Effects of Postprandial Blood Pressure on Gait Parameters in Older People
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Experimental Design
2.3. Assessment of Subject Health Status
2.4. Blood Pressure and Heart Rate Measurements
2.5. Gait Parameters Acquisition and Analysis
2.6. Cardiovascular Autonomic Function
2.7. Orthostatic Blood Pressure
2.8. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Baseline Gait Parameters
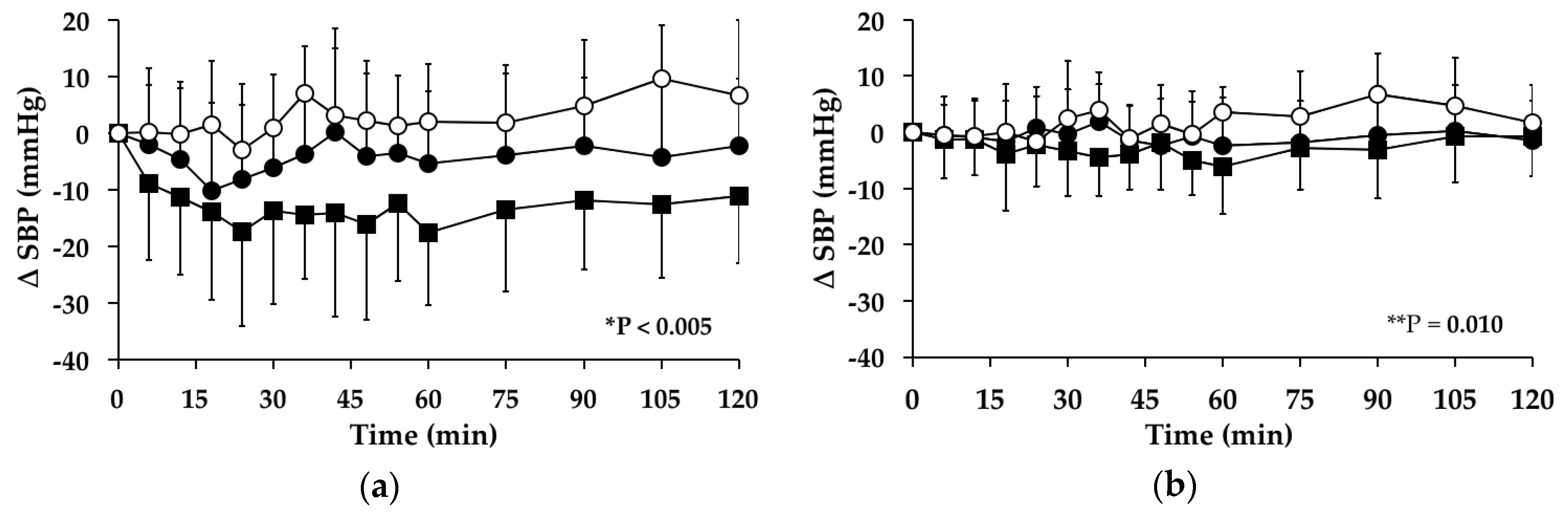
3.3. Systolic Blood Pressure
3.3.1. Subjects with PPH
3.3.2. Subjects without PPH
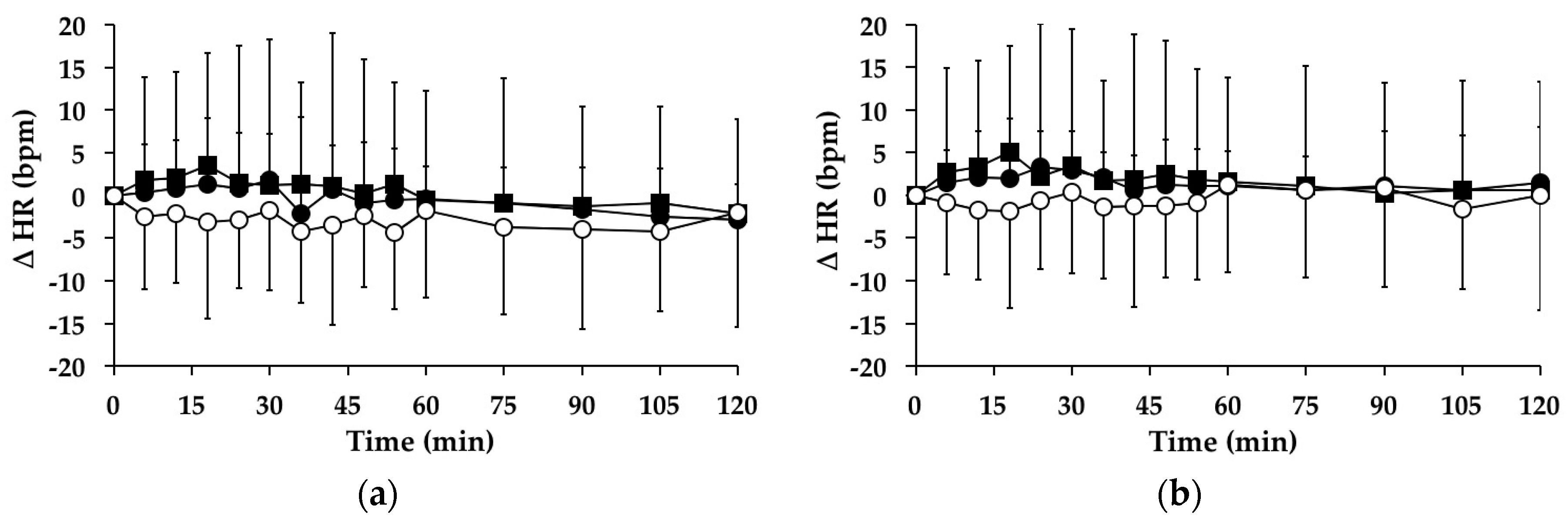
3.4. Heart Rate
3.4.1. Subjects with PPH
3.4.2. Subjects without PPH
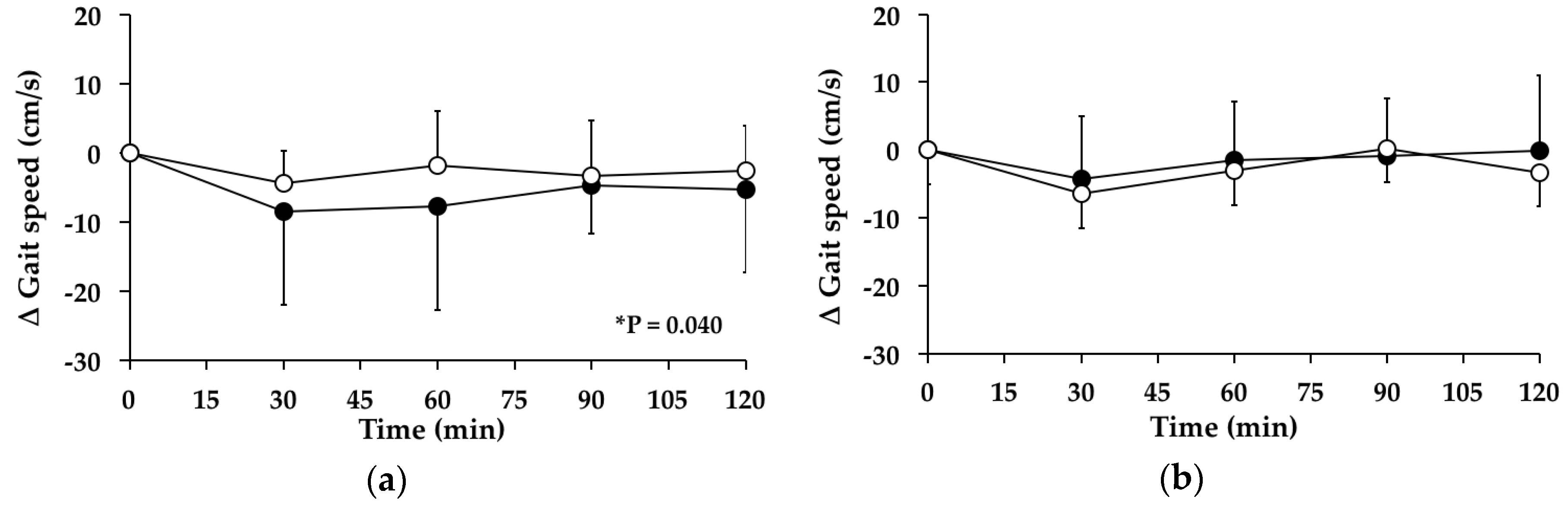
3.5. Gait Speed
3.5.1. Subjects with PPH
3.5.2. Subjects without PPH
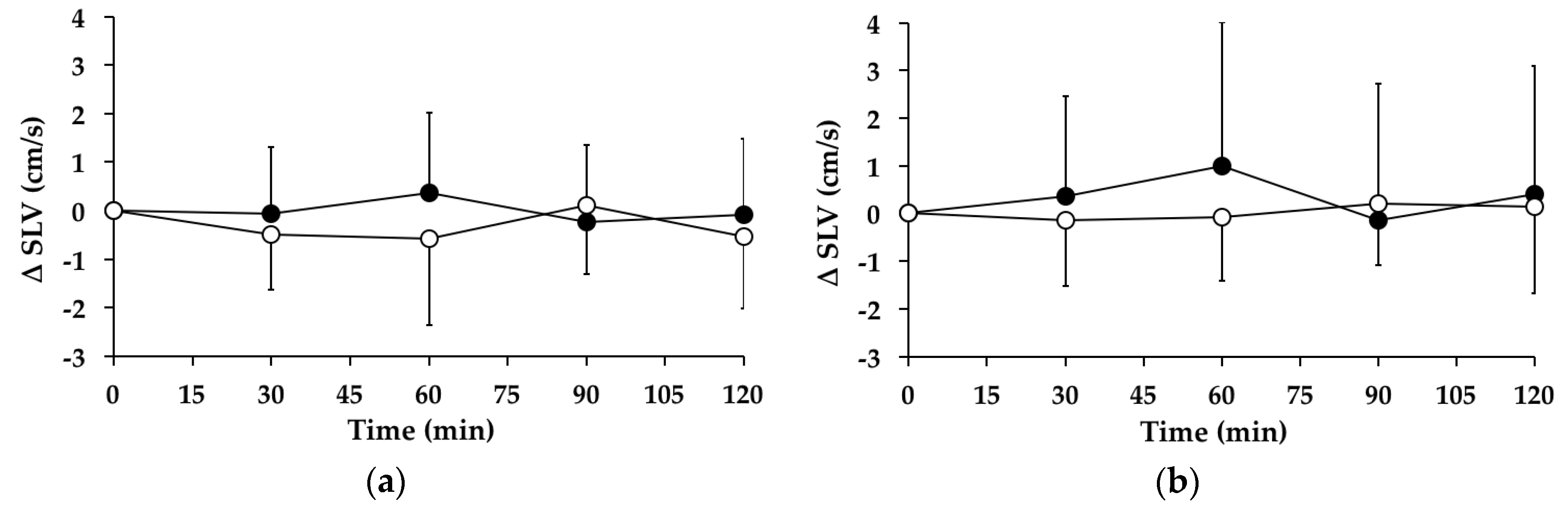
3.6. Stride Length Variability
3.6.1. Subjects with PPH
3.6.2. Subjects without PPH
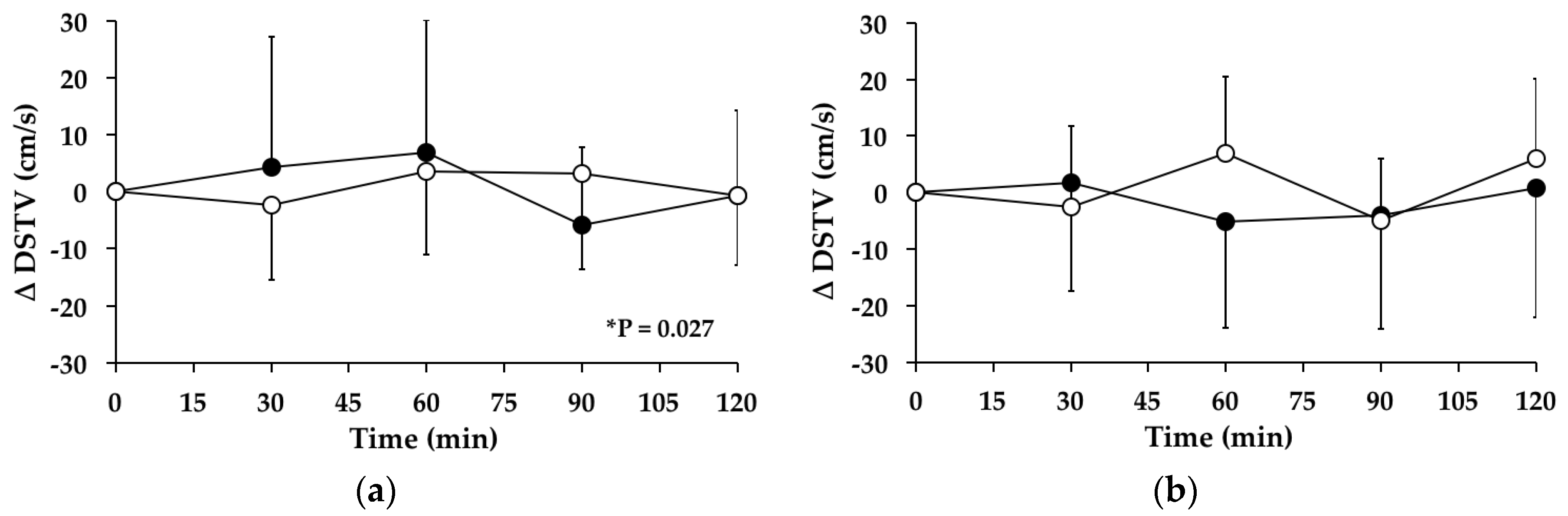
3.7. Double-Support Time Variability
3.7.1. Subjects with PPH
3.7.2. Subjects without PPH
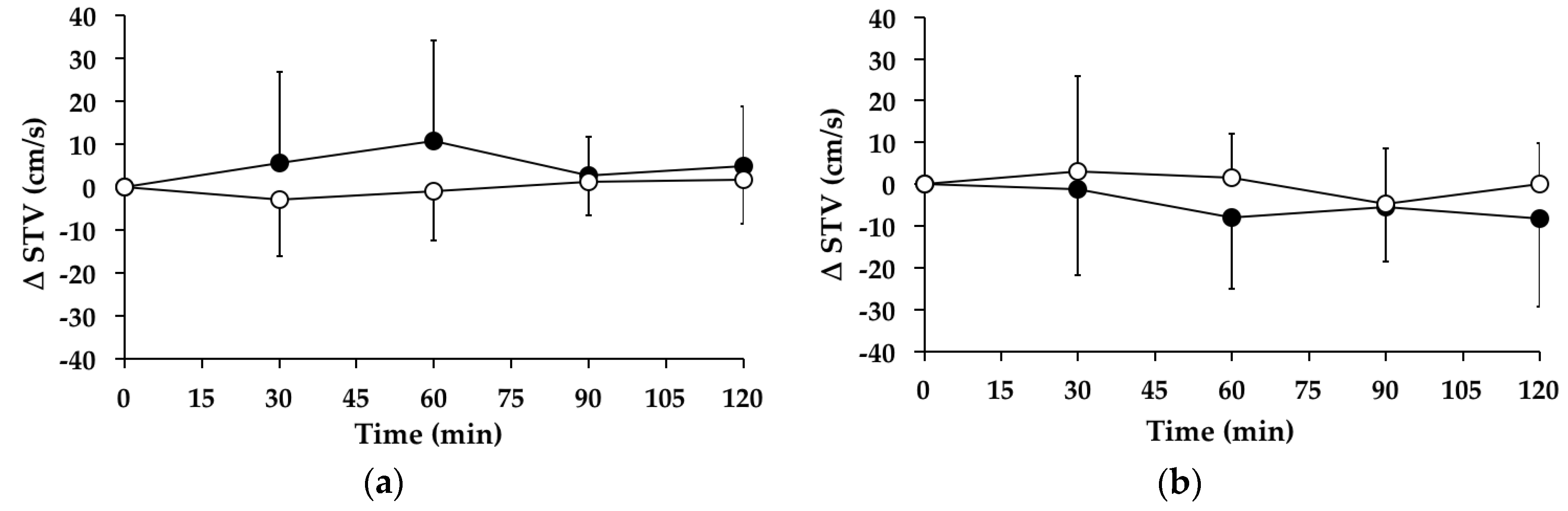
3.8. Swing Time Variability
3.8.1. Subjects with PPH
3.8.2. Subjects without PPH
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PPH | postprandial hypotension |
| SBP | systolic blood pressure |
| HR | heart rate |
| G | glucose |
| WW | water and walk |
| GW | glucose and walk |
| AUC | area under the curve |
| G-TRAC | Adelaide Geriatrics Training and Research with Aged Care |
| CCI | Charlson Comorbidity Index |
| max | maximum |
| SD | standard deviation |
| ACEI | Angiotensin Converting Enzyme Inhibitor |
| ARB | Angiotensin Receptor Blocker |
| CCB | Calcium Channel Blocker. |
| CI | confidence interval |
| △ | change |
| SLV | stride length variability |
| DSTV | double-support time variability |
| STV | swing time variability |
References
- Tinetti, M.E.; Speechley, M. Prevention of falls among the elderly. N. Engl. J. Med. 1989, 320, 1055–1059. [Google Scholar] [PubMed]
- Kannus, P.; Leiponen, P.; Parkkari, J.; Palvanen, M.; Järvinen, M. A sideways fall and hip fracture. Bone 2006, 39, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age Ageing 2006, 35, ii37–ii41. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Parkkari, J.; Koskinen, S.; Niemi, S.; Palvanen, M.; Järvinen, M.; Vuori, I. Fall-induced injuries and deaths among older adults. JAMA 1999, 281, 1895–1899. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Josephson, K.R. The epidemiology of falls and syncope. Clin. Geriatr. Med. 2002, 18, 141–158. [Google Scholar] [CrossRef]
- Moller, J. Projected Costs of Fall Related Injury to Older Persons Due to Demographic Change in Australia; Report No: ISBN 0 642 82313 8; Commonwealth of Australia: Canberra, Australia, July 2003.
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Nyberg, L.; Gustafson, Y.; Lundin-Olsson, L. Fall and injury prevention in residential care—Effects in residents with higher and lower levels of cognition. J. Am. Geriatr. Soc. 2003, 51, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.W.; Lipsitz, L.A. Postprandial hypotension: Epidemiology, pathophysiology, and clinical management. Ann. Intern. Med. 1995, 122, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Mathias, C.J.; da Costa, D.F.; Fosbraey, P.; Bannister, R.; Wood, S.M.; Bloom, S.R.; Christensen, N.J. Cardiovascular, biochemical and hormonal changes during food-induced hypotension in chronic autonomic failure. J. Neurol. Sci. 1989, 94, 255–269. [Google Scholar] [CrossRef]
- Puisieux, F.; Bulckaen, H.; Fauchais, A.L.; Drumez, S.; Salomez-Granier, F.; Dewailly, P. Ambulatory blood pressure monitoring and postprandial hypotension in elderly persons with falls or syncopes. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M535–M540. [Google Scholar] [CrossRef] [PubMed]
- Lagro, J.; Laurenssen, N.C.; Schalk, B.W.; Schoon, Y.; Claassen, J.A.; Olde Rikkert, M.G. Diastolic blood pressure drop after standing as a clinical sign for increased mortality in older falls clinic patients. J. Hypertens. 2012, 30, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Schapira, M.; Soriano, E.R.; Varela, M.; Kaplan, R.; Camera, L.A.; Mayorga, L.M. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J. Gerontol. A Biol. Med. Sci. 2005, 60, 1304–1309. [Google Scholar] [CrossRef]
- Deandrea, S.; Lucenteforte, E.; Bravi, F.; Foschi, R.; La Vecchia, C.; Negri, E. Risk factors for falls in community-dwelling older people. Epidemiology 2010, 21, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Dargent-Molina, P.; Favier, F.; Grandjean, H.; Baudoin, C.; Schott, A.M.; Hausherr, E.; Meunier, P.J.; Bréart, G. Fall-related factors and risk of hip fracture: The EPIDOS prospective study. Lancet 1996, 348, 145–149. [Google Scholar] [CrossRef]
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. Quantitative gait markers and incident fall risk in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Callisaya, M.L.; Blizzard, L.; Schmidt, M.D.; Martin, K.L.; McGinley, J.L.; Sanders, L.M.; Srikanth, V.K. Gait, gait variability and the risk of multiple incident falls in older people: A population-based study. Age Ageing 2011, 40, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Aronow, W.S.; Ahn, C. Postprandial hypotension in 499 elderly persons in a long-term health care facility. J. Am. Geriatr. Soc. 1994, 42, 930–932. [Google Scholar] [CrossRef] [PubMed]
- RANDOM.ORG. Available online: http://www.random.org/ (accessed on 7 August 2014).
- Visvanathan, R.; Chen, R.; Garcia, M.; Horowitz, M.; Chapman, I. The effects of drinks made from simple sugars on blood pressure in healthy older people. Br. J. Nutr. 2005, 93, 575–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, K.; Close, J.C.; Mikolaizak, A.S.; Sachdev, P.S.; Brodaty, H.; Lord, S.R. The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing 2010, 39, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Latt, M.D.; Tiedemann, A.; Mun San Kwan, M.; Lord, S.R. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture 2004, 20, 20–25. [Google Scholar] [CrossRef]
- Bilney, B.; Morris, M.; Webster, K. Concurrent related validity of the GAITRite walkaway system for quantification of the spatial and temporal parameters of gait. Gait Posture 2003, 17, 68–74. [Google Scholar] [CrossRef]
- Wittwer, J.E.; Webster, K.E.; Andrews, P.T.; Menz, H.B. Test-retest reliability of spatial and temporal gait parameters of people with Alzheimer’s disease. Gait Posture 2008, 28, 392–396. [Google Scholar] [CrossRef] [PubMed]
- McDonough, A.L.; Batavia, M.; Chen, F.C.; Kwon, S.; Ziai, J. The validity and reliability of the GAITRite system’s measurements: A preliminary evaluation. Arch. Phys. Med. Rehabil. 2001, 82, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E. Gait changes in older adults: Predictors of falls or indicators of fear. J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M. Gait variability: Methods, modeling and meaning. J. Neuroeng. Rehabil. 2005, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollman, J.H.; McDade, E.M.; Petersen, R.C. Normative spatiotemporal gait parameters in older adults. Gait Posture 2011, 34, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kressig, R.W.; Beauchet, O. European GAITRite Network Group. Guidelines for clinical applications of spatio-temporal gait analysis in older adults. Aging. Clin. Exp. Res. 2006, 18, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, P.A.; Looney, M.A. Walkway length determination for steady state walking in young and older adults. Res. Q. Exerc. Sport. 2008, 79, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ewing, D.J.; Clarke, B.F. Diagnosis and management of diabetic autonomic neuropathy. Br. Med. J. 1982, 285, 916–918. [Google Scholar] [CrossRef]
- Piha, S.J. Cardiovascular autonomic reflex tests: Normal responses and age-related reference values. Clin. Physiol. 1991, 11, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, L.A. Orthostatic hypotension in the elderly. N. Engl. J. Med. 1989, 321, 952–957. [Google Scholar] [PubMed]
- Brach, J.S.; Perera, S.; Studenski, S.; Katz, M.; Hall, C.; Verghese, J. Meaningful change in measures of gait variability in older adults. Gait Posture 2010, 31, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Visvanathan, R.; Gentilcore, D. Intermittent walking: A potential treatment for older people with postprandial hypotension. J. Am. Med. Dir. Assoc. 2015, 16, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Rochester, L. Walking in the real world: Concepts related to functional gait. N. Z. J. Physiother. 2007, 35, 126–130. [Google Scholar]
- Nutt, J.G.; Marsden, C.D.; Thompson, P.D. Human walking and higher-level gait disorders, particularly in the elderly. Neurology 1993, 43, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Sudarsky, L. Gait disorders: Prevalence, morbidity, and etiology. Adv. Neurol. 2001, 87, 111–117. [Google Scholar] [PubMed]
- Barrett, A.; O’Connor, M.; Culhane, K.; Finucane, A.M.; Olaighin, G.; Lyons, D. A footswitch evaluation of the gait of elderly fallers with and without a diagnosis of orthostatic hypotension and healthy elderly controls. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2008, 5101–5104. [Google Scholar]
- Frewen, J.; Finucane, C.; Savva, G.M.; Boyle, G.; Kenny, R.A. Orthostatic hypotension is associated with lower cognitive performance in adults aged 50 plus with supine hypertension. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. Synopsis of function and dysfunction of the frontal lobe. Acta Psychiatr. Scand. Suppl. 1999, 395, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Fisher, A.A.; Davis, M.W.; McLean, A.J. Postprandial systolic blood pressure responses of older people in residential care: Association with risk of falling. Gerontology 2003, 49, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, L.A.; Fullerton, K.J. Postprandial blood pressure reduction in healthy elderly. J. Am. Geriatr. Soc. 1986, 34, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Van Orshoven, N.P.; Jansen, P.A.F.; Oudejans, I.; Schoon, Y.; Oey, P.L. Postprandial hypotension in clinical geriatric patients and healthy elderly: Prevalence related to patient selection and diagnostic criteria. J. Aging Res. 2010, 1, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Goldberger, A.L.; Ruthazer, R.; Mietus, J.; Lipsitz, L.A. Spectral analysis of heart rate dynamics in elderly persons with postprandial hypotension. Am. J. Cardiol. 1992, 69, 201–205. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Balady, G.J.; Amsterdam, E.A.; Chaitman, B.; Eckel, R.; Fleg, J.; Froelicher, V.F.; Leon, A.S.; Piña, I.L.; Rodney, R.; et al. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 104, 1694–1740. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Y.; Hoffman, B.B.; Pershe, R.A.; Blaschke, T.F. Decline in beta adrenergic receptor-mediated vascular relaxation with aging in man. J. Pharmacol. Exp. Ther. 1986, 239, 802–807. [Google Scholar] [PubMed]
- Schniepp, R.; Wuehr, M.; Neuhaeusser, M.; Kamenova, M.; Dimitriadis, K.; Klopstock, T.; Strupp, M.; Brandt, T.; Jahn, K. Locomotion speed determines gait variability in cerebellar ataxia and vestibular failure. Mov. Disord. 2012, 27, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, C.J.; van Deudekom, F.J.; van Campen, J.P.; Appels, B.A.; de Vries, O.J.; Pijnappels, M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J. Neuroeng. Rehabil. 2011, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Sinacore, D.R.; Ehsani, A.A.; Binder, E.F.; Holloszy, J.O.; Kohrt, W.M. Low-intensity exercise as a modifier of physical frailty in older adults. Arch. Phys. Med. Rehabil. 2000, 81, 960–965. [Google Scholar] [CrossRef] [PubMed]
- King, M.B.; Whipple, R.H.; Gruman, C.A.; Judge, J.O.; Schmidt, J.A.; Wolfson, L.I. The performance enhancement project: Improving physical performance in older persons. Arch. Phys. Med. Rehabil. 2002, 83, 1060–1069. [Google Scholar]
- Sayers, S.P.; Guralnik, J.M.; Thombs, L.A.; Fielding, R.A. Effect of Leg Muscle Contraction Velocity on Functional Performance in Older Men and Women. J. Am. Geriatr. Soc. 2005, 53, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Muir, S.W.; Hall, M.; Doherty, T.J.; Kloseck, M.; Beauchet, O.; Speechley, M. Gait variability is associated with frailty in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.M.; McGloin, J.M.; Gahbauer, E.A.; Shepard, D.M.; Bianco, L.M. Two recruitment strategies for a clinical trial of physically frail community-living older persons. J. Am. Geriatr. Soc. 2001, 49, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Rothman, M.D.; Leo-Summers, L.; Gill, T.M. Prognostic significance of potential frailty criteria. J. Am. Geriatr. Soc. 2008, 56, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Gentilcore, D.; Visvanathan, R. The effects of a postprandial blood pressure decline following a glucose drink on gait parameters in healthy older volunteers. Australas. J. Ageing 2012, 31, 2. [Google Scholar]
| PPH | No PPH | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Baseline SBP (mmHg) | Absolute Value of Declined SBP (mmHg) | Max Decline in SBP (mmHg) | Time of Max Decline in SBP (min) | Subject | Baseline SBP (mmHg) | Absolute Value of Declined SBP (mmHg) | Max Decline in SBP (mmHg) | Time of Max Decline in SBP (min) |
| 1 | 145 | 120 | 25 | 105 | 1 | 127 | 113 | 14 | 60 |
| 2 | 169 | 141 | 28 | 30 | 2 | 140 | 129 | 11 | 36 |
| 3 | 145 | 102 | 43 | 105 | 3 | 119 | 107 | 12 | 90 |
| 4 | 136 | 113 | 23 | 105 | 4 | 126 | 123 | 3 | 18 |
| 5 | 153 | 133 | 20 | 18 | 5 | 130 | 119 | 11 | 42 |
| 6 | 134 | 107 | 27 | 24 | 6 | 125 | 113 | 12 | 18 |
| 7 | 166 | 143 | 23 | 75 | 7 | 150 | 134 | 16 | 36 |
| 8 | 158 | 138 | 20 | 105 | 8 | 132 | 118 | 14 | 60 |
| 9 | 144 | 114 | 30 | 18 | 9 | 124 | 113 | 11 | 30 |
| 10 | 173 | 126 | 47 | 60 | 10 | 151 | 135 | 16 | 18 |
| 11 | 145 | 120 | 25 | 120 | 11 | 154 | 151 | 3 | 18 |
| 12 | 132 | 109 | 23 | 105 | 12 | 157 | 143 | 14 | 54 |
| 13 | 133 | 113 | 20 | 42 | 13 | 103 | 95 | 8 | 30 |
| 14 | 136 | 116 | 20 | 18 | |||||
| 15 | 167 | 134 | 33 | 48 | |||||
| 16 | 154 | 134 | 20 | 36 | |||||
| Mean ± SD | 26.69 ± 8.43 | 54.0 ± 36.0 | 11.0 ± 3.0 | 42.0 ± 10.0 |
| Characteristic | Subjects with PPH (n = 16) | Subjects no PPH (n = 13) | p Value | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 76.5 ± 4.1 | 75.8 ± 6.0 | 0.742 | |
| Sex (n/%) | 0.441 | |||
| female | 5 (31) | 6 (46) | ||
| male | 11 (69) | 7 (54) | ||
| History of falls in preceding 12 months (n/%) | 3 (19) | 0 | 0.099 | |
| Falls Efficacy Scale International score (mean ± SD) | 16.8 ± 4.8 | 19.5 ± 6.3 | 0.230 | |
| Orthostatic hypotension (n/%) | 4 (25) | 2 (15) | 0.627 | |
| Autonomic dysfunction (n/%) | 3 (19) | 0 | 0.099 | |
| Charlson Comorbidity Index (mean ± SD) | 2.0 ± 0.9 | 1.2 ± 0.4 | 0.006 | |
| Hypertension (n/%) | 10 (63) | 3 (23) | 0.034 | |
| Heart disease (n/%) | 3 (19) | 1 (8) | 0.390 | |
| Diabetes mellitus (n/%) | 1 (6) | 0 | 0.842 | |
| Hypothyroidism (n/%) | 2 (13) | 1 (8) | 0.672 | |
| Antihypertensives (n/%) | 12 (75) | 4 (31) | 0.017 | |
| ACEI | 2 (13) | 0 | ||
| ARB | 7 (44) | 1 (8) | ||
| Beta Blockers | 1 (6) | 1 (8) | ||
| CCB | 4 (25) | 3 (23) | ||
| Diuretics | 1 (6) | 1 (8) | ||
| Characteristic | Subjects with PPH (n = 16) | Subjects No PPH (n = 13) | p Value |
|---|---|---|---|
| Gait speed “WW” day | 112.9 ± 21.9 | 116.8 ± 27.0 | 0.674 |
| Gait speed “GW” day | 113.8 ± 22.0 | 114.4 ± 24.0 | 0.945 |
| Stride length variability “WW” day | 3.5 ± 1.4 | 2.6 ± 1.3 | 0.085 |
| Stride length variability “GW” day | 3.4 ± 1.4 | 2.6 ± 1.6 | 0.163 |
| Double-support time variability “WW” day | 14.1 ± 10.9 | 17.9 ± 17.9 | 0.484 |
| Double-support time variability “GW” day | 13.9 ± 12.1 | 17.0 ± 24.0 | 0.649 |
| Swing time variability “WW” day | 9.4 ± 11.7 | 11.4 ± 16.1 | 0.702 |
| Swing time variability “GW” day | 6.0 ± 6.3 | 15.0 ± 21.1 | 0.157 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, S.; Visvanathan, R.; Piscitelli, D. Effects of Postprandial Blood Pressure on Gait Parameters in Older People. Nutrients 2016, 8, 219. https://doi.org/10.3390/nu8040219
Nair S, Visvanathan R, Piscitelli D. Effects of Postprandial Blood Pressure on Gait Parameters in Older People. Nutrients. 2016; 8(4):219. https://doi.org/10.3390/nu8040219
Chicago/Turabian StyleNair, Shailaja, Renuka Visvanathan, and Diana Piscitelli. 2016. "Effects of Postprandial Blood Pressure on Gait Parameters in Older People" Nutrients 8, no. 4: 219. https://doi.org/10.3390/nu8040219





