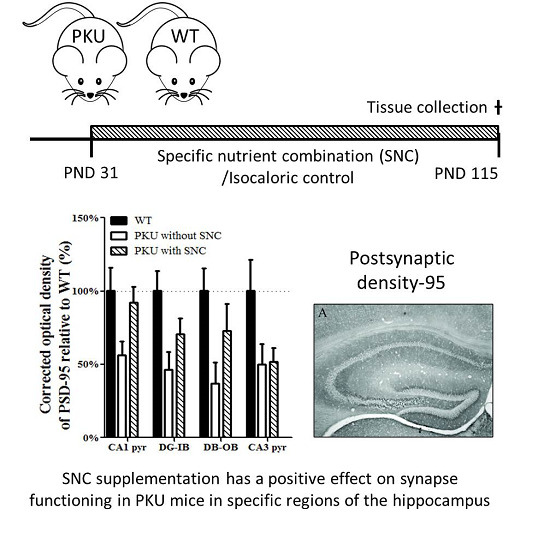
A Specific Nutrient Combination Attenuates the Reduced Expression of PSD-95 in the Proximal Dendrites of Hippocampal Cell Body Layers in a Mouse Model of Phenylketonuria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Dietary Intervention
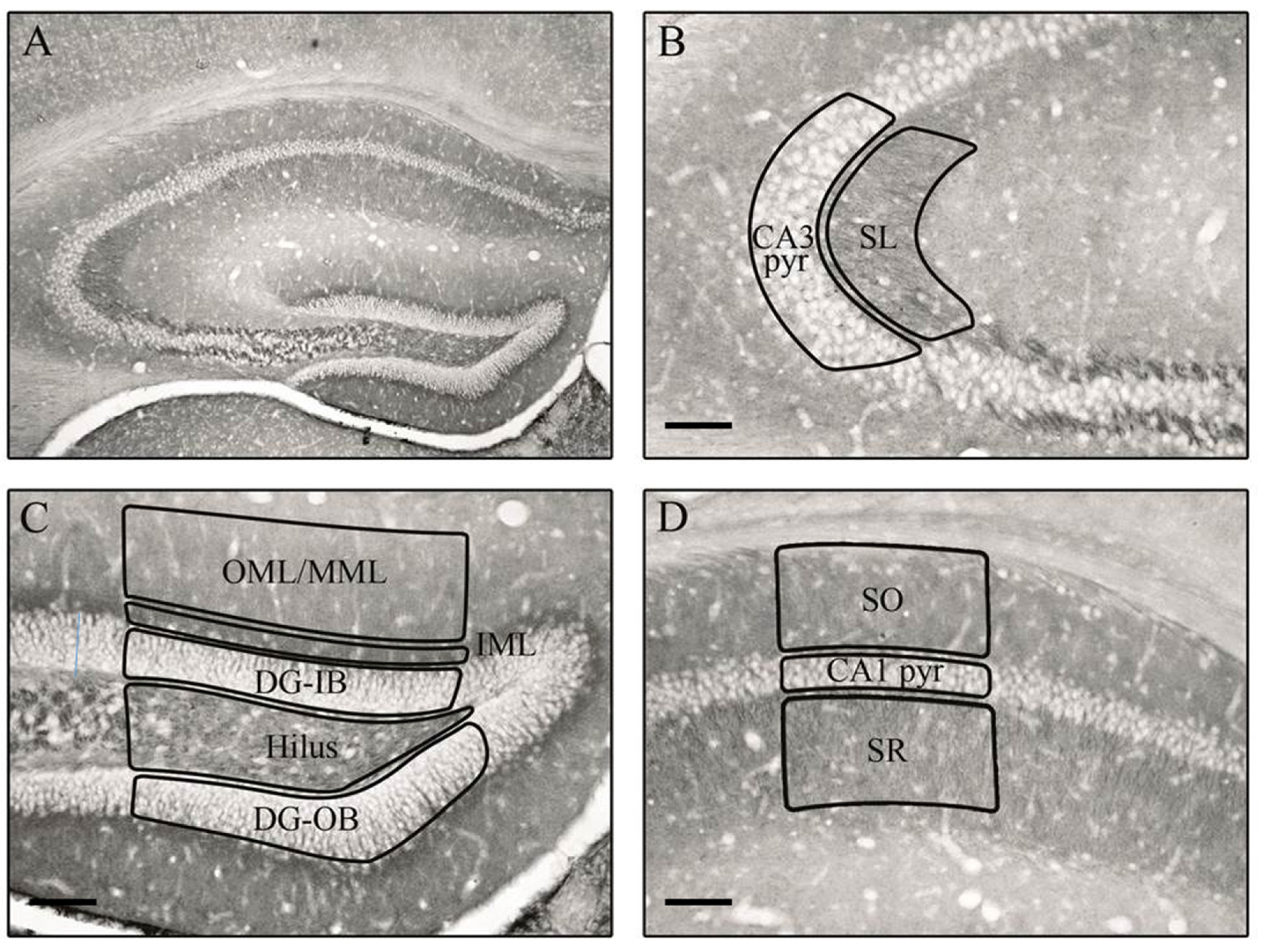
2.2. Tissue Preparation
2.3. Immunohistochemistry
2.4. Statistical Analysis
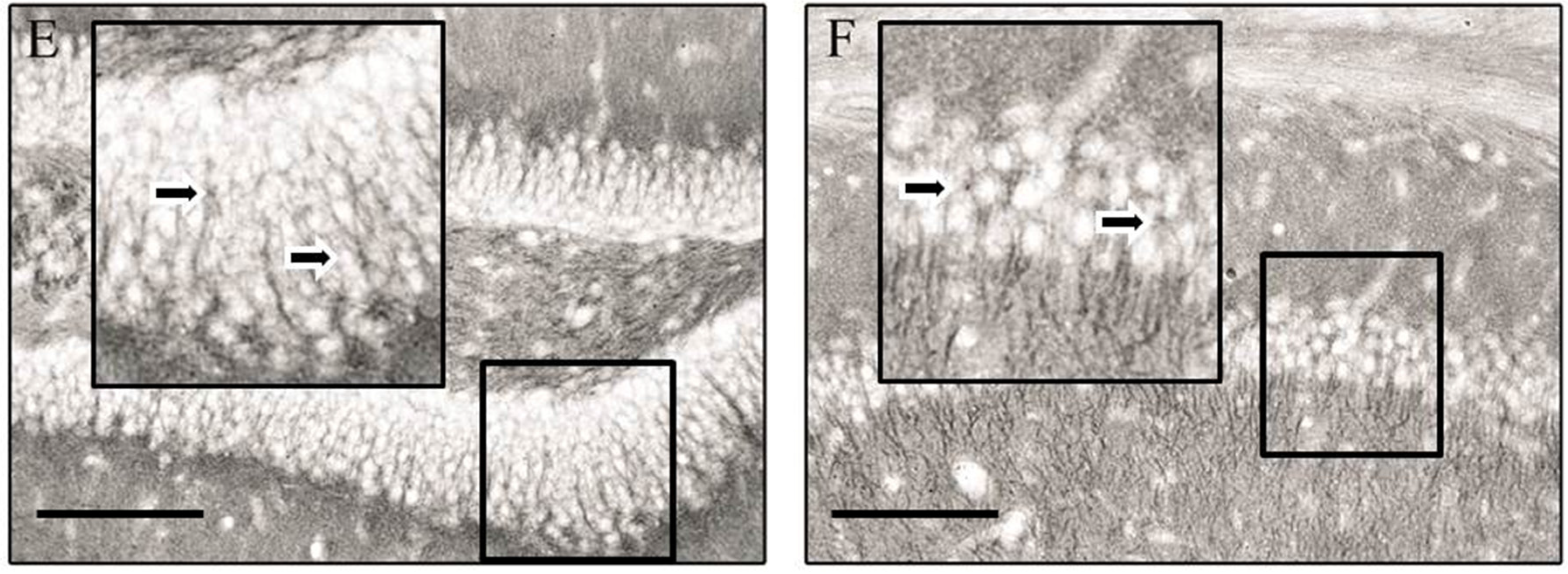
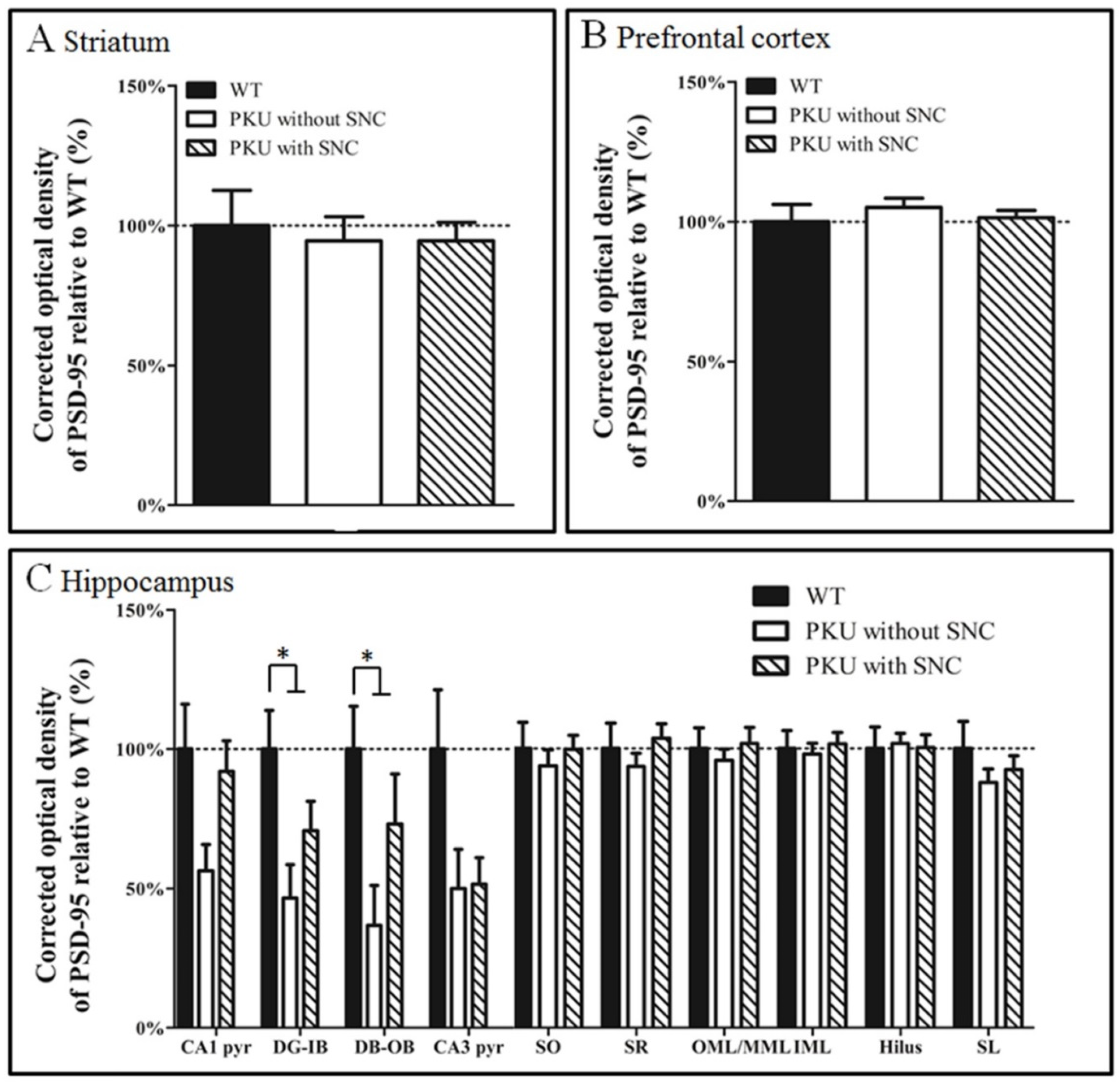
3. Results
4. Discussion
Acknowledgments
Author contribution
Conflicts of Interest
References
- Blau, N.; van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Noel, K.; Fahrbach, K.; Cella, C.; Frame, D.; Dorenbaum, A.; Levy, H. Phenylalanine blood levels and clinical outcomes in phenylketonuria: A systematic literature review and meta-analysis. Mol. Genet. Metab. 2007, 92, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jahja, R.; Huijbregts, S.C.J.; de Sonneville, L.M.J.; van der Meere, J.J.; van Spronsen, F.J. Neurocognitive evidence for revision of treatment targets and guidelines for phenylketonuria. J. Pediatr. 2014, 164, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Dyer, C.A. Relationship between myelin production and dopamine synthesis in the PKU mouse brain. J. Neurochem. 2003, 86, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Gu, X.; Lu, L.; Li, D.; Zhang, X. Phenylketonuria-related synaptic changes in a BTBR-Pah(enu2) mouse model. Neuroreport 2011, 22, 617–622. [Google Scholar] [PubMed]
- Andolina, D.; Conversi, D.; Cabib, S.; Trabalza, A.; Ventura, R.; Puglisi-Allegra, S.; Pascucci, T. 5-Hydroxytryptophan during critical postnatal period improves cognitive performances and promotes dendritic spine maturation in genetic mouse model of phenylketonuria. Int. J. Neuropsychopharmacol. 2011, 14, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.E.; Trejo, M.; Colombo, M.; Aranda, V. Histological maturation of the neocortex in phenylketonuric rats. Early Hum. Dev. 1983, 8, 157–173. [Google Scholar] [CrossRef]
- Loo, Y.H.; Fulton, T.; Miller, K.; Wisniewski, H.M. Phenylacetate and brain dysfunction in experimental phenylketonuria: Synaptic development. Life Sci. 1980, 27, 1283–1290. [Google Scholar] [PubMed]
- Hörster, F.; Schwab, M.A.; Sauer, S.W.; Pietz, J.; Hoffmann, G.F.; Okun, J.G.; Kölker, S.; Kins, S. Phenylalanine reduces synaptic density in mixed cortical cultures from mice. Pediatr. Res. 2006, 59, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Vaks, L.; Carny, O.; Trudler, D.; Magno, A.; Caflisch, A.; Frenkel, D.; Gazit, E. Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria. Nat. Chem. Biol. 2012, 8, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Hood, A.; Antenor-Dorsey, J.A.V.; Hershey, T.; Rutlin, J.; Shimony, J.S.; McKinstry, R.C.; Grange, D.K.; Christ, S.E.; White, D.A. White matter integrity and executive abilities in individuals with phenylketonuria. Mol. Genet. Metab. 2013, 109, 125–131. [Google Scholar]
- Bauman, M.L.; Kemper, T.L. Morphologic and histoanatomic observations of the brain in untreated human phenylketonuria. Acta Neuropathol. 1982, 58, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Imperlini, E.; Orrù, S.; Corbo, C.; Daniele, A.; Salvatore, F. Altered brain protein expression profiles are associated with molecular neurological dysfunction in the PKU mouse model. J. Neurochem. 2014, 129, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Horling, K.; Schlegel, G.; Schulz, S.; Vierk, R.; Ullrich, K.; Santer, R.; Rune, G.M. Hippocampal synaptic connectivity in phenylketonuria. Hum. Mol. Genet. 2014, 24, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- El-Husseini, A.E.; Craven, S.E.; Chetkovich, D.M.; Firestein, B.L.; Schnell, E.; Aoki, C.; Bredt, D.S. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J. Cell Biol. 2000, 148, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Nagura, H.; Ishikawa, Y.; Kobayashi, K.; Takao, K.; Tanaka, T.; Nishikawa, K.; Tamura, H.; Shiosaka, S.; Suzuki, H.; Miyakawa, T.; et al. Impaired synaptic clustering of postsynaptic density proteins and altered signal transmission in hippocampal neurons, and disrupted learning behavior in PDZ1 and PDZ2 ligand binding-deficient PSD-95 knockin mice. Mol. Brain 2012, 5, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Zee, E.A. Synapses, spines and kinases in mammalian learning and memory, and the impact of aging. Neurosci. Biobehav. Rev. 2015, 50, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Embury, J.E.; Charron, C.E.; Martynyuk, A.; Zori, A.G.; Liu, B.; Ali, S.F.; Rowland, N.E.; Laipis, P.J. PKU is a reversible neurodegenerative process within the nigrostriatum that begins as early as 4 weeks of age in Pah(enu2) mice. Brain Res. 2007, 1127, 136–150. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, N.; Broersen, L.M.; de Wilde, M.C.; Hageman, R.J.J.; Groenendijk, M.; Sijben, J.W.C.; Kamphuis, P.J.G.H. Targeting synaptic dysfunction in Alzheimer’s disease by administering a specific nutrient combination. J. Alzheimers. Dis. 2014, 38, 459–479. [Google Scholar] [PubMed]
- Beblo, S.; Reinhardt, H.; Demmelmair, H.; Muntau, A.C.; Koletzko, B. Effect of fish oil supplementation on fatty acid status, coordination, and fine motor skills in children with phenylketonuria. J. Pediatr. 2007, 150, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Beblo, S.; Demmelmair, H.; Hanebutt, F.L. Omega-3 LC-PUFA supply and neurological outcomes in children with phenylketonuria (PKU). J. Pediatr. Gastroenterol. Nutr. 2009, 48 (Suppl. 1), S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.H.L.; Kable, J.A.; Evatt, M.L.; Singh, R.H. A randomized, placebo-controlled, double-blind trial of supplemental docosahexaenoic acid on cognitive processing speed and executive function in females of reproductive age with phenylketonuria: A pilot study. Prostaglandins. Leukot. Essent. Fatty Acids 2011, 85, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, P.N.; Bruinenberg, V.; Anjema, K.; van Vliet, D.; Dutra-Filho, C.S.; van Spronsen, F.J.; van der Zee, E.A. Voluntary Exercise Prevents Oxidative Stress in the Brain of Phenylketonuria Mice. JIMD Rep. 2015. [Google Scholar] [CrossRef]
- APA National Research Council. Nutrient Requirements of Laboratory AnimalsNo Title; Fourth rev.; The National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Hovens, I.B.; Schoemaker, R.G.; van der Zee, E.A.; Absalom, A.R.; Heineman, E.; van Leeuwen, B.L. Postoperative cognitive dysfunction: Involvement of neuroinflammation and neuronal functioning. Brain Behav. Immun. 2014, 38, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, E.A.; Keijser, J.N. Localization of pre- and postsynaptic cholinergic markers in rodent forebrain: A brief history and comparison of rat and mouse. Behav. Brain Res. 2011, 221, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Shors, T.J.; Falduto, J.; Leuner, B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur. J. Neurosci. 2004, 19, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.A.; Yadav, P.N.; Roth, B.L. Insights into the regulation of 5-HT2A serotonin receptors by scaffolding proteins and kinases. Neuropharmacology 2008, 55, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Matt, L.; Patriarchi, T.; Malik, Z.A.; Chowdhury, D.; Park, D.K.; Renieri, A.; Ames, J.B.; Hell, J.W. Capping of the N-terminus of PSD-95 by calmodulin triggers its postsynaptic release. EMBO J. 2014, 33, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Yudowski, G.A.; Olsen, O.; Adesnik, H.; Marek, K.W.; Bredt, D.S. Acute inactivation of PSD-95 destabilizes AMPA receptors at hippocampal synapses. PLoS ONE 2013, 8, e53965. [Google Scholar] [CrossRef] [PubMed]
- MacGillavry, H.D.; Song, Y.; Raghavachari, S.; Blanpied, T.A. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron 2013, 78, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Y.; Zhang, J.; Chen, C. Long-lasting potentiation of hippocampal synaptic transmission by direct cortical input is mediated via endocannabinoids. J. Physiol. 2012, 590, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A.; Buhl, E.H.; Hailer, N.P.; Nitsch, R. Morphological features of the entorhinal-hippocampal connection. Prog. Neurobiol. 1998, 55, 537–562. [Google Scholar] [CrossRef]
- Martynyuk, A.E.; Glushakov, A.V.; Sumners, C.; Laipis, P.J.; Dennis, D.M.; Seubert, C.N. Impaired glutamatergic synaptic transmission in the PKU brain. Mol. Genet. Metab. 2005, 86 (Suppl. 1), S34–S42. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruinenberg, V.M.; Van Vliet, D.; Attali, A.; De Wilde, M.C.; Kuhn, M.; Van Spronsen, F.J.; Van der Zee, E.A. A Specific Nutrient Combination Attenuates the Reduced Expression of PSD-95 in the Proximal Dendrites of Hippocampal Cell Body Layers in a Mouse Model of Phenylketonuria. Nutrients 2016, 8, 185. https://doi.org/10.3390/nu8040185
Bruinenberg VM, Van Vliet D, Attali A, De Wilde MC, Kuhn M, Van Spronsen FJ, Van der Zee EA. A Specific Nutrient Combination Attenuates the Reduced Expression of PSD-95 in the Proximal Dendrites of Hippocampal Cell Body Layers in a Mouse Model of Phenylketonuria. Nutrients. 2016; 8(4):185. https://doi.org/10.3390/nu8040185
Chicago/Turabian StyleBruinenberg, Vibeke M., Danique Van Vliet, Amos Attali, Martijn C. De Wilde, Mirjam Kuhn, Francjan J. Van Spronsen, and Eddy A. Van der Zee. 2016. "A Specific Nutrient Combination Attenuates the Reduced Expression of PSD-95 in the Proximal Dendrites of Hippocampal Cell Body Layers in a Mouse Model of Phenylketonuria" Nutrients 8, no. 4: 185. https://doi.org/10.3390/nu8040185