0.05 | Body weight | −0.094 | NS | −0.042 | NS |
| BMI | −0.102 | <0.01 | −0.095 | <0.05 | BMI | −0.137 | <0.001 | −0.133 | <0.001 |
| WC | −0.096 | <0.05 | −0.093 | <0.05 | WC | −0.114 | <0.05 | −0.125 | <0.05 |
| SBP | −0.143 | <0.001 | −0.076 | <0.05 | SBP | −0.104 | <0.01 | −0.097 | <0.05 |
| DBP | −0.112 | <0.001 | −0.086 | <0.01 | DBP | −0.112 | <0.01 | −0.092 | <0.05 |
| Women | Obesity | ||||||||
| Energy intake | −0.065 | <0.05 | −0.056 | <0.05 | Energy intake | −0.068 | <0.05 | −0.073 | <0.05 |
| Body weight | −0.096 | <0.05 | −0.088 | <0.01 | Body weight | −0.119 | <0.05 | −0.104 | <0.05 |
| BMI | −0.136 | <0.001 | −0.147 | <0.001 | BMI | −0.131 | <0.01 | −0.147 | <0.001 |
| WC | −0.138 | <0.01 | −0.153 | <0.001 | WC | −0.143 | <0.01 | −0.142 | <0.01 |
| SBP | −0.066 | <0.05 | 0.113 | <0.01 | SBP | −0.117 | <0.01 | −0.113 | <0.05 |
| DBP | −0.065 | <0.05 | −0.109 | <0.01 | DBP | −0.143 | <0.05 | −0.131 | <0.05 |
| All | ||||
|---|---|---|---|---|
| Histidine Quartiles | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
| Dietary histidine (% protein) | <1.38 | 1.38–1.59 | 1.59–1.77 | >1.77 |
| Participants, n | 594 | 594 | 594 | 594 |
| Overweight/obesity, n (%) | 267 (44.9) | 242 (40.7) | 210 (35.4) | 170 (28.6) |
| Crude | 1 | 0.768 (0.610, 0.968) * | 0.736 (0.584, 0.928) * | 0.537 (0.423, 0.681) ** |
| Model 1 | 1 | 0.772 (0.602, 0.990) * | 0.752 (0.586, 0.966) * | 0.646 (0.500, 0.834) ** |
| Model 2 | 1 | 0.777 (0.587, 1.029) | 0.744 (0.572, 0.968) * | 0.649 (0.481, 0.875) ** |
| Model 3 | 1 | 0.772 (0.583, 1.023) | 0.745 (0.572, 0.969) * | 0.650 (0.482, 0.876) ** |
| Men | ||||
| Histidine Quartiles | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
| Dietary histidine (% protein) | <1.35 | 1.35–1.57 | 1.57–1.76 | >1.76 |
| Participants, n | 291 | 291 | 291 | 291 |
| Overweight/obesity, n (%) | 132 (45.4) | 117 (40.2) | 98 (33.7) | 85 (29.2) |
| Crude | 1 | 0.926 (0.671, 1.278) | 0.826 (0.592, 1.154) | 0.608 (0.434, 0.853) ** |
| Model 1 | 1 | 0.919 (0.655, 1.289) | 0.843 (0.594, 1.197) | 0.599 (0.490, 0.998) * |
| Model 2 | 1 | 0.973 (0.676, 1.388) | 0.828 (0.561, 1.288) | 0.578 (0.468, 0.991) * |
| Model 3 | 1 | 0.969 (0.672, 1.396) | 0.838 (0.577, 1.302) | 0.575 (0.456, 0.988) * |
| Women | ||||
| Histidine Quartiles | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
| Dietary histidine (% protein) | <1.40 | 1.40–1.61 | 1.61–1.77 | >1.77 |
| Participants, n | 303 | 303 | 303 | 303 |
| Overweight/obesity, n (%) | 135 (44.6) | 125 (41.3) | 112 (37.0) | 85 (28.1) |
| Crude | 1 | 0.639 (0.461, 0.884) ** | 0.624 (0.448, 0.871) ** | 0.463 (0.330, 0.649) ** |
| Model 1 | 1 | 0.642 (0.445, 0.925) * | 0.574 (0.393, 0.837) ** | 0.549 (0.376, 0.803) ** |
| Model 2 | 1 | 0.607 (0.403, 0.915) * | 0.557 (0.375, 0.827) ** | 0.515 (0.333, 0.796) ** |
| Model 3 | 1 | 0.612 (0.406, 0.923) * | 0.556 (0.374, 0.826) ** | 0.518 (0.335, 0.801) ** |
| All | ||||
|---|---|---|---|---|
| Histidine Quartiles | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
| Dietary histidine (% protein) | <1.38 | 1.38–1.59 | 1.59–1.77 | >1.77 |
| Participants, n | 594 | 594 | 594 | 594 |
| Abdominal obesity, n (%) | 191 (32.1) | 174 (29.3) | 168 (28.3) | 143 (24.1) |
| Crude | 1 | 0.771 (0.603, 0.987) * | 0.752 (0.587, 0.963) * | 0.614 (0.477, 0.792) ** |
| Model 1 | 1 | 0.733 (0.563, 0.956) * | 0.774 (0.594, 0.996) * | 0.742 (0.565, 0.974) * |
| Model 2 | 1 | 0.723 (0.545, 0.059) * | 0.782 (0.579, 0.998) * | 0.761 (0.552, 0.985) * |
| Model 3 | 1 | 0.716 (0.539, 0.952) * | 0.809 (0.597, 0.995) * | 0.754 (0.545, 0.943) * |
| Men | ||||
| Histidine Quartiles | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
| Dietary histidine (% protein) | <1.35 | 1.35–1.57 | 1.57–1.76 | >1.76 |
| Participants, n | 291 | 291 | 291 | 291 |
| Abdominal obesity, n (%) | 120 (41.2) | 118 (40.5) | 114 (39.2) | 102 (35.1) |
| Crude | 1 | 0.972 (0.698, 1.353) | 0.918 (0.659, 1.279) | 0.769 (0.550, 1.075) |
| Model 1 | 1 | 0.943 (0.663, 1.341) | 0.923 (0.648, 1.315) | 0.908 (0.634, 1.301) |
| Model 2 | 1 | 0.948 (0.650, 1.383) | 0.940 (0.627, 1.410) | 0.928 (0.600, 1.435) |
| Model 3 | 1 | 0.929 (0.636, 1.357) | 0.959 (0.639, 1.441) | 0.916 (0.592, 1.419) |
| Women | ||||
| Histidine Quartiles | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
| Dietary histidine (% protein) | <1.40 | 1.40–1.61 | 1.61–1.77 | >1.77 |
| Participants, n | 303 | 303 | 303 | 303 |
| Abdominal obesity, n (%) | 71 (23.4) | 56 (18.5) | 54 (17.8) | 41 (13.5) |
| Crude | 1 | 0.639 (0.461, 0.884) ** | 0.624 (0.448, 0.871) ** | 0.463 (0.330, 0.649) ** |
| Model 1 | 1 | 0.499 (0.313, 0.794) ** | 0.539 (0.280, 0.938) * | 0.507 (0.314, 0.816) ** |
| Model 2 | 1 | 0.477 (0.293, 0.777) ** | 0.476 (0.278, 0.802) ** | 0.472 (0.272, 0.821) ** |
| Model 3 | 1 | 0.478 (0.294, 0.778) ** | 0.501 (0.320, 0.840) ** | 0.473 (0.272, 0.822) ** |
| Overall | Men | Women | ||||
|---|---|---|---|---|---|---|
| Parameters | r * | p | r * | p | r * | p |
| FBG | −0.179 | <0.05 | −0.171 | <0.05 | −0.214 | <0.05 |
| Insulin | −0.122 | NS | −0.111 | NS | −0.098 | NS |
| HOMA-IR | −0.233 | <0.05 | −0.221 | <0.05 | −0.237 | <0.05 |
| TC | −0.103 | NS | −0.089 | NS | −0.109 | NS |
| TG | −0.122 | NS | −0.106 | NS | −0.127 | NS |
| HDL | 0.074 | NS | 0.033 | NS | 0.098 | NS |
| LDL | 0.081 | NS | 0.089 | NS | 0.076 | NS |
| 2 h-PG | −0.171 | <0.05 | −0.167 | NS | −0.189 | <0.05 |
| 2 h-Insulin | −0.112 | NS | −0.078 | NS | −0.131 | NS |
| 2 h-TC | −0.081 | NS | −0.065 | NS | −0.057 | NS |
| 2 h-TG | −0.056 | NS | −0.034 | NS | −0.078 | NS |
| 2 h-HDL | 0.098 | NS | 0.035 | NS | 0.101 | NS |
| 2 h-LDL | 0.035 | NS | 0.008 | NS | 0.041 | NS |
| GSH-Px | 0.265 | <0.05 | 0.231 | <0.05 | 0.283 | <0.05 |
| SOD | 0.167 | <0.05 | 0.149 | NS | 0.198 | <0.05 |
| MDA | −0.202 | <0.05 | −0.187 | <0.05 | −0.231 | <0.05 |
| TNF-α | −0.271 | <0.05 | −0.265 | <0.05 | −0.273 | <0.05 |
| IL-1β | −0.178 | <0.05 | −0.172 | <0.05 | −0.189 | <0.05 |
| IL-6 | −0.182 | <0.05 | −0.166 | <0.05 | −0.198 | <0.05 |
| CRP | −0.242 | <0.05 | −0.178 | <0.05 | −0.267 | <0.05 |
| Adiponectin | 0.188 | <0.05 | 0.176 | <0.05 | 0.195 | <0.05 |
| Vaspin | −0.217 | <0.05 | −0.231 | <0.05 | −0.203 | <0.05 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-C.; Li, C.-L.; Qi, J.-Y.; Huang, L.-N.; Shi, D.; Du, S.-S.; Liu, L.-Y.; Feng, R.-N.; Sun, C.-H. Relationships of Dietary Histidine and Obesity in Northern Chinese Adults, an Internet-Based Cross-Sectional Study. Nutrients 2016, 8, 420. https://doi.org/10.3390/nu8070420
Li Y-C, Li C-L, Qi J-Y, Huang L-N, Shi D, Du S-S, Liu L-Y, Feng R-N, Sun C-H. Relationships of Dietary Histidine and Obesity in Northern Chinese Adults, an Internet-Based Cross-Sectional Study. Nutrients. 2016; 8(7):420. https://doi.org/10.3390/nu8070420
Chicago/Turabian StyleLi, Yan-Chuan, Chun-Long Li, Jia-Yue Qi, Li-Na Huang, Dan Shi, Shan-Shan Du, Li-Yan Liu, Ren-Nan Feng, and Chang-Hao Sun. 2016. "Relationships of Dietary Histidine and Obesity in Northern Chinese Adults, an Internet-Based Cross-Sectional Study" Nutrients 8, no. 7: 420. https://doi.org/10.3390/nu8070420




