1. Introduction
Ethanol is the main ingredient used in Chinese spirits and alcoholic beverages and because of its fat-soluble nature, it easily causes stomach injury, so alcohol abuse can cause acute erosive hemorrhagic gastritis and long-term drinking could cause stomach disorders and chronic atrophic gastritis [
1]. At high concentration of ethanol might cause stomach injury through direct erosion, and through metabolism ethanol converts to acetaldehyde, whose toxic effect may lead to stomach cancer [
2]. Different studies explain alcoholic gastric damage mechanism; showing that oxygen free radicals and lipid peroxidation chain reaction is one of the important mechanisms in stomach injury. During ethanol metabolic processes in the body, neutrophils release oxygen free radicals, causing endothelial damage, microcirculation disturbance and inhibits gastric injury healing. Through respiratory burst, neutrophils continue to produce large amounts of oxygen free radicals, forming a vicious circle [
3]. Hydrochloric acid and ethanol could cause imbalance between some endogenous aggressive factors and cytoprotective factors. HCl also could cause damage of gastric mucosa, and ethanol could reach the mucosa and induce cell rupture in the wall of blood vessels [
4].
Malondialdehyde produced by oxygen free radical lipid peroxidation can react with DNA bases, inducing mutation damage [
5]. Ethanol also leads to a decrease in antioxidant active constitutes, such as glutathione, in the body, raising ROS (reactive oxygen species) level and causing antioxidant enzyme inactivation and DNA chain rupture. Cell membrane lipid peroxidation damage causes release of lysosomal enzyme and aggravates gastric lesions. Control of ethanol oxidation and the level of oxygen free radicals in the body are important ways of reducing alcoholic stomach injury [
6].
Yak, is a bovine animal, mainly seen in the Tibetan plateau of China, where yaks’ milk, meat and fur are all available. The amount of milk produced per day is lower with an average of 1.4–2.25 kg, but compared with common milk it contains higher nutrients such as protein, essential amino acids, minerals and so on [
7]. Besides drinking, herdsmen on the Tibetan plateau use it to make yogurt. The difference between yak’s milk and ordinary milk, also due to its special geographic conditions, endows natural-fermented yak yogurt with special qualities. Its special quality was mainly due to the involvement of lactic acid bacteria during natural fermentation, and this team had isolated the lactic acid bacteria from yak yogurt. Early studies showed that lactic acid bacteria isolated from yak yogurt can better resist gastric acid and bile salt than
Lactobacillus bulgaricus, which was commonly used in ordinary food industry [
8]. Studies had shown that lactic acid bacteria isolated from yak yogurt had a stronger ability to survive in the stomach and intestines which could effectively play a vital role in human body. Meanwhile, the study on antioxidant effect of lactic acid bacteria isolated from yak yogurt showed that they all have antioxidant effects
in vitro, and some high-quality lactic acid bacteria showed high antioxidant ability showing high potential for use [
9]. Fujimura
et al. [
10] found that
lactobacillus OLL2716 could stimulate
H. pylori, the
lactobacillus could make
H. pylori retain dormancy, the growth of
H. pylori was inhibited, and that this effect could protect the stomach. Another study also proved that
L. rhamnosus GG could protect the stomach by the animal experiment, the lactobacillus could reduce the gastric mucosa cells apoptosis by increasing the PGE2 level, and this course inhibited the ethanol induced gastric mucosa damage [
11].
Lactobacillus had many functional effects on peptic ulcer prevention and cure through gastric mucosal epithelial cell binding site competition, H. pylori colonization inhibiting, and inflammation related cytokine decreasing [
12]. It has been shown that
Lactobacillus fermentum Suo, a high-quality lactic acid bacteria strain used in this study, can inhibit gastric lesions through its antioxidant ability. The serum levels of MOL, SP, SS, VIP, ET, IL-6, IL-12, TNF-α, IFN-γ and tissue levels of SOD, GSH-Px, NO, MDA of mice were determined by molecular biology methods, such as serum kits and RT-PCR assay. The aim of the study was to determined the inhibitory effects of LF-Suo on gastric injury
in vivo by observing mice stomach injury morphology, tissue damage and oxidation-related serum index, as well as changes of inflammatory cytokines in serum and the expression of oxidation and inflammation-related mRNA in stomach tissue. The further mechanism of anti-gastric injury effects checked through the anti-oxidation effects of LF-Suo were measured. These results and mechanism explanation will help to apply LF-Suo.
4. Discussion
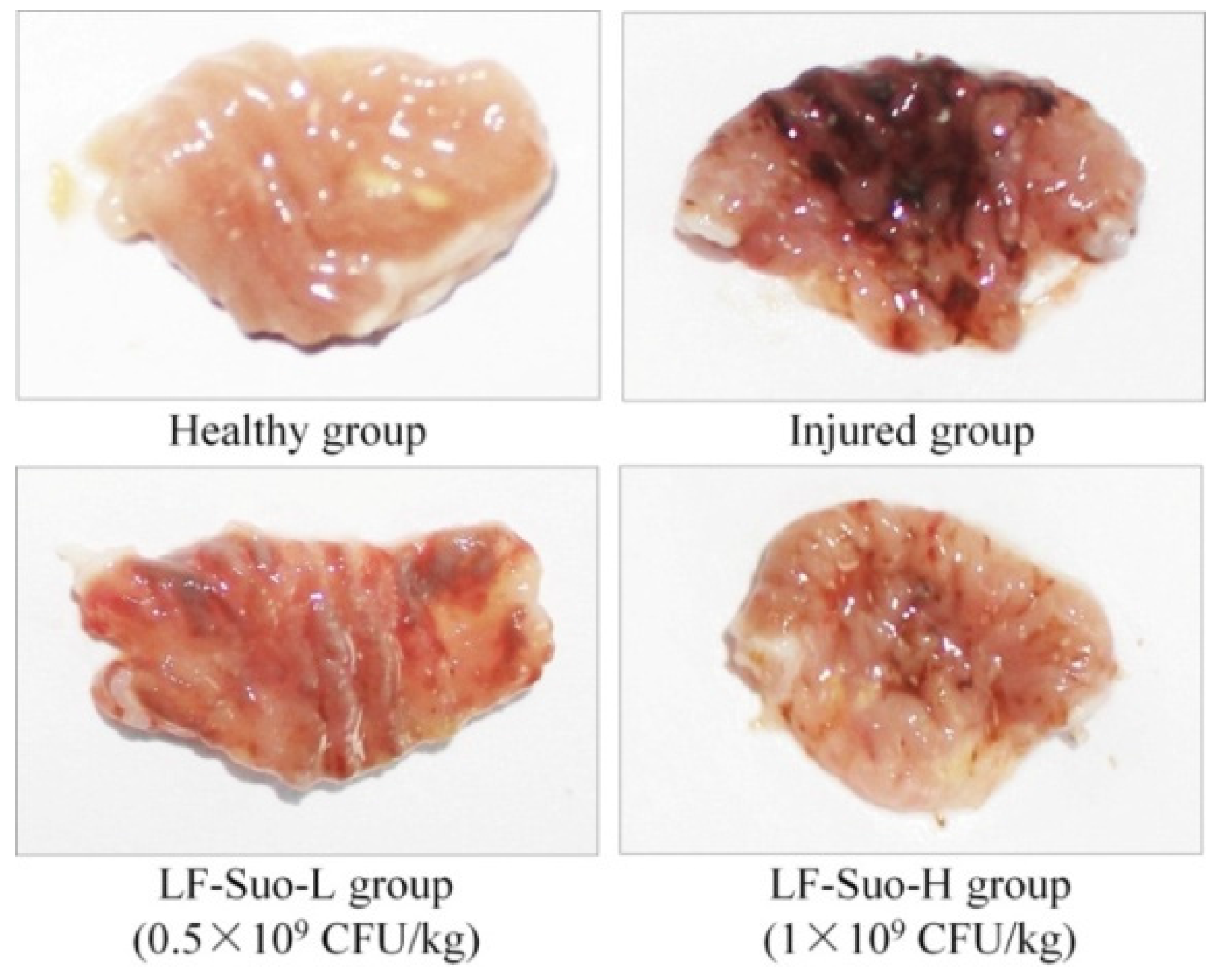
Animal experiments show that through direct observation after stomach injury, gastric mucosa tissues showed festering, and detecting a festered area could determine the degree of the stomach damage [
13]. Ethanol promotes the expression of H
+-K
+-ATP in gastric mucosa and increases the secretion of gastric acid and pepsin, which is one of the important factors of gastric mucosa injury. When ethanol causes stomach hemorrhagic lesions, mucosal blood flow decreases, Na
+ pumps out and K
+ pumps in, causing gastric acid rebound. So present treatment of ethanol gastric mucosa damage is to reduce gastric acid secretion [
16]. The international standard requires that bacteria concentration of lactic acid bacteria drink must be higher than 10
7 CFU/mL, the lactic acid bacteria concentration of 10
9 CFU/mL was also used for experiment in rats [
11]. In our past research, the 10
9 CFU/kg dose LF-Suo had a good preventive effect on constipation [
8], this concentration amount to about 10
7 CFU/mL for human. Based on these studies, we also chose 0.5 × 10
9 CFU/kg and 1.0 × 10
9 CFU/kg for this study. The healthy group mice without induced tissue injury were used as the healthy condition comparison in experiments. The healthy group mice had low MOT, SP, ET, IL-6, IL-12, TNF-α, IFN-γ serum levels and high SS, VIP levels, these trends were also similar to the healthy population [
3,
13].
MOT and SP are excitatory gastrointestinal hormones. Stimulated by stomach injury, the levels of MOT and SP became higher and secretion of gastric acid increases greatly, which makes the internal stomach acidic and aggravates gastric lesions [
14]. SS and VIP are inhibitory gastrointestinal hormones, which could inhibit the secretion of stomach acid [
17]. Animal experiments showed that lowering the levels of MOT and SP and raising the level of SS and VIP could significantly inhibit gastric lesions [
18]. ET could reduce gastric mucosal blood flow, weakening the protection of gastric mucosa, while ET could also affect contraction of gastric smooth muscle and increase stomach movement, which had side effects in the process of gastric lesions; gastric injury could raise the ET level [
19].
Controlling the content of pro-inflammatory factors in the body was also an effective way of inhibiting gastric lesions. Animal experiments showed that ethanol causes the increase of cytokines such as TNF-α, IFN-γ, IL-6, IL-12 in gastric mucosa [
14]. A study found that probiotics could limit the production of TNF-α and IFN-γ and increase the concentration of sIgA [
20]. By increasing sIgA, gastric mucosa epithelium reduces apoptosis, maintains epithelial barrier function, and reduces inflammatory reaction caused by ethanol. Ethanol causes the release of oxygen free radicals, promoting inflammation [
21]. And COX-2 could also play a role in defense by inhibiting pro-inflammatory function [
22].
After gastric damage, some tissues showed oxidation phenomenon. As important antioxidant enzymes, SOD and GSH-Px could convert peroxide caused by stomach tissue oxidation to low-poisonous or harmless substance, which was beneficial to the recovery of gastric damage [
22]. The imbalance between stomach tissue damage and protection factors relatively lowered its defense capability, causing stomach injury. Gastric mucus could prevent gastric mucosa from digestive enzymes and external stimuli, which was a crucial defense factor of gastric mucosa. Excessive drinking (drinking equal to 60 mL absolute alcohol once) could significantly reduce mucus thickness and content of amino hexose in gel layer and reduce mucosal defense capability [
23]. NO could protect gastric mucosa and increase gastric mucosal blood flow. The content of NO declined significantly in patients with gastric ulcer, so NO was proven to be an active component of gastric ulcer prevention [
24]. MDA was the marker of oxidative stress and generated in great quantities after gastric tissue injury, which could be used as an index of gastric ulcer [
25].
The GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene shows high expression in almost all body tissues; GAPDH gene expression is generally invariable in homologous cells or tissue when it is used as housekeeping gene, therefore GAPDH gene is used as an internal control for PCR assay [
26]. β-actin protein is also generally invariable in homologous cells or tissues of mammals; β-actin antibody is used for western blot assay by its internal control effect [
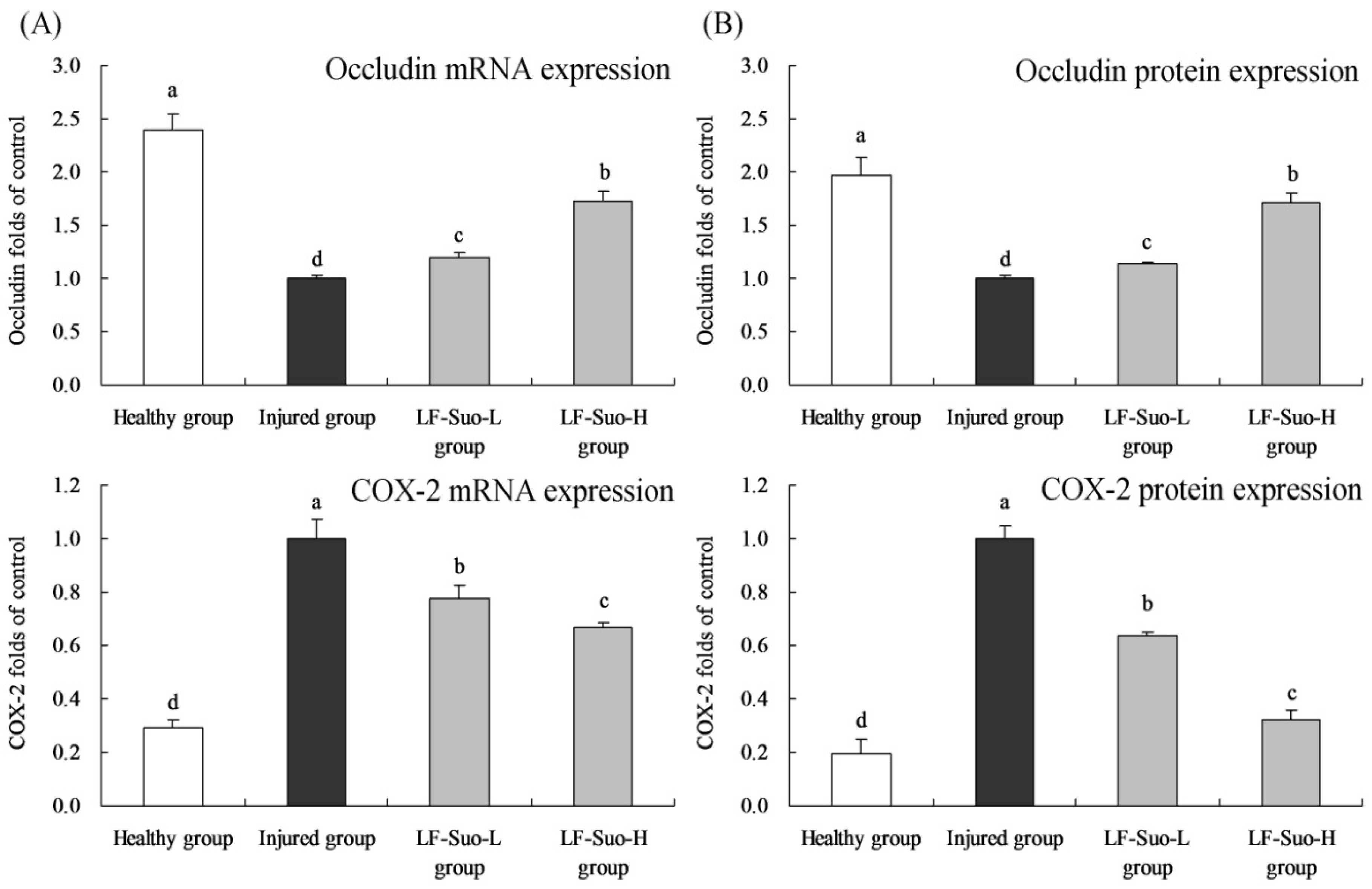
27]. Occludin protein was one of the most important parts in close connection and played a key role to maintain the structure of close connection and membrane barrier. In mucosa epithelium, when occludin expression changed, reduced and missed, mucosal barrier showed dysfunction and mucous membrane permeability increased, causing gastric mucosa injury [
28]. Microcirculation disturbance was one of the reasons for the damaged gastric mucosa barrier. As vasodilation factors, NO and PGE2 could inhibit platelet aggregation and thrombosis and promote gastric mucosa microcirculation, synthesis of protein and cell renewal to regulate immune function and improve the ability to repair mucous membrane, COX-2 was a important factor to synthesize PGE2 [
29]. ET was the strongest vasoconstrictor in the body. Ethanol could inhibit the synthesis and secretion of NO and PGE2 to reduce mucosal blood flow. And through the release of histamine, small arteries expanded and capillary permeability increased, causing mucosal microcirculation. Then lipid peroxide in tissue and oxygen free radicals increased, which promoted the release of ET and causes serious damage of gastric mucosa [
30]. A study has shown that PGE2 could also inhibit the level of TNF-α and IFN-γ
in vivo [
31], these effects might be increased by an increase in COX-2 levels.
Mast cells infiltration plays an important role in ethanol-induced ulcer. Mast cells infiltration causes release of histamine in large quantities, and histamine is closely related to lipid peroxidation. Antioxidants could inhibit lipid peroxidation and improve the activity of antioxidant enzymes, which had been regarded as an adjuvant treatment for alcoholic gastric mucosa damage [
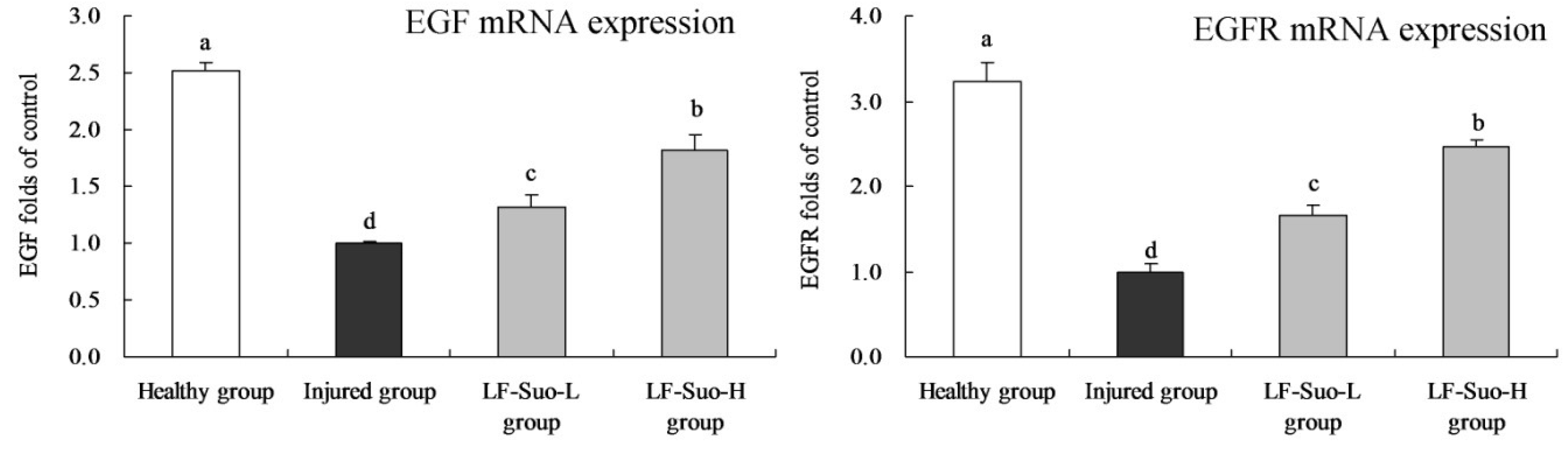
32]. EGF as an antioxidant could stabilize mast cells, while probiotics could inhibit ethanol-induced lipid peroxidation, reduced gastric damage and promoted healing of gastric injury [
33]. EGF is the ligand of EGFR, which could combine and activate EGFR as autocrine or paracrine growth factors to induce EGFR phosphorylation, providing continuous division signals to cells and causing cell proliferation and differentiation [
34]. Studies have shown that EGF and EGFR both are expressed in normal gastric mucosa parietal cells, and that the EGFR autocrine system plays an advantageous role in gastric mucosa epithelium metabolism and maintaining structural integrity [
35]. EGF and EGFR could inhibit the secretion of gastric acid, and their autocrine system plays an important role in cell proliferation and differentiation during ulcer healing [
36].
VEGF acted on vascular endothelial cells and blood vessels and participates in inflammation and damage repair, which was also an important indicator to inspect tissue growth and wound healing. Stomach damage and its healing mechanism were closely related to VEGF [
37]. Experiments demonstrated that single dose VEGF lavage could prevent ethanol-caused acute gastric mucosa damage, which could stimulate new blood vessel formation three weeks later and promote the healing of peptic ulcers [
38]. The VEGF family has three receptors, including VEGFR-l, VEGFR-2 and VEGFR-3, respectively encoded by the Fit-1, Flk-1-KDR and Fit-4 genes [
39]. By increasing the expression of VEGF and Fit-1, tissues regenerated and diluted harmful substances to protect gastric mucosa [
40].
COX-2 is an important inflammatory factor, which could inhibit inflammation in gastric damage and significantly alleviate stomach injury. Controlling the expression of COX-2 in gastric tissue could inhibit stomach inflammation [
41]. Meanwhile, COX-2 and its product prostaglandin could adjust factors such as VEGF and EGFR. As a source, COX-2 could activate VEGF and EGFR in downstream and promote gastric lesions [
42]. Under normal conditions, NF-κB existed in compound proteins in cell plasma, and after activation could move into the nucleus and combine with double-stranded DNA to activate transcription and expression of many downstream genes [
43]. IKK was activated by external stimuli and made IκB phosphorylation degrade and dissociate with NF-κB. Then NF-κB was activated and transferred into the nucleus to regulate gene transcription [
44]. NF-κB is very important in the generation of gastritis, and its expression was higher in gastric damage than that in normal state, while the expression of IκB-α was lower. Mucosal inflammation stimulated by NF-κB might be the key to influence stomach injury, showing that degradation of IκB-α could inhibit the activation of NF-κB [
45].
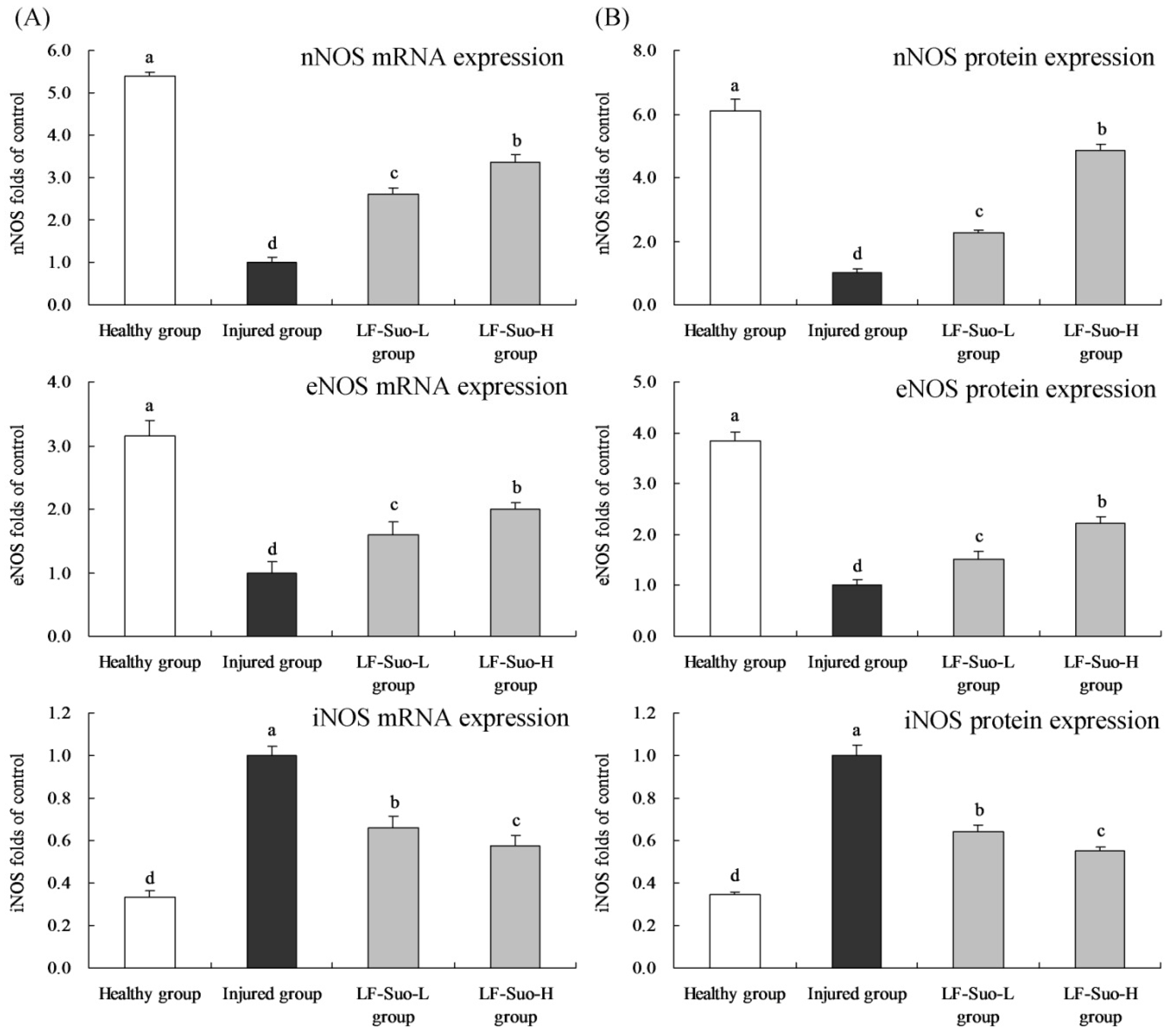
Many forms of enzymes are related to prostaglandins and NO metabolism [
46]. NOS exists both in basic and induced forms, and basic form NOS includes eNOS in endothelial cells and nNOS in intestinal neurons [
47]. After proper stimulation such as endotoxin, NOS could convert into induced iNOS [
48]. Under physiological condition, eNOS and nNOS continuously synthesized NO to regulate stomach tissues. iNOS was not expressed in the normal state, but was activated after stomach tissue injury, intensifying stomach injury. In the process of alleviating stomach injury, increasing the content of eNOS and nNOS and lowering the content of iNOS could effectively play a positive role [
49].
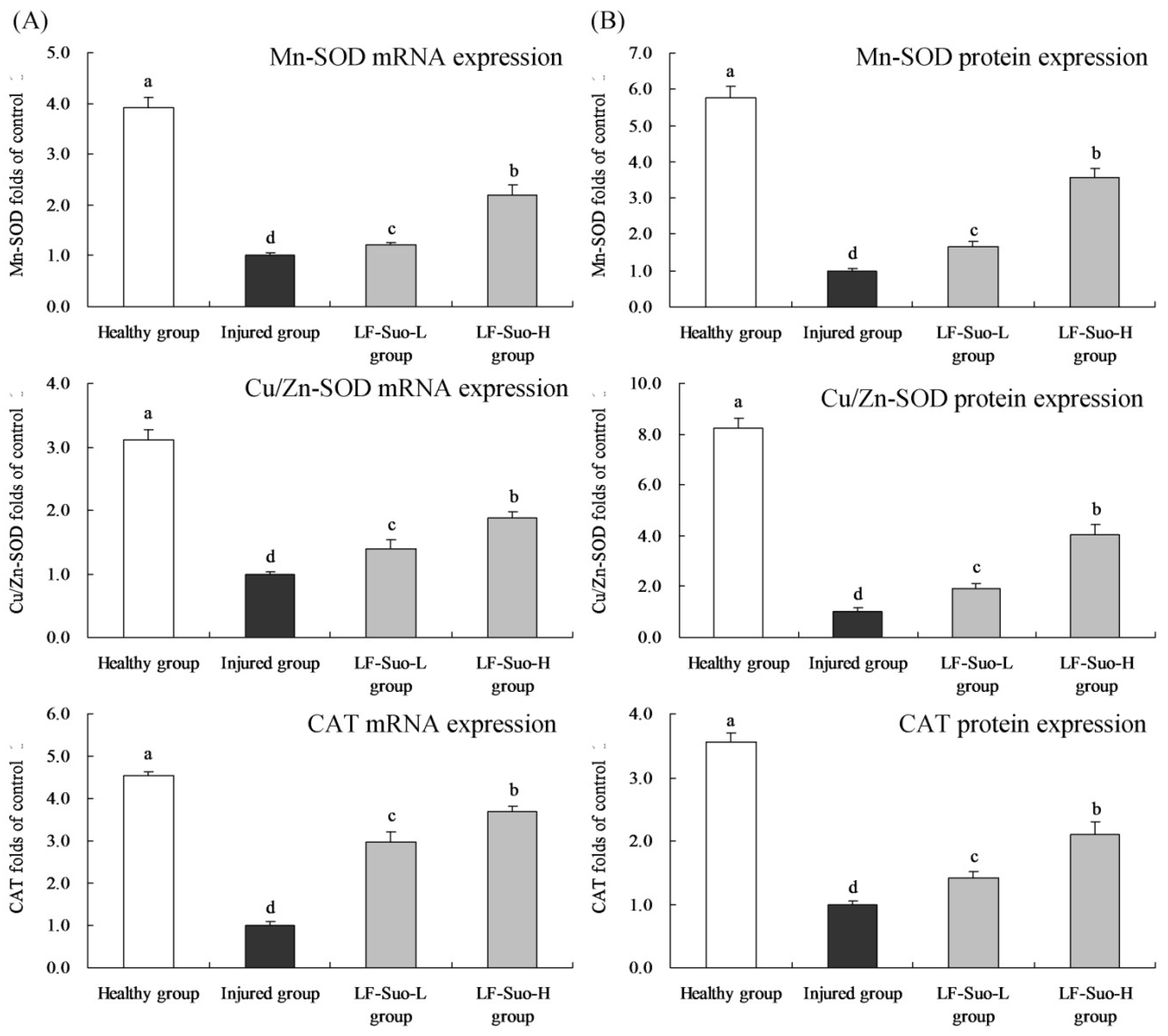
SOD can convert superoxide free radicals into hydrogen peroxide, and in mammalian tissues, there are three kinds of SOD isozymes, including Cu/Zn-SOD (SOD1), Mn-SOD (SOD2) and EC-SOD (SOD3) [
50]. Large amounts of Cu/Zn-SOD exist in the cytoplasm, nucleus, and peroxidase and mitochondrial membranes, whose basic function is to reduce the concentration of O
2− in cells and protect epithelial cells. Mn-SOD is a mitochondrial enzyme and exists in the mitochondrial matrix, which could clear O
2− produced by respiratory chain reactions to prevent peroxidation damage [
51]. Through antioxidant effects, Cu/Zn-SOD and Mn-SOD could inhibit alcoholic gastric lesions. SOD plays a vital role in balancing oxidation and antioxidants in the body, because SOD can remove O
2− by disproportionation reaction to protect cells from damage [
52]. CAT removes oxide free radicals by deoxygenizing H
2O
2 to H
2O and prevented the generation of OH to terminate the radical chain reaction. As antioxidant enzymes, SOD and CAT had been proven to be able to protect gastric mucosa [
53]. In previous research, LF-Suo had strong anti-gastric juice effects and hydrophobic properties, LF-Suo had stronger viability in stomach than
Lactobacillus bulgaricus [
8]. The high survival rate of lactic acid bacteria in gastric acid means the lactic acid bacteria might be used as probiotics [
54], and the mucosa adsorptivity of lactic acid bacteria was in relationship with its hydrophobic property; the high hydrophobic property might help lactic acid bacteria to adsorb in gastric mucosa. These characteristics might make LF-Suo colonize in stomach and show anti-gastric injury effect.
LF-Suo is a new lactic acid bacteria which was found in yak yoghourt; its
in vitro effects have been investigated, but there are not many studies about its
in vivo functional effects; there is one report on its anti-constipation determination [
8]. In this study, the gastric injury treatment effects of LF-Suo were measured and its possible mechanism discussed. These data indicate that this lactic acid bacterial species has gastric injury inhibitory effects similar to those found in others [
10,
11,
12].