Amelioration of Hyperglycaemia, Oxidative Stress and Dyslipidaemia in Alloxan-Induced Diabetic Wistar Rats Treated with Probiotic and Vitamin C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Treatments
- Group 1, Control group: (normal saline only);
- Group 2, Untreated diabetic, UD: (alloxan 150 mg/kg, i.p.);
- Group 3, Diabetic + Treated with insulin, DTI: (alloxan 150 mg/kg, i.p. + s.c. injection of insulin 4 U/kg per day);
- Group 4, Diabetic + Treated with probiotic, DTP: (alloxan 150 mg/kg, i.p. + Saccharomyces cerevisiae, 4.125 × 106 CFU/100 mL, per os);
- Group 5, Diabetic + Treated with Vitamin C, DTVit-C: (alloxan 150 mg/kg, i.p. + vit-C 100 mg/kg, i.m.);
- Group 6, Diabetic + Treated with probiotic and Vit- C, DTPVit-C: (alloxan 150 mg/kg, i.p. + S. cerevisiae, 4.125 × 106 CFU/100 mL, per os. + Vit-C 100 mg/kg, i.m.).
2.3. Induction of Diabetes
2.4. Measurement of Blood Glucose
2.5. Measurements of Body Weight and Feed Intake
2.6. Collection of Blood
2.7. Serum Cholesterol and Triglyceride Assay
2.8. Assessment of Lipid Peroxidation of Organs
2.9. Assay of Antioxidant Enzymes
2.10. Data Analysis
3. Results
3.1. Effects of Treatment with Insulin, Probiotic and Vitamin C on Blood Glucose Concentrations
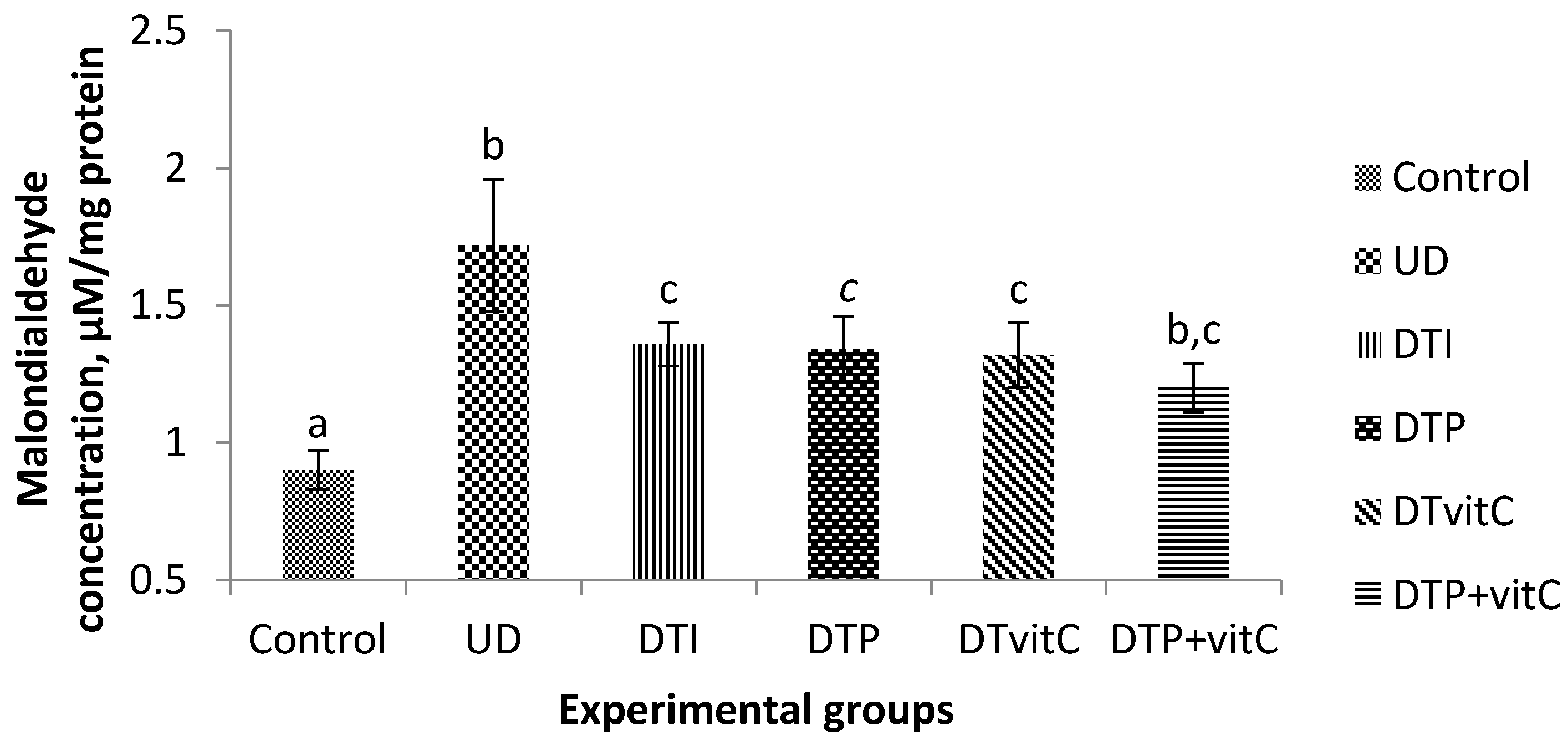
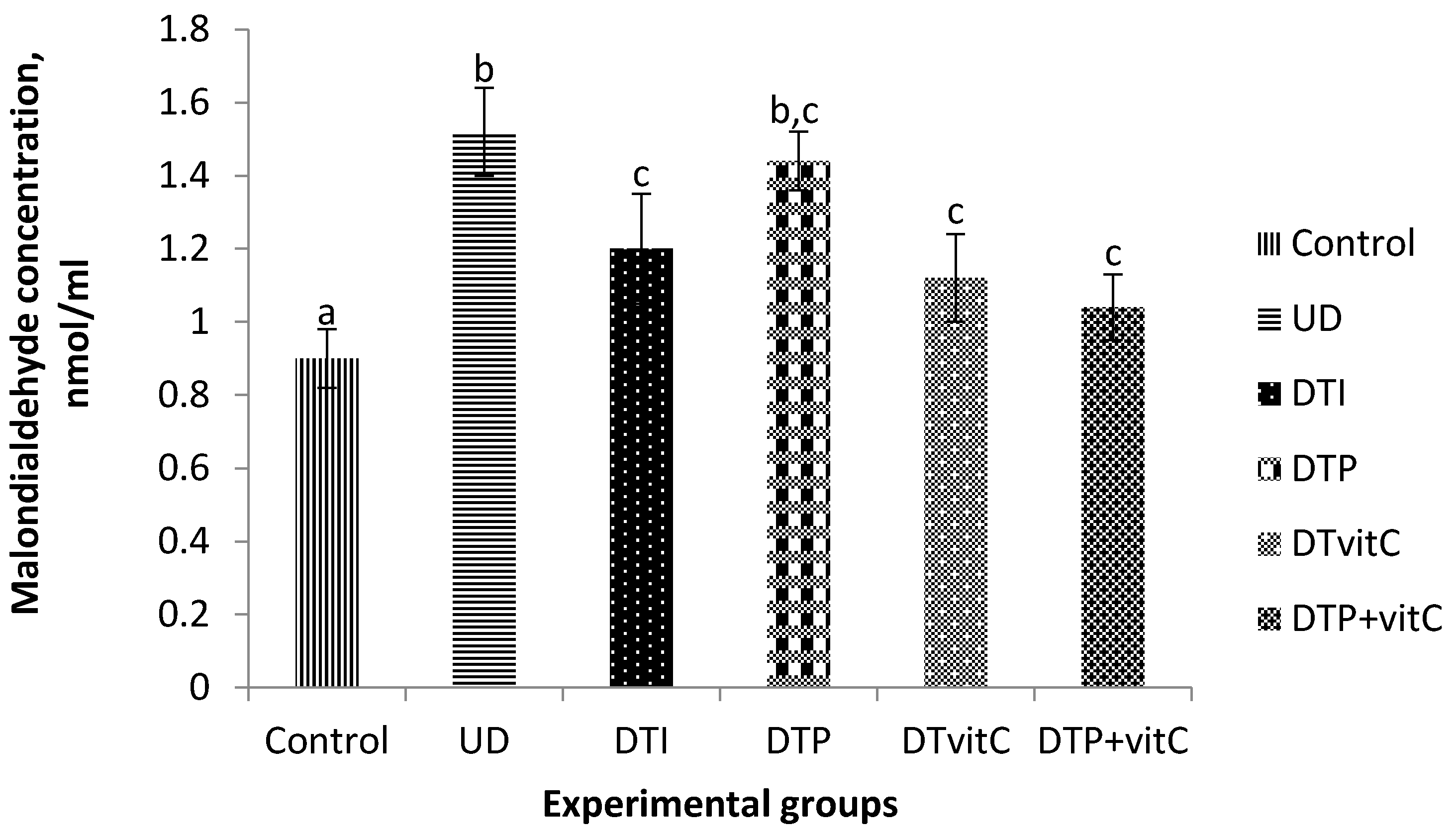
3.2. Effects of Treatment with Insulin, Probiotic and Vitamin C on Serum Malondialdehyde Concentrations
3.3. Effects of Treatment with Insulin, Probiotic, and Vitamin C on the Activities of Serum Antioxidant Enzymes
3.4. Effects of Treatment with Insulin, Probiotic and Vitamin C on Brain Malondialdehyde Concentration
3.5. Effects of Treatment with Insulin, Probiotic and Vitamin C on the Activities of Brain Antioxidant Enzymes
3.6. Effects of Treatment with Insulin, Probiotic and Vitamin C on Kidney Malondialdehyde Concentration
3.7. Effects of Treatment with Insulin, Probiotic and Vitamin C on the Lipid Profile of Alloxan-Induced Diabetic Rats
3.8. Effects of Treatment with Insulin, Probiotic and Vitamin C on the Body Weights and Feed Intake of Alloxan-Induced Diabetic Rats
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aly, H.F.; Mantawy, M.M. Comparative effects of zinc, selenium and vitamin E or their combination on carbohydrate metabolizing enzymes and oxidative stress in streptozotocin-induced diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 66–78. [Google Scholar] [PubMed]
- Vicentini, J.; Valentini, J.; Grotto, D.; Paniz, C.; Roehrs, M.; Brucker, N.; Charao, M.F.; Moro, A.M.; Tonello, R.; Moreira, A.P.; et al. Association among microalbuminuria and oxidative stress biomarkers in patients with type 2 diabetes. J. Invest. Med. 2011, 59, 649–654. [Google Scholar]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.S. Protective and therapeutic effectiveness of taurine in diabetes mellitus: A rationale for antioxidant supplementation. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 55–64. [Google Scholar]
- Yadav, H.; Jain, S.; Sinha, P. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrients 2007, 23, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kangralkar, V.A.; Patil, S.D.; Bandiradekar, R.M. Oxidative stress and diabetes: A review. Int. J. Pharm. Appl. 2010, 1, 38–45. [Google Scholar]
- Ozougwu, J.C.; Obimba, K.C.; Belouwu, C.D.; Unakalamba, C.B. The pathogenesis and pathophysiology of type 1 and 2 diabetes mellitus. J. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef]
- Lenzen, S.; Munday, R. Thiol group reactivity, hydrophilicity and stability of alloxan, its reduction products and its N-methyl derivatives and a comparison with ninhydrin. Biochem. Pharmacol. 1991, 42, 1385–1391. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Perdigon, G.; Corthier, G.; Salminen, S.; Koletzko, B.; Morelli, L. Should yoghurt culture be considered probiotic? Br. J. Nutr. 2005, 93, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Yen, C.L. Antioxidative ability of lactic acid bacteria. J. Agric. Food Chem. 1999, 47, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Uskova, M.A.; Kravchenko, L.V. Antioxidant properties of lactic acid bacteria-probiotic and yogurt strains. Vopr. Pitan. 2009, 78, 18–23. [Google Scholar] [PubMed]
- Donohue, D.C. Safety of probiotics. Asia Pac. J. Clin. Nutr. 2006, 15, 563–569. [Google Scholar] [PubMed]
- Franke, S.I.; Pra, D.; da Silva, J.; Erdtmann, B.; Henriques, J.A. Possible repair action of vitamin C on DNA damage induced by methylmethanesulfonate, cyclophosphamide, FeSO4 and CuSO4 in mouse blood cells in vivo. Mutat. Res. 2005, 583, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Tanko, Y.; Eze, E.D.; Daja, H.S.; Jimoh, A.; Mohammed, K.A.; Musa, K.Y. Ameliorative effects of vitamin C and E on serum lipid profile and liver enzymes on fructose-induced hyperglycaemia in Wistar rats. J. Appl. Pharm. Sci. 2013, 3, S80–S84. [Google Scholar]
- National Research Council (NRC). Guide for the Care and Use of Laboratory Animals (Eight Edition 2011). Available online: www.nap.edu/catalog/12910.html (accessed on 20 August 2014).
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in β-cells of the rat pancreas. Physiol. Res. 2001, 50, 536–546. [Google Scholar]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin- induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.G.; Richmand, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [PubMed]
- Burstein, M.; Scholnick, H.R.; Morfin, R. Rapid method for the isolation of lipoprotein from human serum by precipitation with polyanions. J. Lipid Res. 1970, 11, 583–595. [Google Scholar] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without the use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of lipid peroxidation, malondialdehyde in biochemical system. Ann. Biochem. 1996, 16, 359–367. [Google Scholar] [CrossRef]
- Martin, J.P., Jr.; Dailey, M.; Sugarman, E. Negative and positive assays of superoxide dismutase based on haematoxylin auto-oxidation. Arch. Biochem. Biophys. 1987, 255, 329–336. [Google Scholar] [CrossRef]
- Beers, R.F., Jr.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Verma, L.; Singour, P.K.; Chaurasiya, P.K.; Pawar, R.S.; Patil, U.K. Effect of ethanolic extract of Cassia occidentalis Linn. For the management of alloxan-induced diabetes rats. Pharmacog. Res. 2010, 2, 132–137. [Google Scholar]
- Das, J.; Vasan, V.; Sil, P.C. Taurine exerts hypoglycaemic effect in alloxan-induced diabetic rats, improves insulin-mediated glucose transport signaling pathway in heart and ameliorates cardiac oxidative stress and apoptosis. Toxicol. Appl. Pharmacol. 2012, 258, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Munday, R. Dialuric acid autoxidation: Effects of transition metals on the reaction rate and on the generation of reactive oxygen species. Biochem. Pharmacol. 1988, 37, 409–413. [Google Scholar] [CrossRef]
- Duzguner, V.; Kaya, S. Effect of zinc on the lipid peroxidation and the antioxidant defence systems of the alloxan-induced diabetic rabbits. Free Rad. Biol. Med. 2007, 42, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Oxana, L.; Eleonora, P.; Vyacheslav, B.U. Protective effect of ursodeoxycholic acid on liver mitochondrial function in rats with alloxan-induced diabetes: Link with oxidative stress. Life Sci. 2007, 80, 2397–2402. [Google Scholar]
- Miwa, S.; Watada, H.; Omura, C.; Takayanagi, N.; Nishiyama, K.; Tanaka, Y.; Onuma, T.; Kawamori, R. Anti-oxidative effect of fluvastatin in hyperlipidaemic type 2 diabetic patients. Endocr. J. 2005, 52, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Mendoza-Rodriguez, C.A.; Hiriart, M.; Larrieta, M.E.; Vital, P.; Cerbon, M.A. Protective effect of testosterone on early apoptotic damage induced by streptozotocin in rat pancreas. J. Endocrinol. 2005, 187, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sridulyakul, P.; Chakraphan, D.; Patumraj, S. Vitamin C supplementation could reverse diabetes-induced endothelial cell dysfunction in mesenteric microcirculation in STZ-rats. Clin. Haemorheol. Microcirc. 2006, 34, 315–321. [Google Scholar]
- Franke, S.I.R.; Muller, L.L.; Santos, M.C.; Fishborn, A.; Hermes, L.; Molz, P.; Pereira, C.S.; Wichmann, F.M.A.; Horta, J.A.; Maluf, S.W.; et al. Vitamin C intake reduces the cytotoxicity associated with hyperglycaemia in prediabetes and type 2 diabetes. Biomed. Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, N.D.; Harshal, V.C.; Meena, S. Supplementation of vitamin C reduces blood glucose and improves glycosylated haemoglobin in type 2 diabetes mellitus: A randomized, double-blind study. Adv. Pharm. Sci. 2011. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Naziroglu, M.; Butterworth, J.P.; Sonmez, T.T. Dietary vitamin C and E modulate antioxidant levels in blood, brain, liver, muscle, and testes in diabetic aged rats. Int. J. Vit. Nutr. Res. 2011, 81, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Aluwong, T.; Mohammed, K.; Raji, M.; Dzenda, T.; Govwang, F.; Sinkalu, V.; Ayo, J. Effect of yeast probiotic on growth, antioxidant enzyme activities and malondialdehyde concentration of broiler chickens. Antioxidants 2013, 2, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Chavko, M.; Harabin, A.L. Regional lipid peroxidation and protein oxidation in rat brain after hyperbaric oxygen exposure. Free Rad. Biol. Med. 1996, 20, 973–978. [Google Scholar] [CrossRef]
- Eken, A.; Aydin, A.; Sayal, A.; Ustundag, A.; Duydu, Y.; Dundar, K. The effects of hyperbaric oxygen treatment on oxidative stress and SCE frequencies in humans. Clin. Biochem. 2005, 38, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Opara, E.C. Oxidative stress, micronutrients, diabetes mellitus and its complications. J. R. Soc. Promot. Health 2002, 122, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Hannon-Fletcher, M.P.A.; Hughas, C.; Moles, K.-W.; Barnett, C.R.; Barnett, Y.A. An investigation of in vivo antioxidant status and DNA damage in patients with IDDM. In Antioxidants in Human Health; Basu, T.K., Temple, N.J., Eds.; Oxford CABI Publishing: London, UK, 1999; pp. 259–269. [Google Scholar]
- Kawamura, N.; Ookawara, T.; Suzuki, K.; Konishi, K.; Mino, M.; Taniguchi, N. Increased glycated Cu, Zn-superoxide dismutase levels in erythrocytes of patients with insulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1992, 74, 1352–1354. [Google Scholar] [PubMed]
- Maxwell, S.R.; Thomason, H.; Sandler, D.; Leguen, C.; Baxter, M.A.; Thorpe, G.H.; Jones, A.F.; Barnett, A.H. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes. Eur. J. Clin. Invest. 1997, 27, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Vander Jagt, D.J.; Harrison, J.M.; Ratliff, D.M.; Hunsaker, L.A.; Vander Jagt, D.L. Oxidative stress indices in IDDM subjects with and without long-term diabetic complications. Clin. Biochem. 2001, 34, 265–270. [Google Scholar] [CrossRef]
- Olczyk, K.; Koscielniak-Kocurek, E.; Sonecki, P.; Zdenkowski, W. The lipid peroxidation products and the enzymes of antioxidant system in patients with diabetes mellitus. Rocz. Akad. Med. Bialymst. 1994, 39, 93–99. [Google Scholar] [PubMed]
- Wolff, S.P. Diabetes mellitus and free radicals: Free radicals, transition metals and oxidative Stress in the aetiology of diabetes mellitus and complications. Br. Med. Bull. 1993, 49, 642–652. [Google Scholar] [PubMed]
- Das, J.; Sil, P.C. Taurine ameliorates alloxan-induced diabetic renal injury, oxidative stress-related signaling pathways and apoptosis in rats. Amino Acids 2012, 43, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Patti, L.; Innelli, F.; Di Marino, L.; Annuzzi, G.; Iavicoli, M.; Coronel, G.A.; Riccardi, G.; Rivellese, A.A. Insulin and sulfonylurea therapy in NIDDM patients: Are the effects on lipoprotein metabolism different even with similar blood glucose control? Diabetes 1997, 46, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Vindigni, A.; Chang, K.; Stramm, L.; Chance, R.; Heath, W.F.; DiMarchi, R.D.; di Cera, E.; Williamson, J.R. Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science 1997, 277, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Harisa, G.I.; Taha, E.L.; Khalil, A.F.; Salem, M.M. Oral administration of Lactobacillus acidophilus restores nitric oxide level in diabetic rats. Aust. J. Basic Appl. Sci. 2009, 3, 2963–2969. [Google Scholar]
- Aydemir, T.; Ozturk, R.; Bozkaya, L.A.; Tarhan, L. Effects of antioxidant vitamins A, C, E and the trace elements Cu, Se on CuZn SOD, GSH-Px, CAT and LPO levels in chicken erythrocytes. Cell. Biochem. Funct. 2000, 18, 109–115. [Google Scholar] [CrossRef]
- D’Souza, A.; Fordjour, L.; Ahmad, A.; Cai, C.; Kumar, D.; Valencia, G.; Aranda, J.V.; Beharry, K.D. Effects of probiotics, prebiotics and synbiotics on messenger RNA expression of caveolin-1, NOS, and genes regulating oxidative stress in the terminal ileum of formula fed neonatal rats. Pediatric. Res. 2010, 67, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Islam, M.R.; Waise, T.M.Z.; Hassan, F.; Mondal, S.I.; Kabir, Y. DNA damage and plasma antioxidant indices in Bangladeshi type 2 diabetic patients. Diabetes Metab. 2010, 36, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Elahi-Moghaddam, Z.; Behnam-Rassouli, M.; Mahdavi-Shahri, N.; Hajinejad-Boshroue, R.; Khajouce, E. Comparative study on the effects of type 1 and type 2 diabetes on structural changes and hormonal output of the adrenal cortex in male Wistar rats. J. Diabetes Metab. Disord. 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Kishor, M.W.; Samson, P.N.; Wesley, W.; Brien, B.R. Streptozotocin- and Alloxan-induced diabetes modifies total plasma lipoprotein lipid concentration and composition without altering cholesteryl ester transfer activity. Pharmacol. Toxicol. 1998, 83, 169–175. [Google Scholar]
- Bell, R.H.; Hye, R.J. Animal models of diabetes mellitus: Physiology and pathology. J. Surg. Res. 1983, 35, 433–460. [Google Scholar] [CrossRef]
- Rifai, N.; Warnick, G.R. Lipids, lipoproteins, apolipoproteins, and other cardiovascular risk factors. In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics; Burtis, C.A., Ashwood, E.R., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2006; pp. 903–981. [Google Scholar]
- Salwa, W.R. Health implications of fructose consumption: A review of recent data. Nutr. Metab. 2010, 7, 82. [Google Scholar]
- Saleh, A.A.S. Effects of taurine and/or ginseng and their mixture on lipid profile and some parameters indicative of myocardial status in streptozotocin-diabetic rats. J. Basic Appl. Zool. 2012, 65, 267–273. [Google Scholar] [CrossRef]
- Process, S.; Delgrange, E.; vander Borght, T.V.; Jamart, J.; Donckier, J.E. Minor alterations in thyroid function tests associated with diabetes mellitus and obesity in outpatients without known thyroid illness. Acta Clin. Belg. 2001, 56, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T.; Shah, N.P. Acid and bile tolerance and the cholesterol removal ability of bifidobacteria strains. Biosci. Micro. 2005, 24, 1–10. [Google Scholar] [CrossRef]
- Eriksson, J.; Kohvakka, A. Magnesium and ascorbic acid supplementation in diabetes mellitus. Ann. Nutr. Metab. 1995, 39, 217–223. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Handler, P.; Smith, E.L.; Hill, R.L.; Lehman, I.R. Principles of Biochemistry, 7th ed.; McGrawHill Kogakusha Ltd: Tokyo, Japan, 1994; pp. 619–630. [Google Scholar]
- Stanely, M.P.P.; Menton, V.P.; Gunasekaran, G. Hypolipidaemic action of Tinospora cordifolia roots in alloxan diabetic rats. J. Ethnopharmacol. 1999, 64, 53–57. [Google Scholar]
- An, H.M.; Park, S.Y.; Lee, D.K.; Kim, J.R.; Cha, M.K.; Lee, S.W.; Lim, H.T.; Kim, K.J.; Ha, N.J. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Aleisa, A.M.; Abouhashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S.; Alkhamees, O.A.; Alroujayee, A.S. Ameliorative effects of rutin and ascorbic acid combination on hypercholesterolemia-induced hepatotoxicity in female rats. Afr. J. Pharm. Pharmacol. 2013, 7, 280–288. [Google Scholar] [CrossRef]
- Meigs, J.B.; Hu, F.B.; Rifai, N.; Manson, J.E. Biomarkers of endothelial dysfunction and rsik of type 2 diabetes mellitus. J. Am. Med. Assoc. 2004, 291, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Roselino, N.M.; Pauly-Silveira, D.N.; Cavallini, C.U.D.; Celiberto, S.L.; Pinto, A.R.; Vendramini, C.R.; Rossi, A.E. A potential symbiotic product improves the lipid profile of diabetic rats. Lipids Health Dis. 2012, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Tzeng, E.S. Appearance of different diabetic symptoms after streptozotocin administration: A Comparison Study. Biochem. Mol. Biol. Int. 1993, 30, 1035–1041. [Google Scholar] [PubMed]

| Experimental Groups | ||||||
|---|---|---|---|---|---|---|
| Week | Control | UD | DTI | DTP | DTVit-C | DTP + vit-C |
| 1 | 4.42 ± 0.71 | 21.74 ± 2.98 a | 19.64 ± 4.15 a | 18.20 ± 4.15 a | 18.16 ± 3.83 a | 17.64 ± 4.33 a |
| 2 | 4.48 ± 0.66 | 20.92 ± 2.72 a | 19.00 ± 3.62 a | 17.60 ± 3.50 a | 17.74 ± 3.24 a | 16.60 ± 3.56 a |
| 3 | 4.42 ± 0.71 | 20.30 ± 2.41 a | 21.56 ± 2.43 a | 17.60 ± 2.45 a | 17.30 ± 2.71 a | 15.84 ± 3.04 b |
| 4 | 4.40 ± 0.64 | 19.60 ± 2.71 | 18.10 ± 2.95 a | 16.70 ± 2.09 a | 16.34 ± 2.23 a | 14.80 ± 2.67 b |
| Experimental Groups | SOD (U/mL) | CAT (U/mL) | GPx (µU/mL) |
|---|---|---|---|
| Control | 2.34 ± 0.12 | 49.60 ± 0.75 a | 45.60 ± 0.87 a,b |
| UD | 2.20 ± 0.06 | 47.60 ± 0.68 a | 43.80 ± 0.58 |
| DTI | 2.06 ± 0.07 a | 44.20 ± 1.02 b | 42.00 ± 0.32 b |
| DTP | 2.30 ± 0.09 | 44.20 ± 0.80 a,b | 39.80 ± 0.86 c |
| DTVitC | 2.44 ± 0.05 b | 49.40 ± 0.51 a | 44.60 ± 0.51 a |
| DTP + Vit-C | 2.38 ± 0.07 | 48.60 ± 1.57 a | 45.60 ± 1.63 b,c |
| Experimental Groups | SOD (U/mg Protein) | CAT (U/mg Protein) | GPx (U/mg Protein) |
|---|---|---|---|
| Control | 2.48 ± 0.10 a | 53.40 ± 1.21 a | 48.20 ± 0.97 a |
| UD | 2.02 ± 0.08 b,d | 52.80 ± 1.77 a,b | 49.00 ± 1.64 a |
| DTI | 1.98 ± 0.08 b,d | 54.20 ± 1.39 a | 47.20 ± 1.39 a |
| DTP | 2.00 ± 0.07 b,d | 44.40 ± 1.44 b,c | 42.60 ± 0.93 b,c |
| DTVitC | 1.46 ± 0.08 b,e | 42.40 ± 1.12 b | 38.80 ± 0.73 b,c,d |
| DTP + VitC | 2.30 ± 0.11 a,c | 43.20 ± 0.73 b | 39.80 ± 0.80 b |
| Parameters | Control | UD | DTI | DTP | DTVitC | DTP + VitC |
|---|---|---|---|---|---|---|
| TC, mmol/L | 3.68 ± 0.07 a | 6.26 ± 0.05 a | 3.80 ± 0.33 b | 3.74 ± 0.24 a | 3.28 ± 0.09 a | 3.28 ± 0.12 a |
| TG, mmol/L | 1.58 ± 0.08 b | 2.70 ± 0.07 b | 1.56 ± 0.19 a,b | 1.60 ± 0.07 b | 1.80 ± 0.38 b,c | 1.54 ± 0.15 b |
| HDL, mol/L | 1.48 ± 0.09 b | 0.48 ± 0.04 b | 1.60 ± 0.15 b | 1.64 ± 0.15 b | 1.32 ± 0.10 b,d | 1.42 ± 0.05 b |
| LDL, mmol/L | 2.90 ± 0.10 a | 5.52 ± 0.08 b | 2.06 ± 0.57 b | 1.74 ± 0.63 b | 1.06 ± 0.12 b,d | 1.38 ± 0.29 b |
| Group | Control | UD | DTI | DTP | DTVitC | DTP+vitC | |
|---|---|---|---|---|---|---|---|
| Body weight, g: | Week 1 | 127.6 ± 2.86 | 117.4 ± 8.02 | 125.6 ± 5.52 | 120.6 ± 4.55 | 123.2 ± 7.02 | 123.4 ± 6.31 |
| Week 2 | 140.8 ± 4.81 a | 106.6 ± 5.11 b | 137.8 ± 2.94 b | 125.4 ± 4.24 b,c | 128.4 ± 7.23 a,c | 129.4 ± 5.37 a,c | |
| Week 3 | 150.4 ± 4.07 a | 105.0 ± 4.89 b,c | 134.8 ± 3.37 b | 142.8 ± 4.09 a,e | 138.4 ± 7.23 a,e | 139.6 ± 5.41 a,e | |
| Week 4 | 158.8 ± 5.45 a | 103.8 ± 4.75 b | 147.4 ± 3.57 a,b | 139.4 ± 4.51 c | 142.2 ± 7.15 c | 142.6 ± 5.30 c | |
| Feed intake, g: | Week 1 | 118.3 ± 9.25 a | 87.3 ± 7.49 b,c | 63.6 ± 12.44 b,d | 80.6 ± 10.44 b,c | 53.4 ± 10.54 b,c | 74.4 ± 7.23 b |
| Week 2 | 125.4 ± 8.97 a | 98.9 ± 8.82 b,c | 89.9 ± 6.08 b | 105.7 ± 4.59 b | 79.7 ± 5.04 b,d | 86.9 ± 5.47 b | |
| Week 3 | 123.6 ± 5.67 a | 96.4 ± 6.09 b,c | 86.3 ± 3.79 b | 115.0 ± 5.49 a,d | 82.6 ± 5.58 b | 93.3 ± 4.78 b | |
| Week 4 | 110.0 ± 3.51 a | 104.3 ± 5.14 a | 84.0 ± 6.96 b | 112.4 ± 4.21 a | 83.7 ± 6.17 b | 104.0 ± 3.70 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aluwong, T.; Ayo, J.O.; Kpukple, A.; Oladipo, O.O. Amelioration of Hyperglycaemia, Oxidative Stress and Dyslipidaemia in Alloxan-Induced Diabetic Wistar Rats Treated with Probiotic and Vitamin C. Nutrients 2016, 8, 151. https://doi.org/10.3390/nu8050151
Aluwong T, Ayo JO, Kpukple A, Oladipo OO. Amelioration of Hyperglycaemia, Oxidative Stress and Dyslipidaemia in Alloxan-Induced Diabetic Wistar Rats Treated with Probiotic and Vitamin C. Nutrients. 2016; 8(5):151. https://doi.org/10.3390/nu8050151
Chicago/Turabian StyleAluwong, Tagang, Joseph O. Ayo, Alkali Kpukple, and Olusola Olalekan Oladipo. 2016. "Amelioration of Hyperglycaemia, Oxidative Stress and Dyslipidaemia in Alloxan-Induced Diabetic Wistar Rats Treated with Probiotic and Vitamin C" Nutrients 8, no. 5: 151. https://doi.org/10.3390/nu8050151






