Co-Ingestion of Whey Protein with a Carbohydrate-Rich Breakfast Does Not Affect Glycemia, Insulinemia or Subjective Appetite Following a Subsequent Meal in Healthy Males
Abstract
:1. Introduction
2. Methods
2.1. Participants
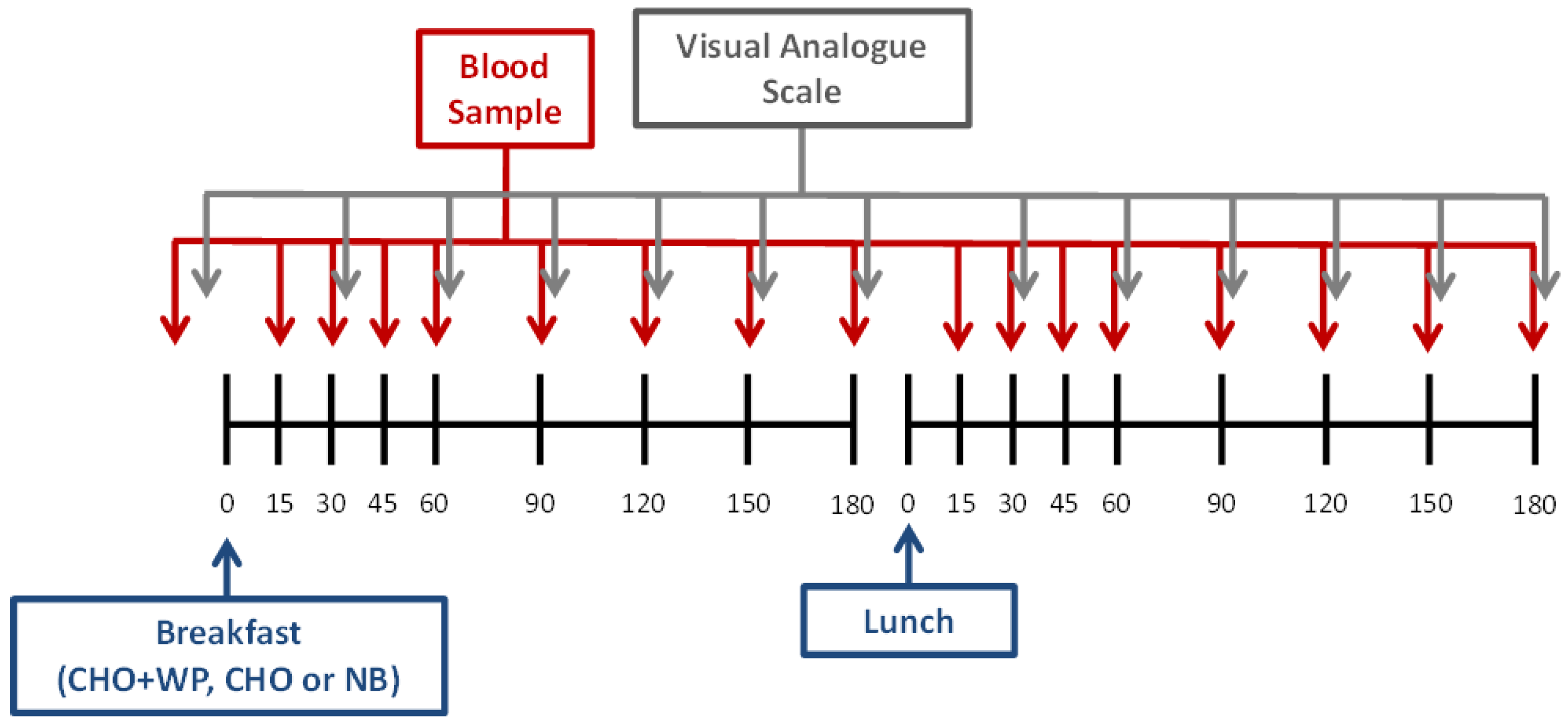
2.2. Experimental Design
2.3. Test Meals
2.4. Blood Sampling and Analysis
2.5. Subjective Ratings
2.6. Statistical Analysis
3. Results
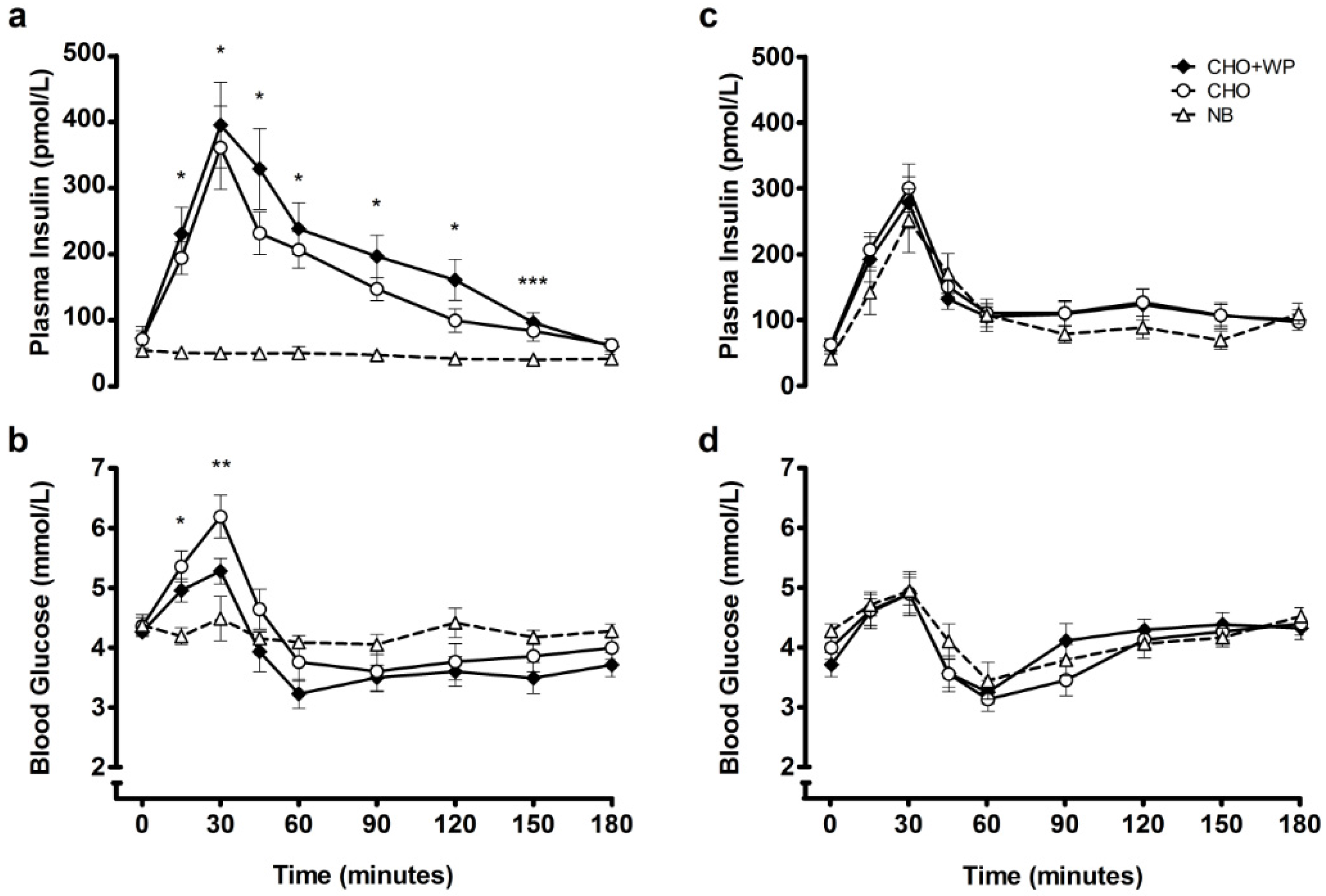
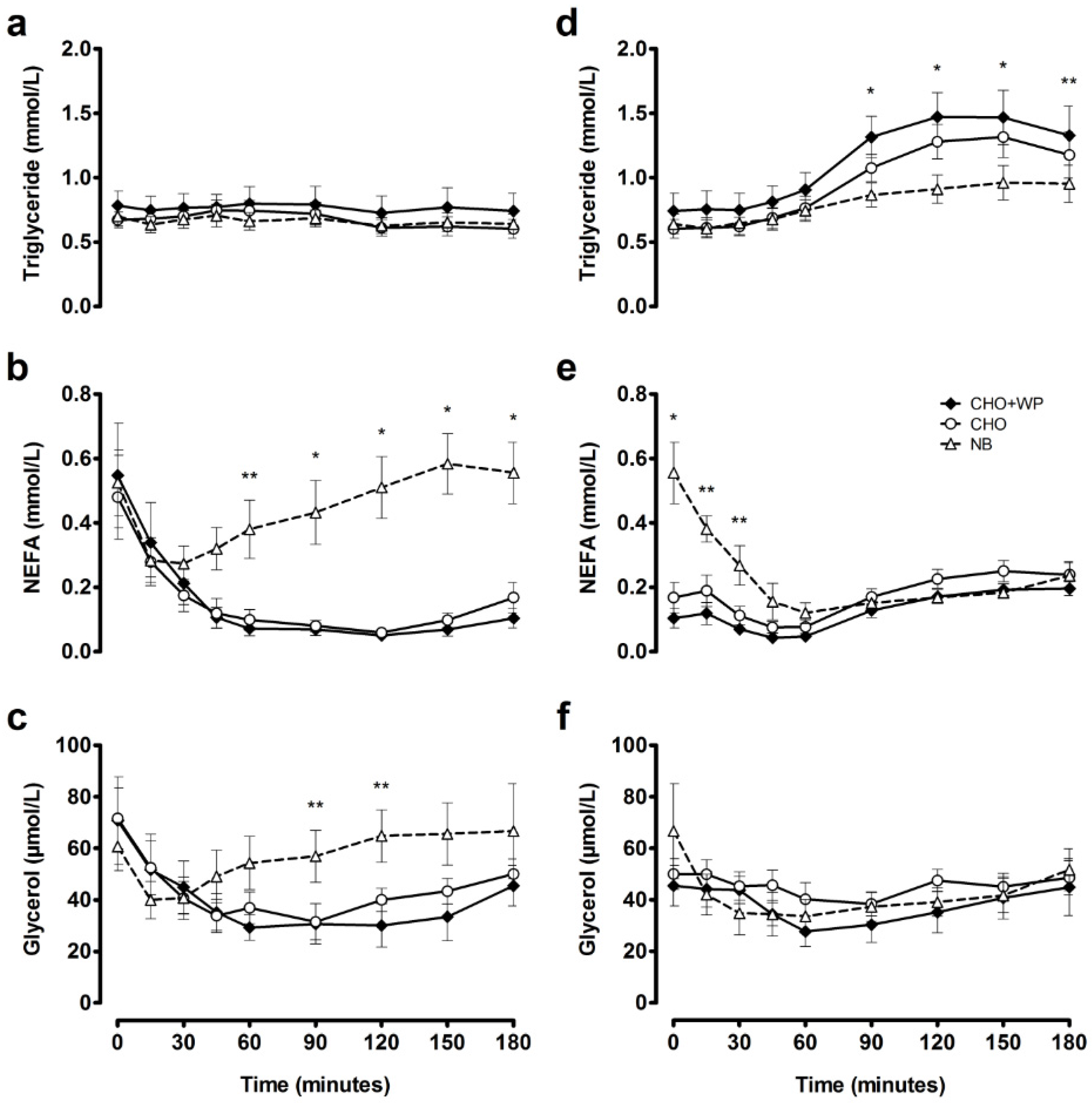
3.1. Post-Breakfast Responses
3.2. Post-Lunch Responses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein—its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108 (Suppl. 2), S105–S112. [Google Scholar] [CrossRef] [PubMed]
- Pasin, G.; Comerford, K.B. Dairy foods and dairy proteins in the management of type 2 diabetes: A systematic review of the clinical evidence. Adv. Nutr. 2015, 6, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Bjørnshave, A.; Hermansen, K. Effects of dairy protein and fat on the metabolic syndrome and type 2 diabetes. Rev. Diabet. Stud. 2014, 11, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, T.; Luhovyy, B.L.; Brown, P.H.; Cho, C.E.; Anderson, G.H. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am. J. Clin Nutr. 2010, 91, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Bjorck, I.M. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75. [Google Scholar] [PubMed]
- Mortensen, L.S.; Hartvigsen, M.L.; Brader, L.J.; Astrup, A.; Schrezenmeir, J.; Holst, J.J.; Thomsen, C.; Hermansen, K. Differential effects of protein quality on postprandial lipemia in response to a fat-rich meal in type 2 diabetes: Comparison of whey, casein, gluten, and cod protein. Am. J. Clin. Nutr. 2009, 90, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gunnerud, U.J.; Ostman, E.M.; Bjorck, I.M. Effects of whey proteins on glycaemia and insulinaemia to an oral glucose load in healthy adults; a dose-response study. Eur. J. Clin. Nutr. 2013, 67, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Holst, J.J.; Bjorck, I.M. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: Studies using glucose-equivalent drinks. Am. J. Clin. Nutr. 2007, 85, 996–1004. [Google Scholar] [PubMed]
- Zafar, T.A.; Waslien, C.; AlRaefaei, A.; Alrashidi, N.; AlMahmoud, E. Whey protein sweetened beverages reduce glycemic and appetite responses and food intake in young females. Nutr. Res. 2013, 33, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Radavelli-Bagatini, S.; Hagger, M.; Ellis, V. Comparative effects of whey and casein proteins on satiety in overweight and obese individuals: A randomized controlled trial. Eur. J. Clin. Nutr. 2014, 68, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V. Clinical significance of targeting postprandial and fasting hyperglycemia in managing type 2 diabetes mellitus. Curr. Med. Res. Opin. 2003, 19, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.D.; Mikhailidis, D.P.; Kovar, J.; Lairon, D.; Nordestgaard, B.G.; Ooi, T.C.; Perez-Martinez, P.; Bilianou, H.; Anagnostopoulou, K.; Panotopoulos, G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: An expert panel statement. Curr. Vasc. Pharmacol. 2011, 9, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Ansar, S.; Koska, J.; Reaven, P.D. Postprandial hyperlipidemia, endothelial dysfunction and cardiovascular risk: Focus on incretins. Cardiovasc. Diabetol. 2011, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.D.R.R.; Wright, J.W.; Williams, C.M.; Lovegrove, J.A. Meal ingestion provokes entry of lipoproteins containing fat from the previous meal: Possible metabolic implications. Eur. J. Nutr. 2005, 44, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Ellis, V. The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br. J. Nutr. 2010, 104, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Esteves de Oliveira, F.C.; Pinheiro Volp, A.C.; Alfenas, R.C. Impact of different protein sources in the glycemic and insulinemic responses. Nutr. Hosp. 2011, 26, 669–676. [Google Scholar] [PubMed]
- Pal, S.; Radavelli-Bagatini, S. The effects of whey protein on cardiometabolic risk factors. Obes. Rev. 2013, 14, 324–343. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Cabello, I.; Herrera, M.O.; Artacho, R. Possible role of milk-derived bioactive peptides in the treatment and prevention of metabolic syndrome. Nutr. Rev. 2012, 70, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.T.; Lira, F.S.; Rosa, J.C.; de Oliveira, E.P.; Oyama, L.M.; Santos, R.V.; Pimentel, G.D. Dietary whey protein lessens several risk factors for metabolic diseases: A review. Lipids Health Dis. 2012, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and type 2 diabetes. J. Nutr. Biochem. 2013, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gunnerud, U.J.; Heinzle, C.; Holst, J.J.; Ostman, E.M.; Bjorck, I.M. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS ONE 2012, 7, e44731. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.L.; Ward, L.S.; Bastian, E.D.; Jenkins, A.L.; Campbell, J.; Vuksan, V. A whey protein supplement decreases post-prandial glycemia. Nutr. J. 2009, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O.; Ahren, B.; Boaz, M.; Landau, Z.; Bar-Dayan, Y.; Ganz, T.; Barnea, M.; Wainstein, J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: A randomised clinical trial. Diabetologia 2014, 57, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Duffey, K.J. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the united states. Am. J. Clin. Nutr. 2010, 91, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Chungchunlam, S.M.S.; Henare, S.J.; Ganesh, S.; Moughan, P.J. Dietary whey protein influences plasma satiety-related hormones and plasma amino acids in normal-weight adult women. Eur. J. Clin. Nutr. 2015, 69, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hoefle, A.S.; Bangert, A.M.; Stamfort, A.; Gedrich, K.; Rist, M.J.; Lee, Y.-M.; Skurk, T.; Daniel, H. Metabolic responses of healthy or prediabetic adults to bovine whey protein and sodium caseinate do not differ. J. Nutr. 2015, 145, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Frape, D.L.; Williams, N.R.; Scriven, A.J.; Palmer, C.R.; O’Sullivan, K.; Fletcher, R.J. Diurnal trends in responses of blood plasma concentrations of glucose, insulin, and c-peptide following high- and low-fat meals and their relation to fat metabolism in healthy middle-aged volunteers. Br. J. Nutr. 1997, 77, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Stevenson, E.J. Calcium co-ingestion augments postprandial glucose-dependent insulinotropic peptide(1–42), glucagon-like peptide-1 and insulin concentrations in humans. Eur. J. Nutr. 2014, 53, 375–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, J.N.; Altman, D.G.; Campbell, M.J.; Royston, P. Analysis of serial measurements in medical research. BMJ 1990, 300, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q.; Westphal, S.A.; Neil, B.J.; Seaquist, E.R. Effects of dose of ingested glucose on plasma metabolite and hormone responses in type ii diabetic subjects. Diabetes Care 1989, 12, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, T.; Luhovyy, B.L.; Panahi, S.; Kubant, R.; Brown, P.H.; Anderson, G.H. Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J. Nutr. Biochem. 2014, 25, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Plat, L.; Byrne, M.M.; Sturis, J.; Polonsky, K.S.; Mockel, J.; Fery, F.; Van Cauter, E. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am. J. Physiol. Endocrinol. Metab. 1996, 270, E36–E42. [Google Scholar]
- Smith, G.I.; Yoshino, J.; Stromsdorfer, K.L.; Klein, S.J.; Magkos, F.; Reeds, D.N.; Klein, S.; Mittendorfer, B. Protein ingestion induces muscle insulin resistance independent of leucine-mediated mtor activation. Diabetes 2015, 64, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Lopez, X.; Cypess, A.; Manning, R.; O’Shea, S.; Kulkarni, R.N.; Goldfine, A.B. Exogenous insulin enhances glucose-stimulated insulin response in healthy humans independent of changes in free fatty acids. J. Clin. Endocrinol. Metab. 2011, 96, 3811–3821. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Noakes, M.; Clifton, P.M. Appetite hormones and energy intake in obese men after consumption of fructose, glucose and whey protein beverages. Int. J. Obes. 2007, 31, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Potier, M.; Fromentin, G.; Lesdema, A.; Benamouzig, R.; Tome, D.; Marsset-Baglieri, A. The satiety effect of disguised liquid preloads administered acutely and differing only in their nutrient content tended to be weaker for lipids but did not differ between proteins and carbohydrates in human subjects. Br. J. Nutr. 2010, 104, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; van der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol. Int. 2014, 31, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Donin, A.S.; Nightingale, C.M.; Owen, C.G.; Rudnicka, A.R.; Perkin, M.R.; Jebb, S.A.; Stephen, A.M.; Sattar, N.; Cook, D.G.; Whincup, P.H. Regular breakfast consumption and type 2 diabetes risk markers in 9- to 10-year-old children in the child heart and health study in England (chase): A cross-sectional analysis. PLoS Med. 2014, 11, e1001703. [Google Scholar] [CrossRef]
- Betts, J.A.; Richardson, J.D.; Chowdhury, E.A.; Holman, G.D.; Tsintzas, K.; Thompson, D. The causal role of breakfast in energy balance and health: A randomized controlled trial in lean adults. Am. J. Clin. Nutr. 2014, 100, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, E.J.; Dawson, J.; Alcorn, A.; Larsen, L.H.; Thomas, E.A.; Cardel, M.; Bourland, A.C.; Astrup, A.; St-Onge, M.-P.; Hill, J.O.; et al. The effectiveness of breakfast recommendations on weight loss: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Astbury, N.M.; Taylor, M.A.; Macdonald, I.A. Breakfast consumption affects appetite, energy intake, and the metabolic and endocrine responses to foods consumed later in the day in male habitual breakfast eaters. J. Nutr. 2011, 141, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Veasey, R.C.; Rumbold, P.L.; Stevenson, E.J. Breakfast and exercise contingently affect postprandial metabolism and energy balance in physically active males. Br. J. Nutr. 2013, 110, 721–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, A.; Bellisle, F. Nutrients, satiety, and control of energy intake. Appl. Physiol. Nutr. Metab. 2015, 40, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Bano, G. Glucose homeostasis, obesity and diabetes. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Wessman, Y.; Almgren, P.; Groop, L. High levels of nonesterified fatty acids are associated with increased familial risk of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty acids, obesity, and insulin resistance: Time for a reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-M.; Heden, T.D.; Liu, Y.; Nyhoff, L.M.; Thyfault, J.P.; Leidy, H.J.; Kanaley, J.A. A high-protein breakfast induces greater insulin and glucose-dependent insulinotropic peptide responses to a subsequent lunch meal in individuals with type 2 diabetes. J. Nutr. 2015, 145, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; Gardiner, J.; McBurney, M.I.; Anderson, S.; Weatherspoon, L.J.; Henry, D.N.; Hord, N.G. Effects of breakfast meal composition on second meal metabolic responses in adults with type 2 diabetes mellitus. Eur. J. Clin. Nutr. 2006, 60, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Del Prato, S.; Leonetti, F.; Simonson, D.C.; Sheehan, P.; Matsuda, M.; DeFronzo, R.A. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia 1994, 37, 1025–1035. [Google Scholar] [CrossRef] [PubMed]

| Energy kJ (kcal) | Carbohydrate g (E%) | Fat g (E%) | Protein g (E%) | ||
|---|---|---|---|---|---|
| CHO | 1799 (430) | 93 (87) | 2 (4) | 9 (8) | |
| Breakfast | CHO + WP | 2172 (519) | 95 (74) | 2 (3) | 29 (22) |
| NB | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Lunch | 3446 (824) | 104 (50) | 33 (36) | 29 (14) |
| CHO + WP 1,2 | CHO | NB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose (mmol/L) | ||||||||||
| Post-breakfast | AUC | 3.8 | ± | 0.2 | 4.2 | ± | 0.2 | 4.2 | ± | 0.1 |
| iAUC | −0.4 | ± | 0.1 | −0.2 | ± | 0.1 | −0.2 | ± | 0.1 | |
| Post-lunch | AUC | 4.1 | ± | 0.1 | 4.0 | ± | 0.1 | 4.1 | ± | 0.2 |
| iAUC | 0.4 | ± | 0.2 | −0.0 | ± | 0.1 | −0.1 | ± | 0.2 | |
| Insulin (pmol/L) | ||||||||||
| Post-breakfast | AUC | 193.1 | ± | 26.3 a | 154.7 | ± | 18.5 b | 46.1 | ± | 8.0 c |
| iAUC | 120.2 | ± | 15.3 a | 83.5 | ± | 8.6 b | −8.2 | ± | 2.8 c | |
| Post-lunch | AUC | 130.7 | ± | 18.8 | 136.9 | ± | 15.7 | 110.8 | ± | 18.6 |
| iAUC | 70.8 | ± | 11.1 | 74.2 | ± | 9.4 | 68.9 | ± | 13.5 | |
| Triglyceride (mmol/L) | ||||||||||
| Post-breakfast | AUC | 0.77 | ± | 0.13 | 0.67 | ± | 0.07 | 0.66 | ± | 0.07 |
| iAUC | −0.02 | ± | 0.03 | 0.00 | ± | 0.02 | −0.04 | ± | 0.02 | |
| Post-lunch | AUC | 1.16 | ± | 0.16 a | 0.99 | ± | 0.11 a | 0.82 | ± | 0.10 b |
| iAUC | 0.42 | ± | 0.05 a | 0.39 | ± | 0.04 a | 0.18 | ± | 0.05 b | |
| NEFA (mmol/L) | ||||||||||
| Post-breakfast | AUC | 0.13 | ± | 0.03 a | 0.13 | ± | 0.03 a | 0.44 | ± | 0.08 b |
| iAUC | −0.42 | ± | 0.13 | −0.35 | ± | 0.11 | −0.08 | ± | 0.05 | |
| Post-lunch | AUC | 0.13 | ± | 0.02 a | 0.18 | ± | 0.02 | 0.21 | ± | 0.02 b |
| iAUC | 0.02 | ± | 0.02 a | 0.01 | ± | 0.03 a | −0.35 | ± | 0.08 b | |
| Glycerol (µmol/L) | ||||||||||
| Post-breakfast | AUC | 37 | ± | 7 | 41 | ± | 5 | 57 | ± | 9 |
| iAUC | −34 | ± | 15 | −30 | ± | 8 | −4 | ± | 4 | |
| Post-lunch | AUC | 37 | ± | 6 | 45 | ± | 3 | 40 | ± | 7 |
| iAUC | −9 | ± | 4 | −5 | ± | 6 | −26 | ± | 13 | |
| CHO + WP 1,2 | CHO | NB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Combined appetite score (mm) | ||||||||||
| Post-breakfast | AUC | 53.0 | ± | 3.9 a | 55.4 | ± | 4.6 a | 82.1 | ± | 2.5 b |
| iAUC | −23.1 | ± | 4.5 a | −18.4 | ± | 5.7 a | 8.3 | ± | 0.8 b | |
| Post-lunch | AUC | 52.2 | ± | 4.8 | 57.4 | ± | 5.2 | 61.0 | ± | 5.7 |
| iAUC | −16.4 | ± | 4.4 | −16.5 | ± | 3.5 | −28.2 | ± | 5.9 | |
| Hunger (mm) | ||||||||||
| Post-breakfast | AUC | 48.7 | ± | 3.8 a | 52.5 | ± | 4.3 a | 74.5 | ± | 3.2 b |
| iAUC | −24.1 | ± | 4.4 a | −16.8 | ± | 5.7 a | 9.8 | ± | 2.1 b | |
| Post-lunch | AUC | 48.7 | ± | 4.7 | 54.8 | ± | 5.0 | 57.3 | ± | 5.4 |
| iAUC | −16.4 | ± | 4.6 | −17.0 | ± | 3.8 | −26.7 | ± | 5.7 | |
| Fullness (mm) | ||||||||||
| Post-breakfast | AUC | 47.6 | ± | 4.0 a | 46.1 | ± | 4.6 a | 15.9 | ± | 2.4 b |
| iAUC | 25.9 | ± | 4.7 a | 23.1 | ± | 5.1 a | −8.9 | ± | 1.6 b | |
| Post-lunch | AUC | 49.2 | ± | 4.5 | 43.9 | ± | 5.5 | 39.2 | ± | 5.7 |
| iAUC | 17.3 | ± | 4.8 a | 17.8 | ± | 3.4 | 30.8 | ± | 6.2 b | |
| Prospective Consumption (mm) | ||||||||||
| Post-breakfast | AUC | 58.1 | ± | 5.0 a | 60.4 | ± | 6.0 a | 85.0 | ± | 2.4 b |
| iAUC | −19.4 | ± | 5.0 a | −13.1 | ± | 7.3 | 6.8 | ± | 1.2 b | |
| Post-lunch | AUC | 56.8 | ± | 6.3 | 62.0 | ± | 5.7 | 65.4 | ± | 6.4 |
| iAUC | −15.6 | ± | 4.6 | −14.3 | ± | 3.9 | −24.0 | ± | 6.4 | |
| Satisfaction (mm) | ||||||||||
| Post-breakfast | AUC | 47.1 | ± | 3.5 a | 45.4 | ± | 4.3 a | 15.4 | ± | 2.5 b |
| iAUC | 23.1 | ± | 5.0 a | 20.7 | ± | 5.1 a | −7.8 | ± | 1.7 b | |
| Post-lunch | AUC | 47.6 | ± | 4.1 | 43.2 | ± | 5.1 | 39.7 | ± | 5.5 |
| iAUC | 16.1 | ± | 4.8 | 16.8 | ± | 3.5 | 31.1 | ± | 5.7 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allerton, D.M.; Campbell, M.D.; Gonzalez, J.T.; Rumbold, P.L.S.; West, D.J.; Stevenson, E.J. Co-Ingestion of Whey Protein with a Carbohydrate-Rich Breakfast Does Not Affect Glycemia, Insulinemia or Subjective Appetite Following a Subsequent Meal in Healthy Males. Nutrients 2016, 8, 116. https://doi.org/10.3390/nu8030116
Allerton DM, Campbell MD, Gonzalez JT, Rumbold PLS, West DJ, Stevenson EJ. Co-Ingestion of Whey Protein with a Carbohydrate-Rich Breakfast Does Not Affect Glycemia, Insulinemia or Subjective Appetite Following a Subsequent Meal in Healthy Males. Nutrients. 2016; 8(3):116. https://doi.org/10.3390/nu8030116
Chicago/Turabian StyleAllerton, Dean M., Matthew D. Campbell, Javier T. Gonzalez, Penny L. S. Rumbold, Daniel J. West, and Emma J. Stevenson. 2016. "Co-Ingestion of Whey Protein with a Carbohydrate-Rich Breakfast Does Not Affect Glycemia, Insulinemia or Subjective Appetite Following a Subsequent Meal in Healthy Males" Nutrients 8, no. 3: 116. https://doi.org/10.3390/nu8030116





