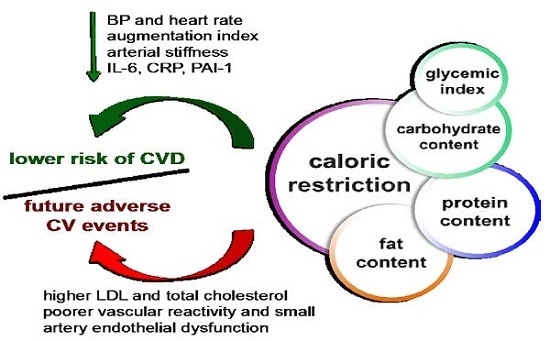
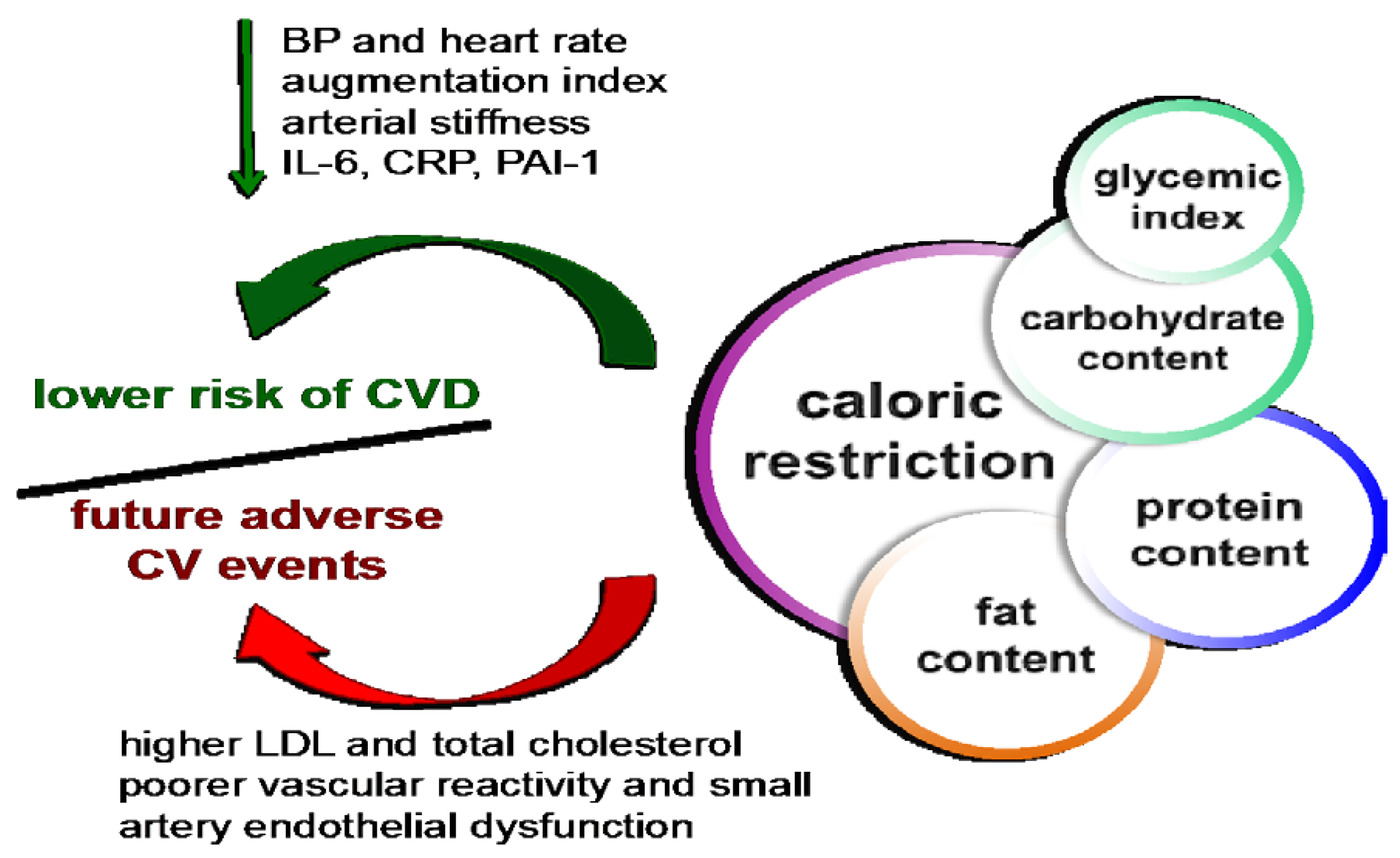
Caloric Restriction as a Strategy to Improve Vascular Dysfunction in Metabolic Disorders
Abstract
:1. Introduction
2. CR Reduces CV Risk Factors
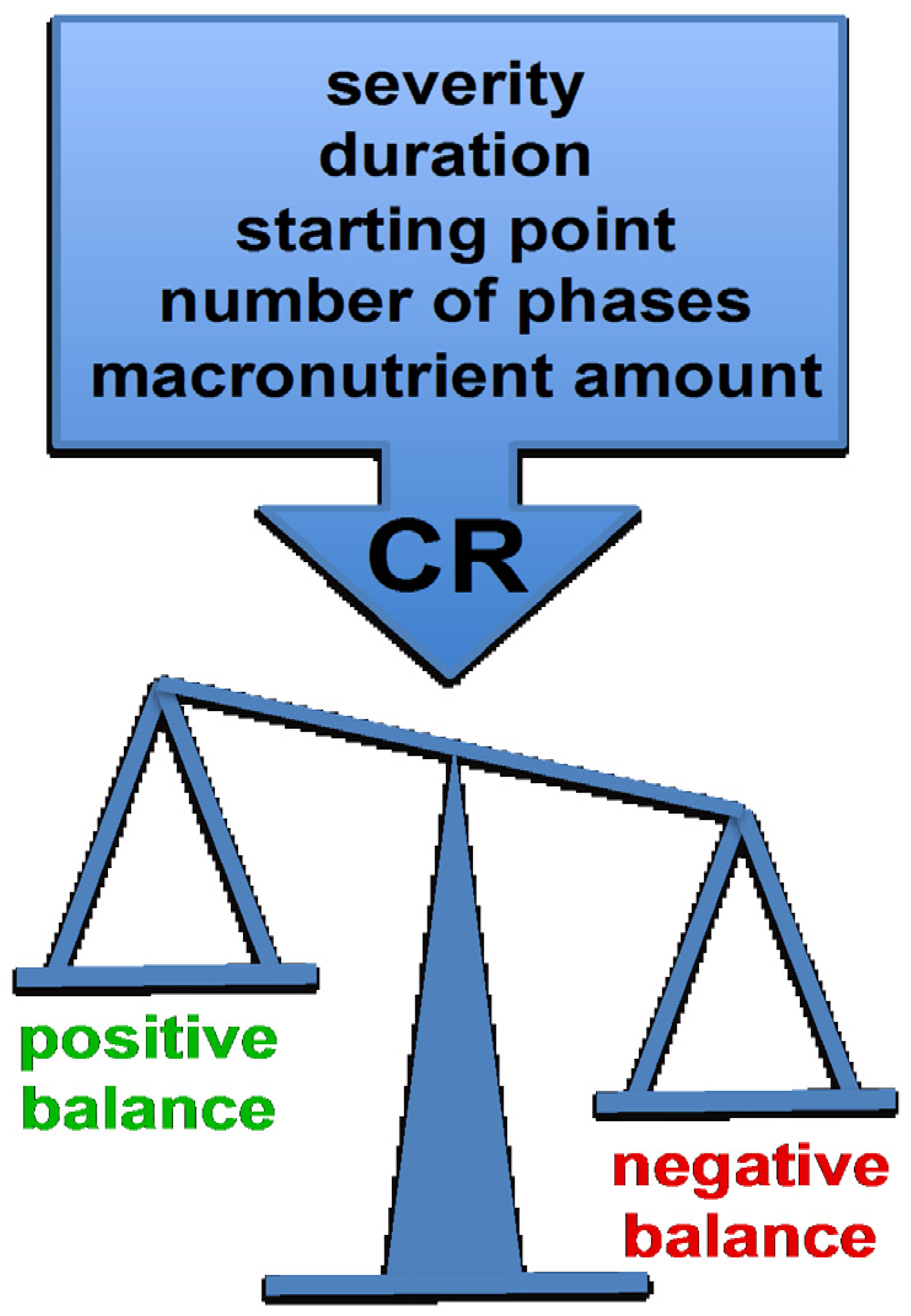
3. CR Protocols Differ in Their Starting Point, Severity, Duration and Number of Phases
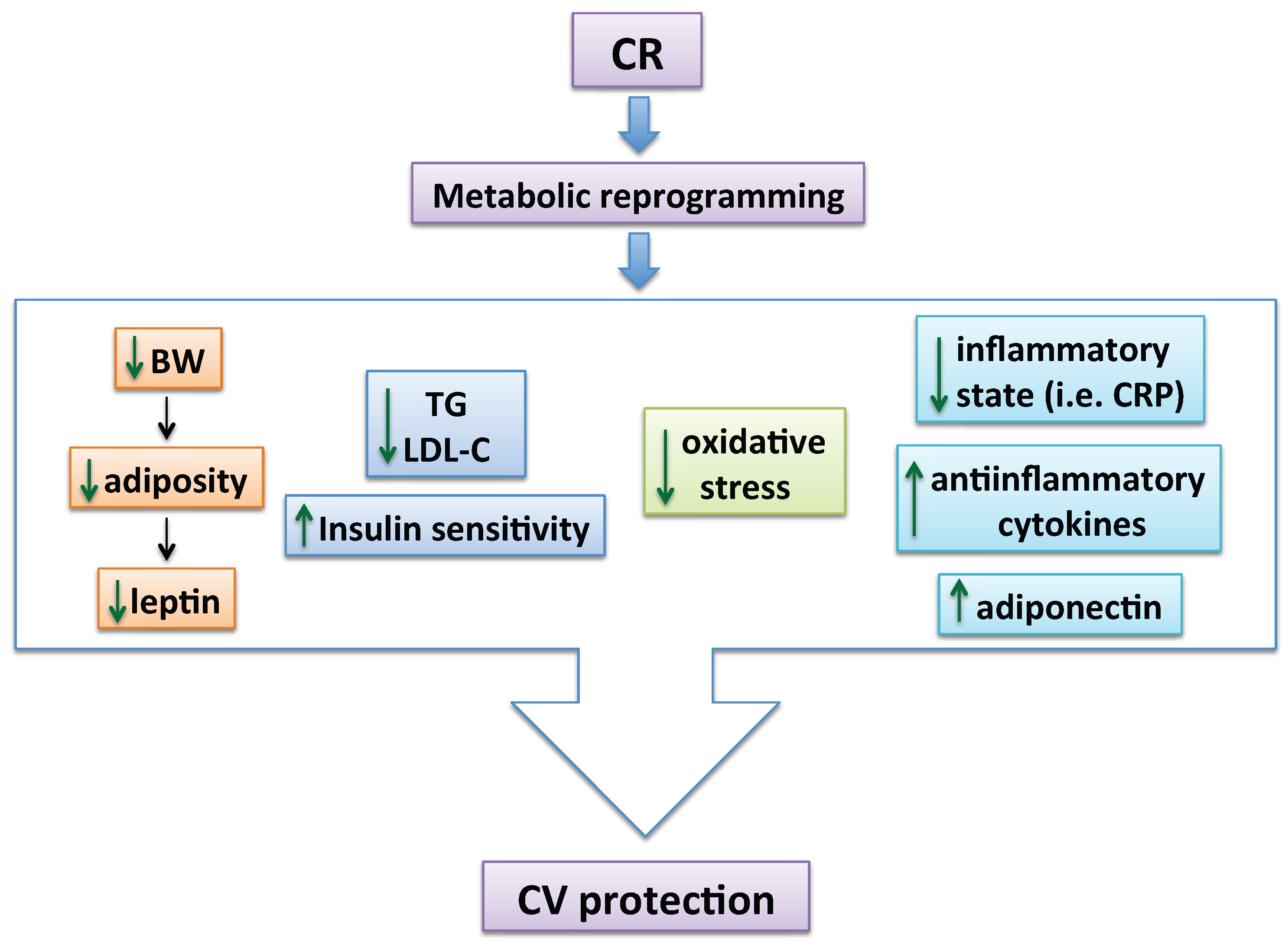
4. Mechanisms by Which CR Exerts Vascular Protection in Metabolic Disorders
4.1. Effects of CR on Endothelial Function
4.2. Effects of CR on Arterial Wall Structure and Remodeling
4.3. Effects of CR on PVAT Dysfunction in Obesity
4.4. Effects of CR on Vascular Actions of Leptin and Adiponectin
5. Dietary Strategies Based on Macronutrients Modification
5.1. Low-Carbohydrate Diets
5.2. Higher Protein Content: Does It Make the Difference?
5.3. Diet Glycemic Index Variation
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| AL | ad libitum |
| AMPK | adenosine monophosphate-activated protein kinase |
| AP-1 | activator protein-1 |
| BP | blood pressure |
| BW | body weight |
| CR | caloric restriction |
| CRP | high-sensitivity C-reactive protein |
| CV | cardiovascular |
| CVD | cardiovascular disease |
| eNOS | endothelial nitric oxide synthase |
| FA | fatty acid |
| FFAs | free fatty-acids |
| FMD | flow-mediated dilation |
| GI | glycemic index |
| H2O2 | hydrogen peroxide |
| HDL | high-density lipoprotein |
| HFD | high-fat diet |
| HP | low-fat, high-protein, reduced-carbohydrate |
| IL | interleukin |
| JNK | c-Jun N-terminal kinase |
| LDL | low-density lipoprotein |
| MAPK | mitogen-activated protein kinases |
| MCP-1 | monocyte chemoattractant protein-1 |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor κB |
| NO | nitric oxide |
| O2− | superoxide anion |
| PAI-1 | plasminogen activator inhibitor-1 |
| PI3K | phosphoinositide 3-kinase |
| PVAT | perivascular adipose tissue |
| SHR | spontaneously hypertensive rats |
| SIRT1 | sirtuin-1 |
| SOD | superoxide dismutase |
| T2D | type 2 diabetes |
| TG | triglycerides |
| TGFβ1 | transforming growth factor beta-1 |
| TNF-α | tumor necrosis factor α |
| VLCD | very low-calorie diet |
References
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, V.G.; Aroor, A.R.; Sowers, J.R. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 2014, 10, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Fontana, L.; Young, V.L.; Coggan, A.R.; Kilo, C.; Patterson, B.W.; Mohammed, B.S. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N. Engl. J. Med. 2004, 350, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Tamboli, R.A.; Magkos, F.; Marks-Shulman, P.A.; Eckhauser, A.W.; Richards, W.O.; Klein, S.; Abumrad, N.N. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 2010, 139, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Stevens, V.J.; Obarzanek, E.; Cook, N.R.; Lee, I.M.; Appel, L.J.; Smith West, D.; Milas, N.C.; Mattfeldt-Beman, M.; Belden, L.; Bragg, C.; et al. Long-term weight loss and changes in blood pressure: Results of the trials of hypertension prevention, phase II. Ann. Intern. Med. 2001, 134, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Higashi, Y.; Nakagawa, K.; Kimura, M.; Noma, K.; Hara, K.; Matsuura, H.; Goto, C.; Oshima, T.; Chayama, K. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am. J. Hypertens. 2002, 15, 302–309. [Google Scholar] [CrossRef]
- Davì, G.; Guagnano, M.T.; Ciabattoni, G.; Basili, S.; Falco, A.; Marinopiccoli, M.; Nutini, M.; Sensi, S.; Patrono, C. Platelet activation in obese women: Role of inflammation and oxidant stress. JAMA 2002, 288, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, P.; Nappo, F.; Giugliano, G.; Esposito, K.; Marfella, R.; Cioffi, M.; D’Andrea, F.; Molinari, A.M.; Giugliano, D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002, 105, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Raitakari, M.; Ilvonen, T.; Ahotupa, M.; Lehtimäki, T.; Harmoinen, A.; Suominen, P.; Elo, J.; Hartiala, J.; Raitakari, O.T. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: Role of plasma glucose. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Swain, J.; Goldfine, A.B.; Rifai, N.; Ludwig, D.S. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA 2004, 292, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Villareal, D.T.; Weiss, E.P.; Racette, S.B.; Steger-May, K.; Klein, S.; Holloszy, J.O.; Washington University School of Medicine CALERIE Group. Calorie restriction or exercise: Effects on coronary heart disease risk factors. A randomized, controlled trial. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E197–E202. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Park, H.S.; Kim, K.S.; Choi, W.H.; Ahn, C.W.; Kim, B.T.; Kim, S.M.; Lee, S.Y.; Ahn, S.M.; Kim, Y.K.; et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. J. Nutr. Biochem. 2008, 19, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.N.; Columbus, M.L.; Shields, K.J.; Asubonteng, J.; Meyer, M.L.; Sutton-Tyrrell, K.; Goodpaster, B.H.; DeLany, J.P.; Jakicic, J.M.; Barinas-Mitchell, E. Effects of an intensive behavioral weight loss intervention consisting of caloric restriction with or without physical activity on common carotid artery remodeling in severely obese adults. Metabolism 2012, 61, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Samaras, K.; Viardot, A.; Lee, P.N.; Jenkins, A.; Botelho, N.K.; Bakopanos, A.; Lord, R.V.; Hayward, C.S. Reduced arterial stiffness after weight loss in obese type 2 diabetes and impaired glucose tolerance: The role of immune cell activation and insulin resistance. Diabetes Vasc. Dis. Res. 2013, 10, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Soare, A.; Weiss, E.P.; Pozzilli, P. Benefits of caloric restriction for cardiometabolic health, including type 2 diabetes mellitus risk. Diabetes Metab. Res. Rev. 2014, 30, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.P.; Zhao, X.; Courville, A.B.; Linderman, J.D.; Smith, S.; Sebring, N.; Della Valle, D.M.; Fitzpatrick, B.; Simchowitz, L.; Celi, F.S. Effects of a 12-month moderate weight loss intervention on insulin sensitivity and inflammation status in nondiabetic overweight and obese subjects. Horm. Metab. Res. 2015, 47, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Kume, S.; Takeda-Watanabe, A.; Tsuda, S.; Kanasaki, K.; Koya, D. Calorie restriction in overweight males ameliorates obesity-related metabolic alterations and cellular adaptations through anti-aging effects, possibly including AMPK and SIRT1 activation. Biochim. Biophys. Acta 2013, 1830, 4820–4827. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 6659–6663. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.D.; Bartke, A. Diet and aging. Cell Metab. 2008, 8, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Hellerstein, M.K. Alternate-day fasting and chronic disease prevention: A review of human and animal trials. Am. J. Clin. Nutr. 2007, 86, 7–13. [Google Scholar] [PubMed]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-day fasting in nonobese subjects: Effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [PubMed]
- Ahmet, I.; Tae, H.J.; de Cabo, R.; Lakatta, E.G.; Talan, M.I. Effects of calorie restriction on cardioprotection and cardiovascular health. J. Mol. Cell. Cardiol. 2011, 51, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Shibata, R.; Miura, R.; Shimano, M.; Kondo, K.; Li, P.; Ohashi, T.; Kihara, S.; Maeda, N.; Walsh, K.; et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J. Biol. Chem. 2009, 284, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.X.; Dhahbi, J.M.; Mote, P.L.; Spindler, S.R. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc. Natl. Acad. Sci. USA 2001, 98, 10630–10635. [Google Scholar] [CrossRef] [PubMed]
- Higami, Y.; Barger, J.L.; Page, G.P.; Allison, D.B.; Smith, S.R.; Prolla, T.A.; Weindruch, R. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J. Nutr. 2006, 136, 343–352. [Google Scholar] [PubMed]
- Donato, A.J.; Walker, A.E.; Magerko, K.A.; Bramwell, R.C.; Black, A.D.; Henson, G.D.; Lawson, B.R.; Lesniewski, L.A.; Seals, D.R. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 2013, 12, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Ouyang, C.; Ding, Q.; Song, J.; Cao, W.; Mao, L. A moderate low-carbohydrate low-calorie diet improves lipid profile, insulin sensitivity and adiponectin expression in rats. Nutrients 2015, 7, 4724–4738. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Lee, Y.C.; Huang, C.F.; Wang, Y.R.; Yu, H.P.; Lau, Y.T. Gender-specific effects of caloric restriction on the balance of vascular nitric oxide and superoxide radical. Cardiovasc. Res. 2010, 87, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Morton, J.S.; Oka, T.; Robillard-Frayne, I.; Bagdan, M.; Lopaschuk, G.D.; Des Rosiers, C.; Walsh, K.; Davidge, S.T.; Dyck, J.R. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension 2010, 56, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, B.; Nelson, J.F.; Colston, J.T.; Freeman, G.L. Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2094–H2102. [Google Scholar] [PubMed]
- Csiszar, A.; Labinskyy, N.; Jimenez, R.; Pinto, J.T.; Ballabh, P.; Losonczy, G.; Pearson, K.J.; de Cabo, R.; Ungvari, Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mech. Ageing Dev. 2009, 130, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Gortan Cappellari, G.; Burekovic, I.; Barazzoni, R.; Stebel, M.; Guarnieri, G. Caloric restriction improves endothelial dysfunction during vascular aging: Effects on nitric oxide synthase isoforms and oxidative stress in rat aorta. Exp. Gerontol. 2010, 45, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Castello, L.; Froio, T.; Cavallini, G.; Biasi, F.; Sapino, A.; Leonarduzzi, G.; Bergamini, E.; Poli, G.; Chiarpotto, E. Calorie restriction protects against age-related rat aorta sclerosis. FASEB J. 2005, 19, 1863–1865. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, E.; Simsek, A.; Ozbek, M.; Somay, A. Caloric restriction increases internal iliac artery and penil nitric oxide synthase expression in rat: Comparison of aged and adult rats. Arch. Ital. Urol. Androl. 2013, 85, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Minamiyama, Y.; Bito, Y.; Takemura, S.; Takahashi, Y.; Kodai, S.; Mizuguchi, S.; Nishikawa, Y.; Suehiro, S.; Okada, S. Calorie restriction improves cardiovascular risk factors via reduction of mitochondrial reactive oxygen species in type II diabetic rats. J. Pharmacol. Exp. Ther. 2007, 320, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Prieto, C.F.; Pulido-Olmo, H.; Ruiz-Hurtado, G.; Gil-Ortega, M.; Aranguez, I.; Rubio, M.A.; Ruiz-Gayo, M.; Somoza, B.; Fernandez-Alfonso, M.S. Mild caloric restriction reduces blood pressure and activates endothelial AMPK-PI3K-Akt-eNOS pathway in obese Zucker rats. Vascul. Pharmacol. 2015, 65–66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ketonen, J.; Pilvi, T.; Mervaala, E. Caloric restriction reverses high-fat diet-induced endothelial dysfunction and vascular superoxide production in C57Bl/6 mice. Heart Vessel. 2010, 25, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Singh, N.; Wharton, S.; Sharma, A.M. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 2008, 16, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Siklova-Vitkova, M.; Klimcakova, E.; Polak, J.; Kovacova, Z.; Tencerova, M.; Rossmeislova, L.; Bajzova, M.; Langin, D.; Stich, V. Adipose tissue secretion and expression of adipocyte-produced and stromavascular fraction-produced adipokines vary during multiple phases of weight-reducing dietary intervention in obese women. J. Clin. Endocrinol. Metab. 2012, 97, E1176–E1181. [Google Scholar] [CrossRef] [PubMed]
- Capel, F.; Klimcakova, E.; Viguerie, N.; Roussel, B.; Vitkova, M.; Kovacikova, M.; Polak, J.; Kovacova, Z.; Galitzky, J.; Maoret, J.J.; et al. Macrophages and adipocytes in human obesity: Adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes 2009, 58, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Luca, F.; Grunebaum, L.; Jesel, L.; Meyer, N.; Desprez, D.; Robert, S.; Dignat-George, F.; Toti, F.; Simon, C.; et al. Short-term very low-calorie diet in obese females improves the haemostatic balance through the reduction of leptin levels, PAI-1 concentrations and a diminished release of platelet and leukocyte-derived microparticles. Int. J. Obes. 2011, 35, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Horigome, H.; Tanaka, K.; Nakata, Y.; Ohkawara, K.; Katayama, Y.; Matsui, A. Impact of weight reduction on production of platelet-derived microparticles and fibrinolytic parameters in obesity. Thromb. Res. 2007, 119, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.T.; Mitchell, J.R. Benefits of short-term dietary restriction in mammals. Exp. Gerontol. 2013, 48, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Dean, D.J. Glucose transport with brief dietary restriction: Heterogenous responses in muscles. Am. J. Physiol. 1994, 266, E946–E952. [Google Scholar] [PubMed]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Weindruch, R.; Walford, R.L. Dietary restriction in mice beginning at 1 year of age: Effect on life-span and spontaneous cancer incidence. Science 1982, 215, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Weindruch, R.; Gottesman, S.R.; Walford, R.L. Modification of age-related immune decline in mice dietarily restricted from or after midadulthood. Proc. Natl. Acad. Sci. USA 1982, 79, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.J.; Morris, P.; Sohal, R.S. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003, 17, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, E.; Viguerie, N.; Andersson, I.; Verdich, C.; Langin, D.; Arner, P. Effects of different hypocaloric diets on protein secretion from adipose tissue of obese women. Diabetes 2004, 53, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Fornieri, C.; Taparelli, F.; Quaglino, D.; Contri, M.B.; Davidson, J.M.; Algeri, S.; Ronchetti, I.P. The effect of caloric restriction on the aortic tissue of aging rats. Connect. Tissue Res. 1999, 40, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Barazzoni, R.; Vadori, M.; Stebel, M.; Biolo, G.; Guarnieri, G. Lack of direct effect of moderate hyperleptinemia to improve endothelial function in lean rat aorta: Role of calorie restriction. Atherosclerosis 2004, 175, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Clifton, P.M. C-reactive protein and coronary artery disease: Influence of obesity, caloric restriction and weight loss. J. Nutr. Biochem. 2002, 13, 316–321. [Google Scholar] [CrossRef]
- Garcia-Prieto, C.F.; Gil-Ortega, M.; Aranguez, I.; Ortiz-Besoain, M.; Somoza, B.; Fernandez-Alfonso, M.S. Vascular ampk as an attractive target in the treatment of vascular complications of obesity. Vascul. Pharmacol. 2015, 67–69, 10–20. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, C.F.; Hernández-Nuño, F.; Rio, D.D.; Ruiz-Hurtado, G.; Aránguez, I.; Ruiz-Gayo, M.; Somoza, B.; Fernández-Alfonso, M.S. High-fat diet induces endothelial dysfunction through a down-regulation of the endothelial AMPK-PI3K-Akt-eNOS pathway. Mol. Nutr. Food Res. 2015, 59, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Lobato, N.S.; Filgueira, F.P.; Prakash, R.; Giachini, F.R.; Ergul, A.; Carvalho, M.H.; Webb, R.C.; Tostes, R.C.; Fortes, Z.B. Reduced endothelium-dependent relaxation to anandamide in mesenteric arteries from young obese Zucker rats. PLoS ONE 2013, 8, e63449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blume, C.; Benz, P.M.; Walter, U.; Ha, J.; Kemp, B.E.; Renné, T. Amp-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J. Biol. Chem. 2007, 282, 4601–4612. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lee, I.K.; Kim, H.S.; Kim, Y.M.; Koh, E.H.; Won, J.C.; Han, S.M.; Kim, M.S.; Jo, I.; Oh, G.T.; et al. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2488–2494. [Google Scholar] [CrossRef] [PubMed]
- Dagher, Z.; Ruderman, N.; Tornheim, K.; Ido, Y. Acute regulation of fatty acid oxidation and amp-activated protein kinase in human umbilical vein endothelial cells. Circ. Res. 2001, 88, 1276–1282. [Google Scholar] [CrossRef]
- McCarty, M.F. Ampk activation as a strategy for reversing the endothelial lipotoxicity underlying the increased vascular risk associated with insulin resistance syndrome. Med. Hypotheses 2005, 64, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.S.; Heilbronn, L.K.; Smith, C.; Hunt, N.D.; Ingram, D.K.; Ravussin, E.; de Cabo, R.; Team, P.C. In vitro cellular adaptations of indicators of longevity in response to treatment with serum collected from humans on calorie restricted diets. PLoS ONE 2008, 3, e3211. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Aras-Lopez, R.; Alonso, M.J.; Salaices, M. Small artery remodeling in obesity and insulin resistance. Curr. Vasc. Pharmacol. 2014, 12, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Walford, R.L.; Harris, S.B.; Gunion, M.W. The calorically restricted low-fat nutrient-dense diet in biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc. Natl. Acad. Sci. USA 1992, 89, 11533–11537. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Clifton, P.M.; Lister, N.; Keogh, J.B. Effect of weight loss induced by energy restriction on measures of arterial compliance: A systematic review and meta-analysis. Atherosclerosis 2016, 247, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Alfonso, M.S.; Gil-Ortega, M.; Garcia-Prieto, C.F.; Aranguez, I.; Ruiz-Gayo, M.; Somoza, B. Mechanisms of perivascular adipose tissue dysfunction in obesity. Int. J. Endocrinol. 2013, 2013, 402053. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Ribaudo, M.C.; Assael, F.; Vecci, E.; Tiberti, C.; Zappaterreno, A.; di Mario, U.; Leonetti, F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 2003, 88, 5163–5168. [Google Scholar] [CrossRef] [PubMed]
- Somoza, B.; Guzman, R.; Cano, V.; Merino, B.; Ramos, P.; Diez-Fernandez, C.; Fernandez-Alfonso, M.S.; Ruiz-Gayo, M. Induction of cardiac uncoupling protein-2 expression and adenosine 5′-monophosphate-activated protein kinase phosphorylation during early states of diet-induced obesity in mice. Endocrinology 2007, 148, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ortega, M.; Condezo-Hoyos, L.; García-Prieto, C.F.; Arribas, S.M.; González, M.C.; Aranguez, I.; Ruiz-Gayo, M.; Somoza, B.; Fernández-Alfonso, M.S. Imbalance between pro and anti-oxidant mechanisms in perivascular adipose tissue aggravates long-term high-fat diet-derived endothelial dysfunction. PLoS ONE 2014, 9, e95312. [Google Scholar]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ma, S.; He, H.; Yang, D.; Chen, X.; Luo, Z.; Liu, D.; Zhu, Z. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res. 2010, 33, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Fésüs, G.; Dubrovska, G.; Gorzelniak, K.; Kluge, R.; Huang, Y.; Luft, F.C.; Gollasch, M. Adiponectin is a novel humoral vasodilator. Cardiovasc. Res. 2007, 75, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.K.; Witzmann, F.A.; McKenney, M.L.; Lai, X.; Berwick, Z.C.; Moberly, S.P.; Alloosh, M.; Sturek, M.; Tune, J.D. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: Influence of obesity. Circulation 2013, 128, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hou, N.; Han, F.; Guo, Y.; Hui, Z.; Du, G.; Zhang, Y. Effect of high free fatty acids on the anti-contractile response of perivascular adipose tissue in rat aorta. J. Mol. Cell. Cardiol. 2013, 63, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, A.; Rebolledo, O.R.; Marra, C.A.; Garcia, M.E.; Roldan Palomo, A.R.; Rimorini, L.; Gagliardino, J.J. Early alterations in vascular contractility associated to changes in fatty acid composition and oxidative stress markers in perivascular adipose tissue. Cardiovasc. Diabetol. 2010, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Dubrovska, G.; Tsang, S.Y.; Essin, K.; Luft, F.C.; Huang, Y.; Gollasch, M. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension 2004, 44, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, B.; de Castro, J.; Herold, D.; Dubrovska, G.; Arribas, S.; González, M.C.; Aranguez, I.; Luft, F.C.; Ramos, M.P.; Gollasch, M.; et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Willens, H.J.; Byers, P.; Chirinos, J.A.; Labrador, E.; Hare, J.M.; de Marchena, E. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am. J. Cardiol. 2007, 99, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadzadeh, R.; Greenstein, A.S.; Yadav, R.; Jeziorska, M.; Hama, S.; Soltani, F.; Pemberton, P.W.; Ammori, B.; Malik, R.A.; Soran, H.; et al. Effects of bariatric surgery on human small artery function: Evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J. Am. Coll. Cardiol. 2013, 62, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K.; Correia, M.L.; Haynes, W.G.; Mark, A.L. Obesity-associated hypertension: New insights into mechanisms. Hypertension 2005, 45, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Blanquicett, C.; Graves, A.; Kleinhenz, D.J.; Hart, C.M. Attenuation of signaling and nitric oxide production following prolonged leptin exposure in human aortic endothelial cells. J. Investig. Med. 2007, 55, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Knudson, J.D.; Dincer, U.D.; Zhang, C.; Swafford, A.N., Jr.; Koshida, R.; Picchi, A.; Focardi, M.; Dick, G.M.; Tune, J.D. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H48–H56. [Google Scholar] [CrossRef] [PubMed]
- Bouloumie, A.; Marumo, T.; Lafontan, M.; Busse, R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999, 13, 1231–1238. [Google Scholar] [PubMed]
- Shinmura, K.; Tamaki, K.; Bolli, R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J. Mol. Cell. Cardiol. 2005, 39, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Miura, J.; Lu, L.X.; Bernier, M.; DeCabo, R.; Lane, M.A.; Roth, G.S.; Ingram, D.K. Circulating adiponectin levels increase in rats on caloric restriction: The potential for insulin sensitization. Exp. Gerontol. 2004, 39, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ortega, M.; Stucchi, P.; Guzmán-Ruiz, R.; Cano, V.; Arribas, S.; González, M.C.; Ruiz-Gayo, M.; Fernández-Alfonso, M.S.; Somoza, B. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology 2010, 151, 3299–3306. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Margaritis, M.; Coutinho, P.; Shirodaria, C.; Psarros, C.; Herdman, L.; Sanna, F.; De Silva, R.; Petrou, M.; Sayeed, R.; et al. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: The regulatory role of perivascular adipose tissue. Diabetes 2015, 64, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Lavi, T.; Karasik, A.; Koren-Morag, N.; Kanety, H.; Feinberg, M.S.; Shechter, M. The acute effect of various glycemic index dietary carbohydrates on endothelial function in nondiabetic overweight and obese subjects. J. Am. Coll. Cardiol. 2009, 53, 2283–2287. [Google Scholar] [CrossRef] [PubMed]
- Gögebakan, O.; Kohl, A.; Osterhoff, M.A.; van Baak, M.A.; Jebb, S.A.; Papadaki, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Hlavaty, P.; Weickert, M.O.; et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: The diet, obesity, and genes (diogenes) study: A randomized, controlled trial. Circulation 2011, 124, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Cosentino, L.; Rosafio, G.; Morgana, M.; Mattina, A.; Sprini, D.; Verga, S.; Rini, G.B. Effects of hypocaloric diets with different glycemic indexes on endothelial function and glycemic variability in overweight and in obese adult patients at increased cardiovascular risk. Clin. Nutr. 2013, 32, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Fernandez, M.L.; Feinman, R.D.; Phinney, S.D. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog. Lipid Res. 2008, 47, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Ballard, K.D.; Silvestre, R.; Judelson, D.A.; Quann, E.E.; Forsythe, C.E.; Fernandez, M.L.; Kraemer, W.J. Effects of dietary carbohydrate restriction versus low-fat diet on flow-mediated dilation. Metabolism 2009, 58, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.M.; Keogh, J.B.; Foster, P.R.; Noakes, M. Effect of weight loss on inflammatory and endothelial markers and fmd using two low-fat diets. Int. J. Obes. 2005, 29, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, T.P.; Brinkworth, G.D.; Keogh, J.B.; Noakes, M.; Buckley, J.D.; Clifton, P.M. Long-term effects of weight loss with a very low carbohydrate and low fat diet on vascular function in overweight and obese patients. J. Intern. Med. 2010, 267, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Ballard, K.D.; Quann, E.E.; Kupchak, B.R.; Volk, B.M.; Kawiecki, D.M.; Fernandez, M.L.; Seip, R.L.; Maresh, C.M.; Kraemer, W.J.; Volek, J.S. Dietary carbohydrate restriction improves insulin sensitivity, blood pressure, microvascular function, and cellular adhesion markers in individuals taking statins. Nutr. Res. 2013, 33, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Alessi, M.C.; Juhan-Vague, I. PAI-1 and the metabolic syndrome: Links, causes, and consequences. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, T.; Imaizumi, T.; Endo, T.; Shiramoto, M.; Harasawa, Y.; Takeshita, A. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circulation 1994, 90, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.; Kones, R.; Ferré, R.; Plana, N.; Girona, J.; Aragonés, G.; Ibarretxe, D.; Heras, M.; Masana, L. Negative effect of a low-carbohydrate, high-protein, high-fat diet on small peripheral artery reactivity in patients with increased cardiovascular risk. Br. J. Nutr. 2013, 109, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Hite, A.H.; Berkowitz, V.G.; Berkowitz, K. Low-carbohydrate diet review: Shifting the paradigm. Nutr. Clin. Pract. 2011, 26, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.M.; Dalskov, S.M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunešová, M.; Pihlsgård, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, G.D.; Noakes, M.; Parker, B.; Foster, P.; Clifton, P.M. Long-term effects of advice to consume a high-protein, low-fat diet, rather than a conventional weight-loss diet, in obese adults with type 2 diabetes: One-year follow-up of a randomised trial. Diabetologia 2004, 47, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Khoo, J.; Ling, P.S.; Tan, J.; Teo, A.; Ng, H.L.; Chen, R.Y.; Tay, T.L.; Tan, E.; Cheong, M. Comparing the effects of meal replacements with reduced-fat diet on weight, sexual and endothelial function, testosterone and quality of life in obese asian men. Int. J. Impot. Res. 2014, 26, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Rizkalla, S.W.; Prifti, E.; Cotillard, A.; Pelloux, V.; Rouault, C.; Allouche, R.; Laromiguière, M.; Kong, L.; Darakhshan, F.; Massiera, F.; et al. Differential effects of macronutrient content in 2 energy-restricted diets on cardiovascular risk factors and adipose tissue cell size in moderately obese individuals: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. High-sensitivity C-reactive protein: Potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001, 103, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Sacks, F.M.; Carey, V.J.; Obarzanek, E.; Swain, J.F.; Miller, E.R.; Conlin, P.R.; Erlinger, T.P.; Rosner, B.A.; Laranjo, N.M.; et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the omniheart randomized trial. JAMA 2005, 294, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef] [PubMed]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Lagiou, P.; Sandin, S.; Lof, M.; Trichopoulos, D.; Adami, H.O.; Weiderpass, E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in swedish women: Prospective cohort study. BMJ 2012, 344, e4026. [Google Scholar] [CrossRef] [PubMed]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Low-carbohydrate diets and all-cause mortality: A systematic review and meta-analysis of observational studies. PLoS ONE 2013, 8, e55030. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [PubMed]
- McMillan-Price, J.; Petocz, P.; Atkinson, F.; O’neill, K.; Samman, S.; Steinbeck, K.; Caterson, I.; Brand-Miller, J. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: A randomized controlled trial. Arch. Intern. Med. 2006, 166, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Recio-Rodriguez, J.I.; Gomez-Marcos, M.A.; Patino-Alonso, M.C.; Rodrigo-De Pablo, E.; Cabrejas-Sánchez, A.; Arietaleanizbeaskoa, M.S.; Repiso-Gento, I.; Gonzalez-Viejo, N.; Maderuelo-Fernandez, J.A.; Agudo-Conde, C.; et al. Glycemic index, glycemic load, and pulse wave reflection in adults. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Block, G.; Norkus, E.P.; Morrow, J.D.; Dietrich, M.; Hudes, M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am. J. Clin. Nutr. 2006, 84, 70–76, quiz 266–267. [Google Scholar] [PubMed]
- Sacks, F.M.; Carey, V.J.; Anderson, C.A.; Miller, E.R.; Copeland, T.; Charleston, J.; Harshfield, B.J.; Laranjo, N.; McCarron, P.; Swain, J.; et al. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: The omnicarb randomized clinical trial. JAMA 2014, 312, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Long-term effects of low glycemic index/load vs. High glycemic index/load diets on parameters of obesity and obesity-associated risks: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 699–706. [Google Scholar] [CrossRef]
| Reference | Model | CR Protocol |
|---|---|---|
| Kondo et al., 2009 [26] | C57/BL6 mice | 35% CR for 4 weeks |
| Donato et al., 2013 [29] | mice | 10% CR (1 week) + 25% CR (1 week) + 40% CR throughout the life of the animal |
| Chen et al., 2015 [30] | Wistar rats | 20% CR or 40% CR for 12 weeks—only reduction of starch |
| Chou et al., 2010 [31] | Wistar rats | 40% CR for 2 weeks |
| Dolinsky et al., 2010 [32] | Wistar and SHR rats | 10% CR (2 weeks) + 40% CR (3 weeks) |
| Chandrasekar et al., 2001 [33] | Fisher344 rats | 40% CR for 10 months |
| Csiszar et al., 2009 [34] | Fisher344 rats | 40% CR (life-long; age-related studies) |
| Ahmet et al., 2011 [24] | Fisher344 rats | 40% CR for 22 months (age-related studies) |
| Zanetti et al., 2010 [35] | Fisher344 rats | 26% CR for 3 weeks (age-related studies) |
| Castello et al., 2005 [36] | Sprague Dawley rats | 40% CR for 4, 10 or 22 months (age-related studies) |
| Ozbek et al., 2013 [37] | Sprague Dawley rats | 40% CR for 3 months |
| Minamiyama et al., 2007 [38] | type II diabetic rats (OLETF) | 30% CR for 13 weeks |
| García-Prieto et al., 2015 [39] | Zucker obese rats | 20% CR for 2 weeks |
| Ketonen et al., 2010 [40] | C57Bl/6J mice under HFD | 30% CR for 50 days (with HFD) |
| Iacobellis et al., 2008 [41] | patients | VLCD (900 kcal/day). Phase 1—complete meal replacement (12 weeks); phase 2—transition period including healthy foods and partial meal replacement (4–6 weeks); phase 3—long-term maintenance |
| Kitada et al., 2013 [19] | overweight patients | 25% CR for 7 weeks |
| Siklova-Vitkova et al., 2012 [42] | obese patients | 800 kcal/day (1 month) + weight stabilization period (low-calorie diet for 2 months + weight maintenance diet for 3 months) |
| Capel et al, 2009 [43] | obese patients | 800 kcal/day (1 month) + weight stabilization period (low-calorie diet for 2 months + weight maintenance diet for 3–4 months) |
| Davì et al., 2002 [9] | obese patients | 1200 kcal/day for 12 weeks |
| Ziccardi et al., 2002 [10] | obese patients | 1300 kcal/day for 12 months |
| Raitakari et al., 2004 [11] | obese patients | 580 kcal/day for 6 weeks |
| Cooper et al., 2012 [15] | obese patients | CR to produce a 8%–10% weight loss within 12 months with or without physical activity |
| Morel et al., 2011 [44] | obese patients | 600 kcal/day (1 month) + 1200 kcal/day (1 month) |
| Fontana et al., 2007 [13] | overweight/obese patients | 16% CR (3 months) + 20% CR (9 months) |
| Ho et al., 2015 [18] | overweight/obese patients | CR to produce a 5%–7% weight loss within 12 months |
| Murakami et al., 2007 [45] | overweight/obese patients | ≈1200 kcal/day (women) or 1600 kcal/day (men) for 12 weeks with or without exercise program |
| Sasaki et al., 2002 [8] | obese patients with hypertension | 800 kcal/day for 2 weeks |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Prieto, C.F.; Fernández-Alfonso, M.S. Caloric Restriction as a Strategy to Improve Vascular Dysfunction in Metabolic Disorders. Nutrients 2016, 8, 370. https://doi.org/10.3390/nu8060370
García-Prieto CF, Fernández-Alfonso MS. Caloric Restriction as a Strategy to Improve Vascular Dysfunction in Metabolic Disorders. Nutrients. 2016; 8(6):370. https://doi.org/10.3390/nu8060370
Chicago/Turabian StyleGarcía-Prieto, Concha F., and María S. Fernández-Alfonso. 2016. "Caloric Restriction as a Strategy to Improve Vascular Dysfunction in Metabolic Disorders" Nutrients 8, no. 6: 370. https://doi.org/10.3390/nu8060370