The Octyl Ester of Ginsenoside Rh2 Induces Lysosomal Membrane Permeabilization via Bax Translocation
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Antibodies
2.2. Cell Culture and Treatment
2.3. Lysosomal Stability Assessments
2.4. Assay of Cell Proliferation by MTT
2.5. Flow Cytometric Analysis of Apoptosis
2.6. Measurement of Mitochondrial Membrane Potential (ΔΨmt)
2.7. Culture Conditions to Inhibit Gene Expression by siRNA.
2.8. Preparation of Whole Cell Lysates
2.9. Preparation of Lysosomal Lysates
2.10. Protein Determination and Western Blot Analysis
2.11. Statistical Analysis
3. Results
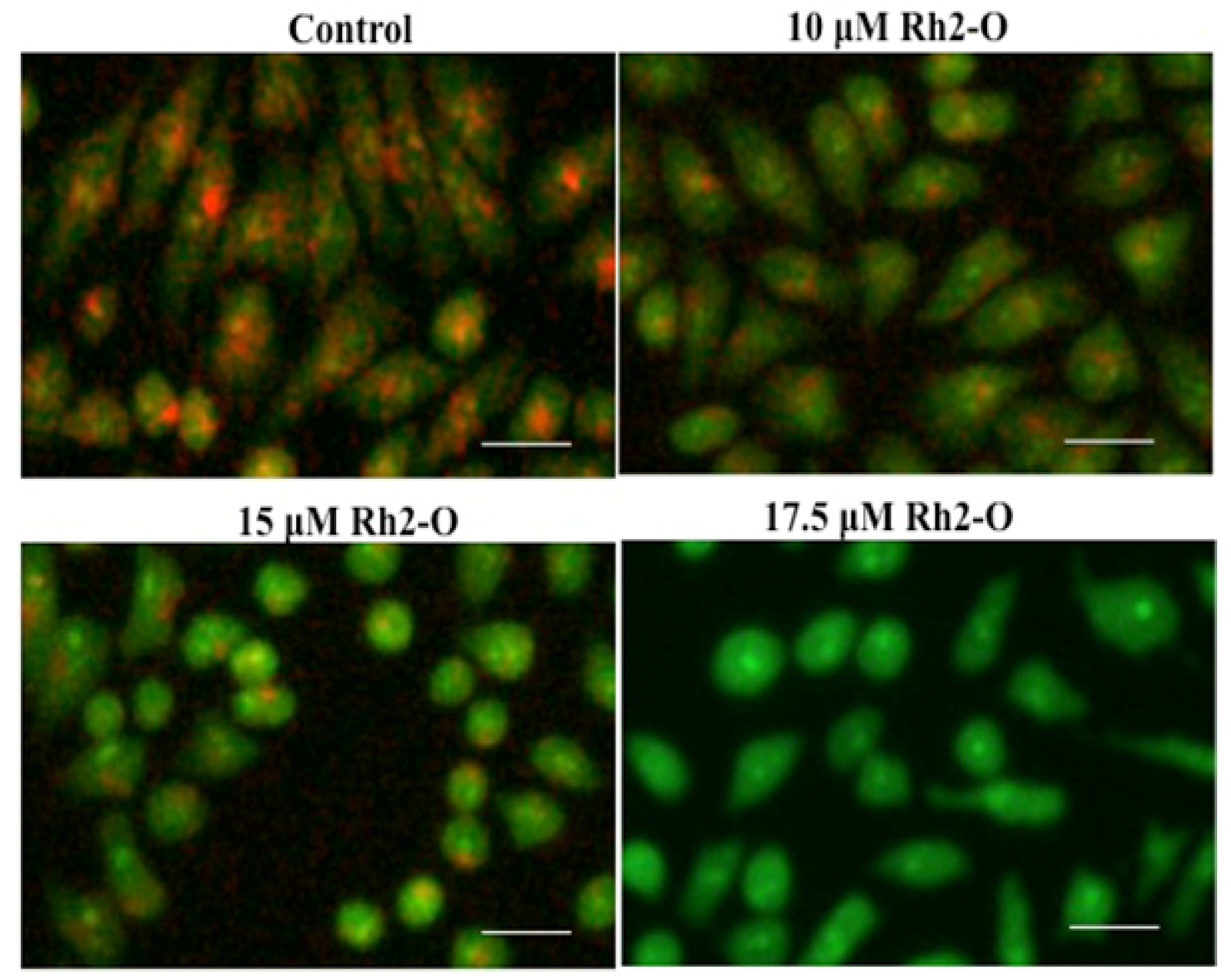
3.1. Rh2-O Induced Lysosomal Membrane Permeabilization in HepG2 Cells
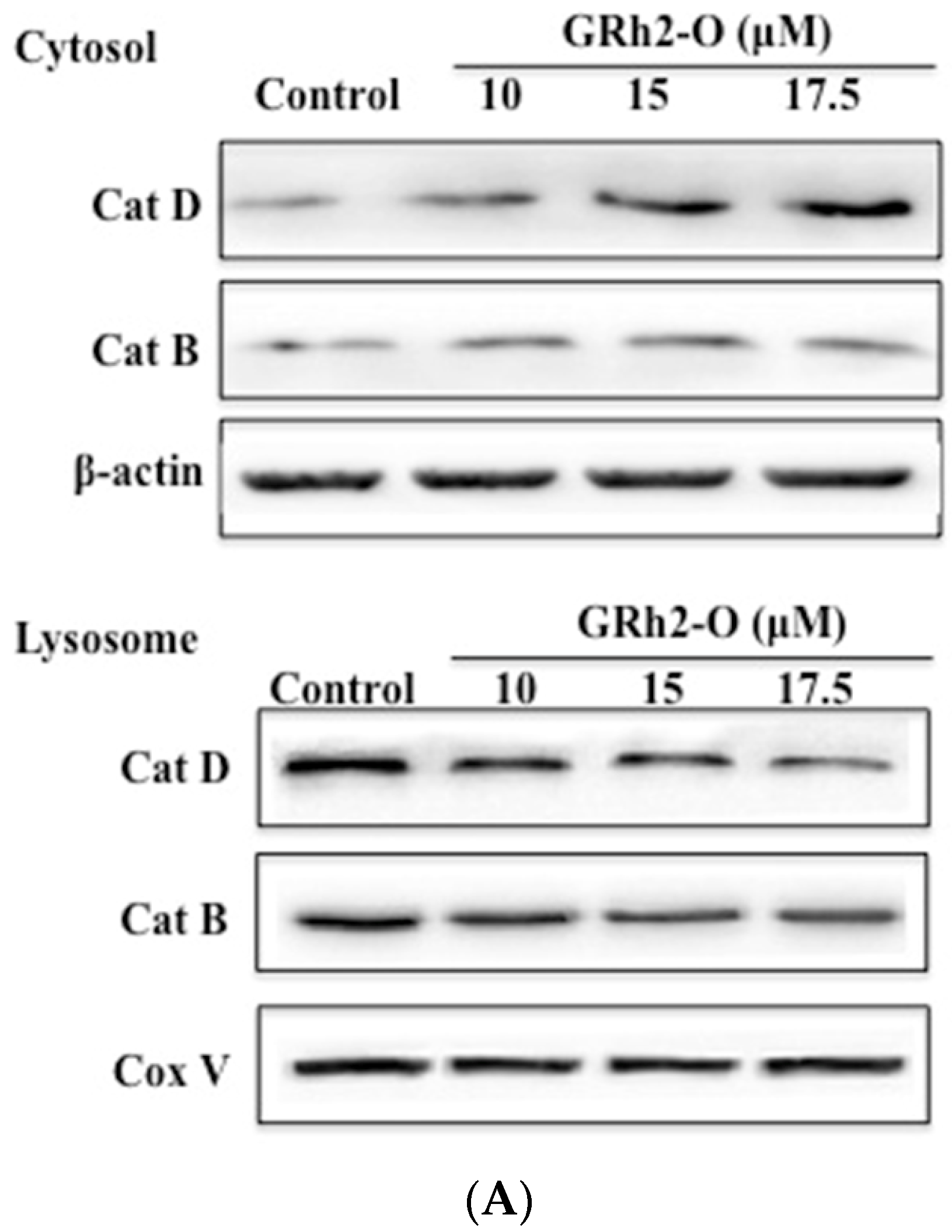
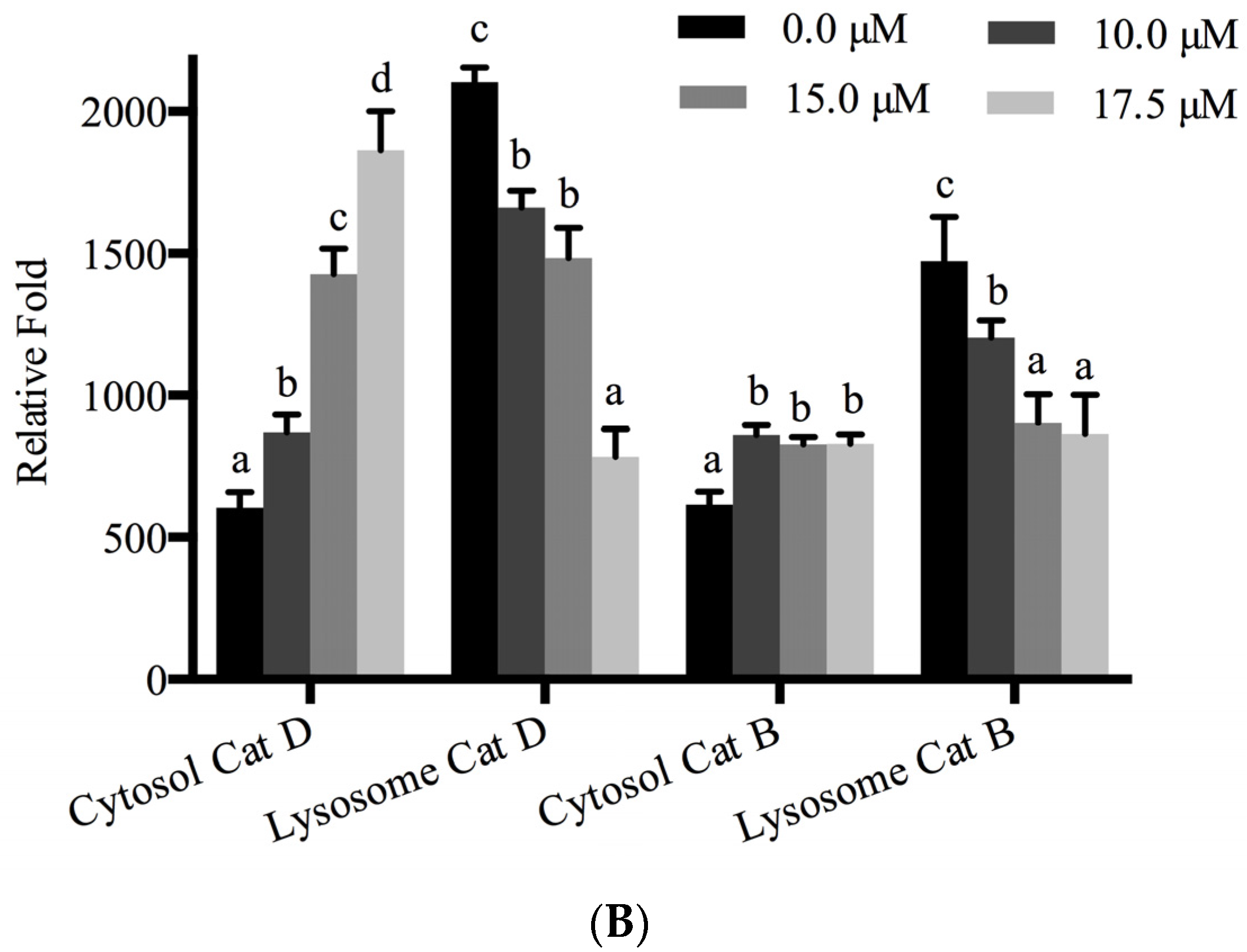
3.2. Rh2-O Triggered the Release of Cathepsin to the Cytosol
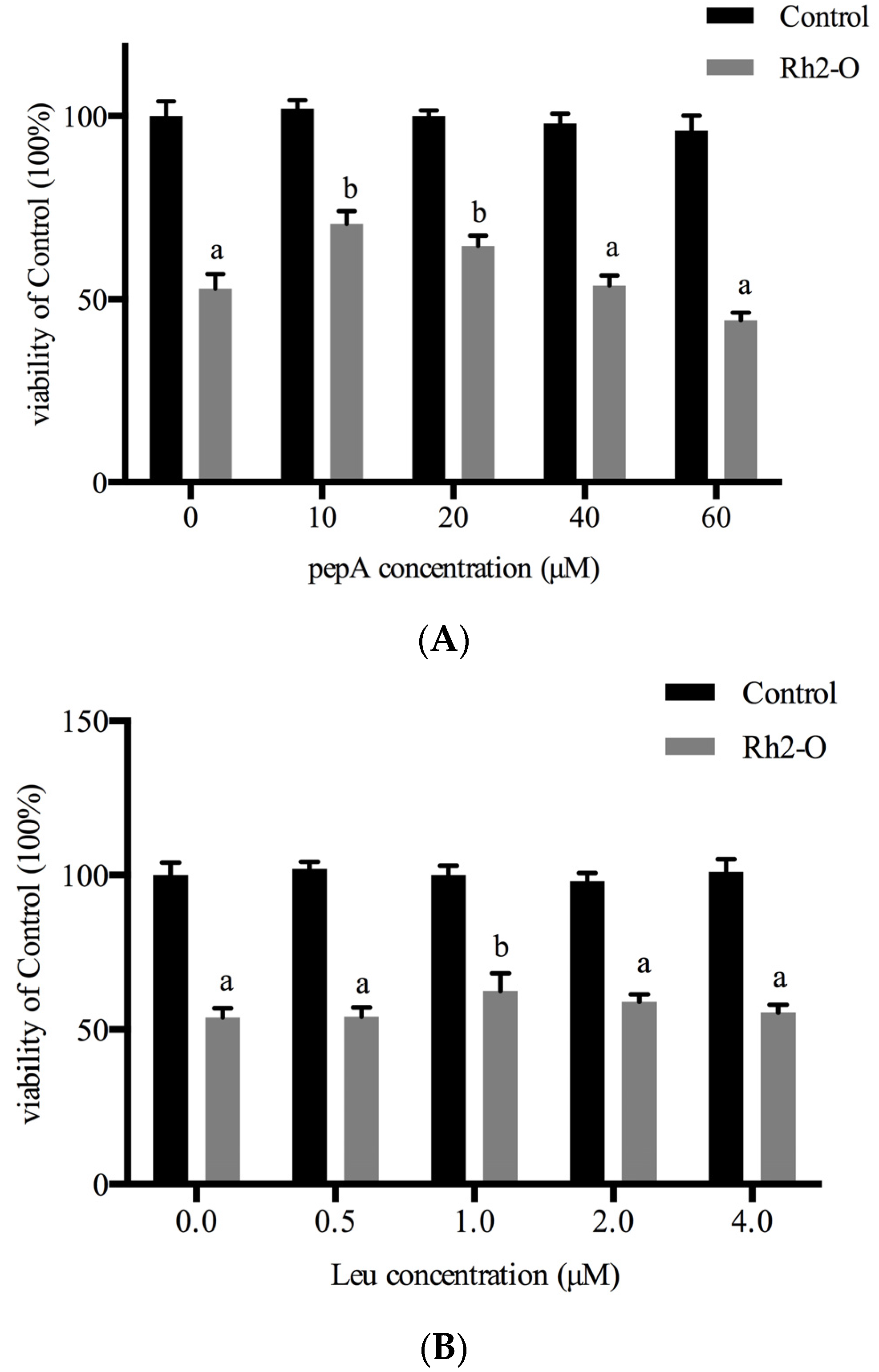
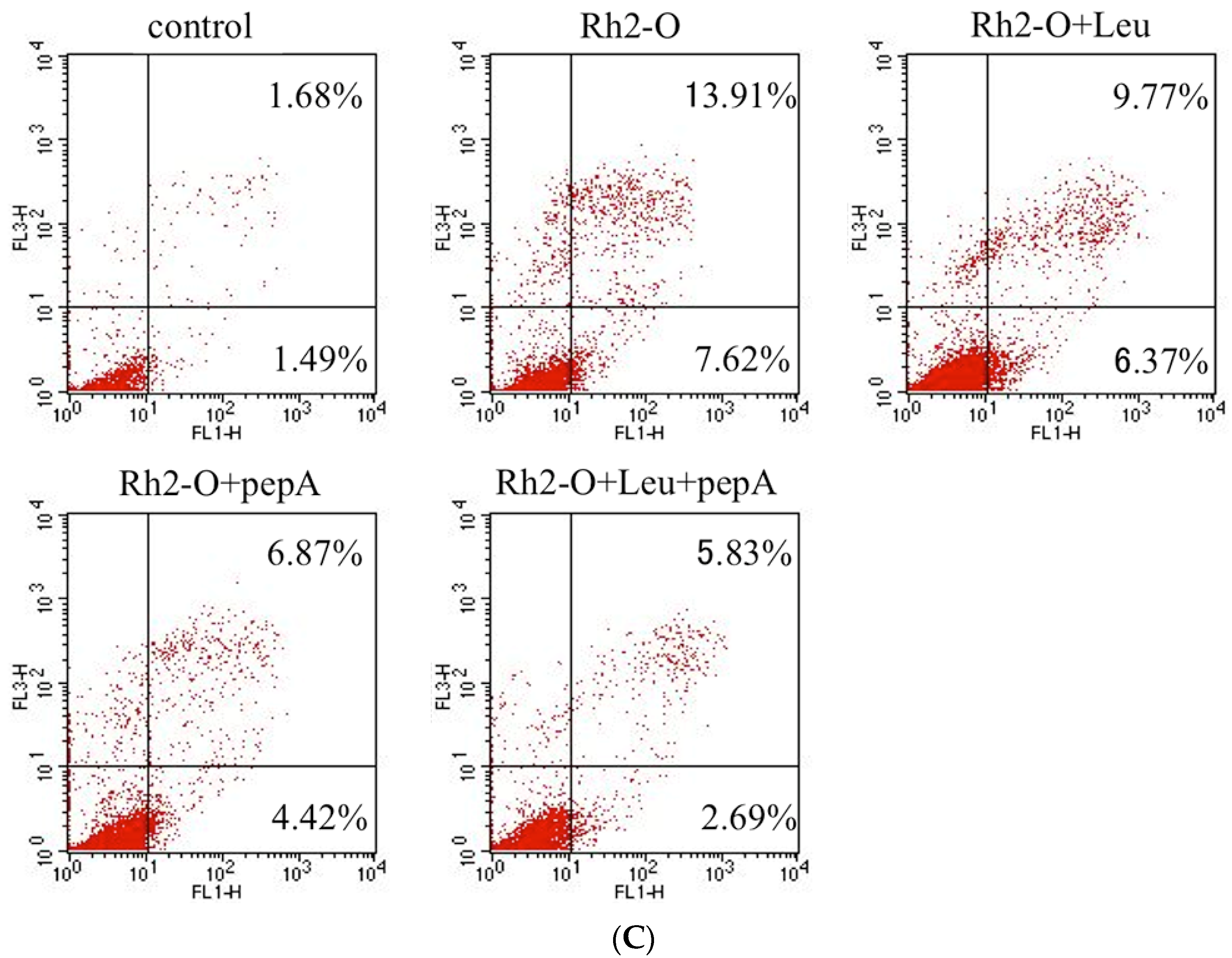
3.3. Effect of Cathepsin Inhibitors on Rh2-O-Induced Apoptosis in HepG2 Cells
3.4. Cathepsins Caused the Cleavage of Bid
3.5. The Release of Lysosomal Proteases Was Upstream of Mitochondrial Membrane Permeabilization
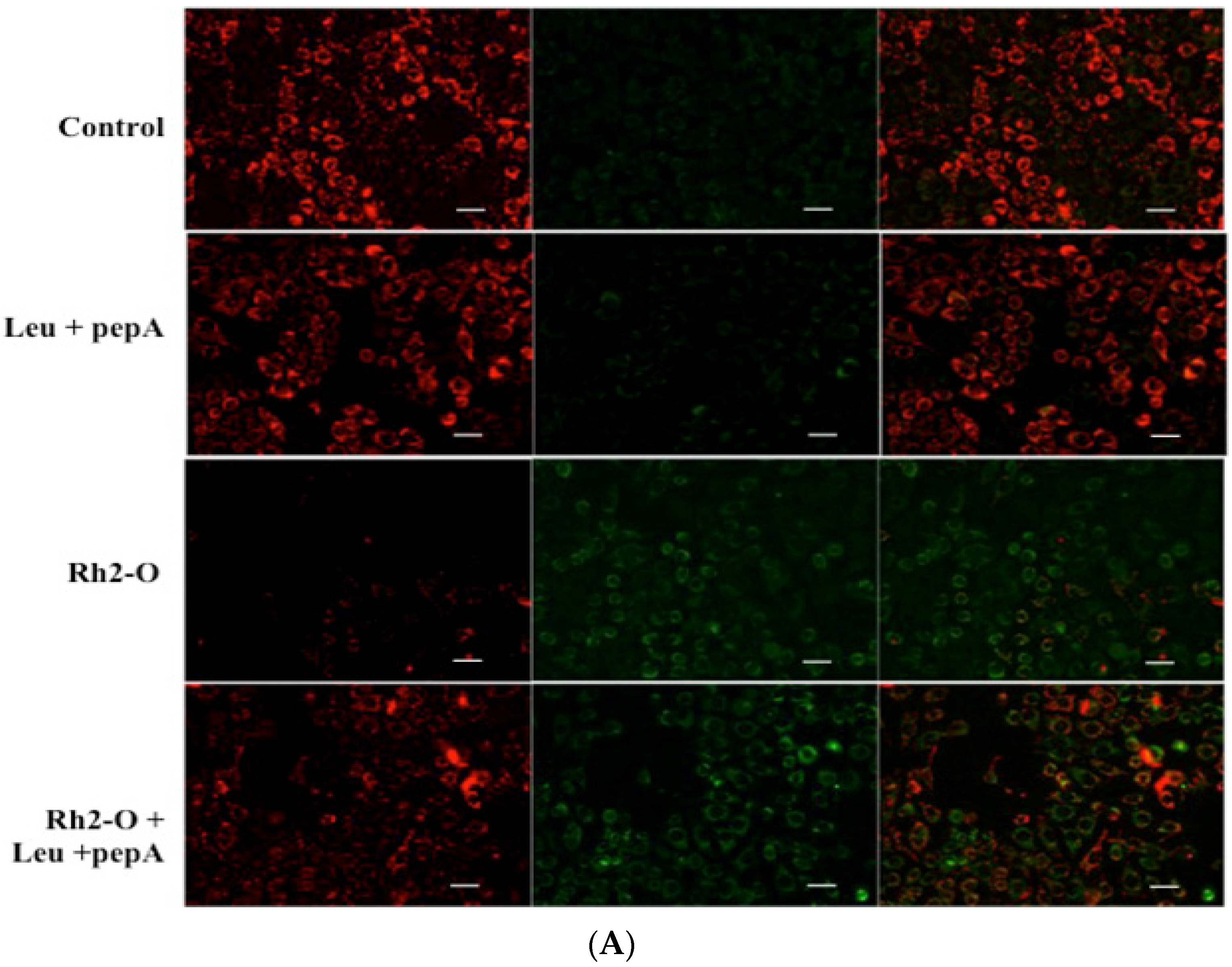
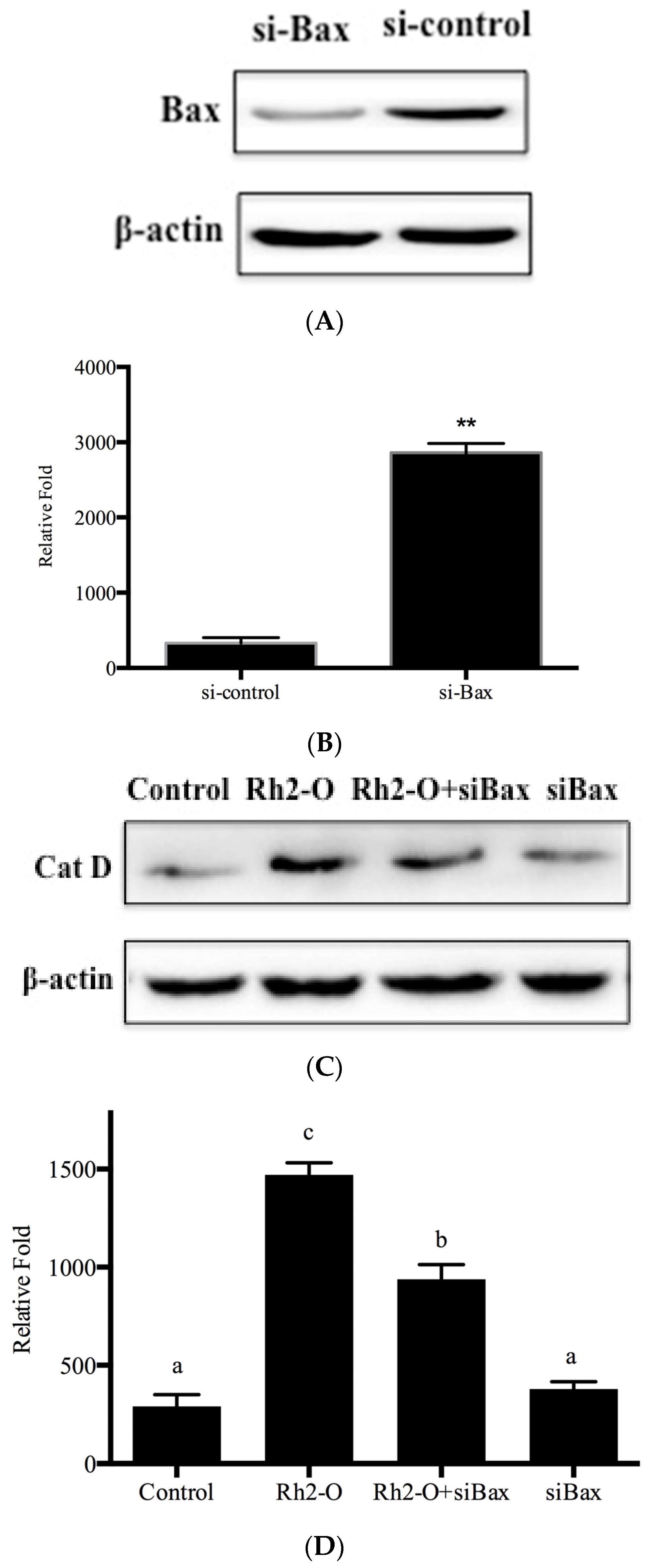
3.6. Bax and DRAM1 Involved in Lysosomal Membrane Permeabilization
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xia, T.; Wang, J.C.; Xu, W.; Xu, L.H.; Lao, C.H.; Ye, Q.X.; Fang, J.P. 20(S)-ginsenoside Rh2 induces apoptosis in human leukaemiareh cells through mitochondrial signaling pathways. Biol. Pharm. Bull. 2014, 37, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Yun, H.; Kim, D.H.; Kang, I.; Choe, W.; Kim, S.S.; Ha, J. AMP-activated protein kinase determines apoptotic sensitivity of cancer cells to ginsenoside-Rh2. J. Ginseng Res. 2014, 38, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.H.; Kim, H.; Hong, M.J.; Yang, M.H.; Kim, J.W.; Yoo, H.; Yang, H.; Park, J.H.; Sung, S.H.; Kim, H.P.; et al. Stereoisomer-specific anticancer activities of ginsenoside Rg3 and Rh2 in HepG2 cells: Disparity in cytotoxicity and autophagy-inducing effects due to 20(S)-epimers. Biol. Pharm. Bull. 2015, 38, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhao, Y.; Liang, X.J. Current evaluation of the millennium phytomedicine-ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr. Med. Chem. 2009, 16, 2924–2942. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.S.T.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Deng, Z.Y.; Zhang, B.; Xiong, Z.X.; Zheng, S.L.; Tan, C.L.; Hu, J.N. Esterification of ginsenoside Rh2 enhanced its cellular uptake and antitumor activity in human HepG2 cells. J. Agric. Food Chem. 2016, 64, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Borlak, J.; Zwadlo, C. Expression of drug-metabolizing enzymes, nuclear transcription factors and ABC transporters in Caco-2 cells. Xenobiotica 2003, 33, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ye, H.; Zhu, X.M.; Hu, J.N.; Li, H.Y.; Tsao, R.; Deng, Z.Y.; Zheng, Y.N.; Li, W. Esterification enhanced intestinal absorption of ginsenoside Rh2 in Caco-2 cells without impacts on its protective effects against H2O2-induced cell injury in human umbilical vein endothelial cells (HUVECs). J. Agric. Food Chem. 2014, 62, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad, J.; Hosseini, M.J.; Eskandari, M.R.; Shekarabi, S.M.; Daraei, B. Mitochondrial/lysosomal toxic cross-talk plays a key role in cisplatin nephrotoxicity. Xenobiotica 2010, 40, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Repnik, U.; Turk, B. Lysosomal-mitochondrial cross-talk during cell death. Mitochondrion 2010, 10, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.T.; Korsmeyer, S.J. Bcl-2 family: Regulators of cell death. Annu. Rev. Immunol. 1998, 16, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Kagedal, K.; Johansson, A.C.; Johansson, U.; Heimlich, G.; Roberg, K.; Wang, N.S.; Jurgensmeier, J.M.; Ollinger, K. Lysosomal membrane permeabilization during apoptosis—Involvement of Bax? Int. J. Exp. Path. 2005, 86, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.J.; Zhang, X.D.; Sun, W.; Qi, L.; Wu, J.C.; Qin, Z.H. DRAM1 regulates apoptosis through increasing protein levels and lysosomal localization of BAX. Cell Death Dis. 2015, 6, 1624. [Google Scholar] [CrossRef] [PubMed]
- Chwieralski, C.E.; Welte, T.; Buhling, F. Cathepsin-regulated apoptosis. Apoptosis 2006, 11, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Shi, J.G.; Yao, Q.H.; Jiao, D.M.; Wang, Y.Y.; Hu, H.Z.; Wu, Y.Q.; Song, J.; Yan, J.; Wu, L.J. Lysosomal membrane permeabilization is involved in curcumin-induced apoptosis of A549 lung carcinoma cells. Mol. Cell Biochem. 2012, 359, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.C.; Appelqvist, H.; Nilsson, C.; Kagedal, K.; Roberg, K.; Ollinger, K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis 2010, 15, 527–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Laskar, A.; Sultana, N.; Osman, E.; Ghosh, M.; Li, Q.Q.; Yuan, X.M. Cell death induced by 7-oxysterols via lysosomal and mitochondrial pathways is p53-dependent. Free Radical. Biol. Med. 2012, 53, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Brunk, U.T.; Svensson, I. Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak. Redox Rep. 1999, 4, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, A.M.; Appelqvist, H.; Ederth, T.; Ollinger, K. Lysosomotropic agents: Impact on lysosomal membrane permeabilization and cell death. Biochem. Soc. Trans. 2014, 42, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, N.; Guicciardi, M.E.; Yin, X.M.; Gores, G.J. TNF-α-mediated lysosomal permeabilization is FAN and caspase 8/Bid dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef]
- Gao, C.P.; Ding, Y.; Zhong, L.F.; Jiang, L.P.; Geng, C.G.; Yao, X.F.; Cao, J. Tacrine induces apoptosis through lysosome- and mitochondria-dependent pathway in HepG2 cells. Toxicol. Vitro 2014, 28, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, M.F.; Tahara, Y.; Chechetka, S.; Miyako, E.; Iijima, S.; Yudasaka, M. Lysosomal membrane permeabilization: Carbon nanohorn-induced reactive oxygen species generation and toxicity by this neglected mechanism. Toxicol. Appl. Pharm. 2014, 280, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Neumeyer, J.; Jakob, M.; Hallas, C.; Tchikov, V.; Winoto-Morbach, S.; Wickel, M.; Schneider-Brachert, W.; Trauzold, A.; Hethke, A.; et al. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004, 11, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Cirman, T.; Oresic, K.; Mazovec, G.D.; Turk, V.; Reed, J.C.; Myers, R.M.; Salvesen, G.S.; Turk, B. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J. Biol. Chem. 2004, 279, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Gustafsson, B.; Brunkc, U.T. The lysosomal-mitochondrial axis theory of postmitotic aging and cell death. Chem. Biol. Interact. 2006, 163, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Buettner, C.; Yeh, G.Y.; Phillips, R.S.; Mittleman, M.A.; Kaptchuk, T.J. Systematic review of the effects of ginseng on cardiovascular risk factors. Ann. Pharmacother. 2006, 40, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Deng, Z.Y.; Xiong, Z.X.; Zhang, B.; Yang, J.Y.; Hu, J.N. A ROS-mediated lysosomal-mitochondrial pathway is induced be ginsenoside Rh2 in hepatoma HepG2 cells. Food Funct. 2015, 6, 3828–3837. [Google Scholar] [CrossRef] [PubMed]
- Oberle, C.; Huai, J.; Reinheckel, T.; Tacke, M.; Rassner, M.; Ekert, P.G. Lysosomal membrane permeabilization and cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes. Cell Death Differ. 2010, 17, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Appelqvist, H.; Johansson, A.C.; Linderoth, E.; Johansson, U.; Antonsson, B.; Steinfeld, R.; Kagedal, K.; Ollinger, K. Lysosome-mediated apoptosis is associated with cathepsin D-specific processing of bid at Phe24, Trp48, and Phe183. Ann. Clin. Lab Sci. 2012, 42, 231–242. [Google Scholar] [PubMed]
- Tanase, C.; Albulescu, R.; Codrici, E.; Calenic, B.; Popescu, I.D.; Mihai, S.; Necula, L.; Cruceru, M.L.; Hinescu, M.E. Decreased expression of APAF-1 and increased expression of cathepsin B in invasive pituitary adenoma. OncoTargets Ther. 2015, 8, 81–90. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Zhang, B.; Sun, Y.; Xiong, Z.-X.; Peng, H.; Deng, Z.-Y.; Hu, J.-N. The Octyl Ester of Ginsenoside Rh2 Induces Lysosomal Membrane Permeabilization via Bax Translocation. Nutrients 2016, 8, 244. https://doi.org/10.3390/nu8050244
Chen F, Zhang B, Sun Y, Xiong Z-X, Peng H, Deng Z-Y, Hu J-N. The Octyl Ester of Ginsenoside Rh2 Induces Lysosomal Membrane Permeabilization via Bax Translocation. Nutrients. 2016; 8(5):244. https://doi.org/10.3390/nu8050244
Chicago/Turabian StyleChen, Fang, Bing Zhang, Yong Sun, Zeng-Xing Xiong, Han Peng, Ze-Yuan Deng, and Jiang-Ning Hu. 2016. "The Octyl Ester of Ginsenoside Rh2 Induces Lysosomal Membrane Permeabilization via Bax Translocation" Nutrients 8, no. 5: 244. https://doi.org/10.3390/nu8050244



