Feeding a Diet Enriched in Docosahexaenoic Acid to Lactating Dams Improves the Tolerance Response to Egg Protein in Suckled Pups
Abstract
:1. Introduction
2. Materials and Methods
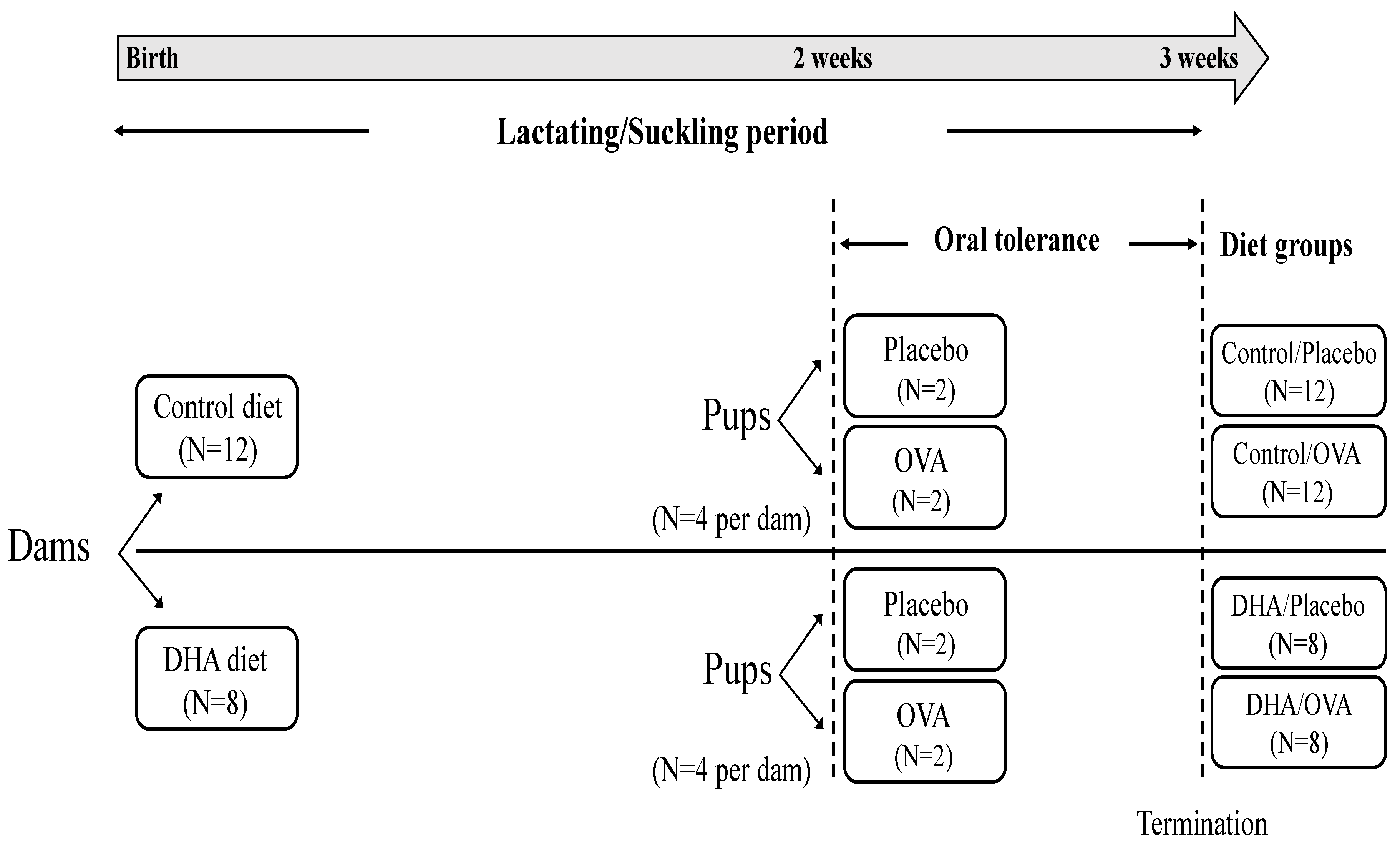
2.1. Animals and Diets
2.2. Ovalbumin Administration and Immunization
2.3. Tissue Collection
2.4. Immune Cell Isolation
2.5. Immune Cell Phenotype Analysis
2.6. Measurements of Ex Vivo Cytokine Secretion by Mitogen-Stimulated Splenocytes and Plasma Ova-Specific Immunoglobulin Concentrations
2.7. Statistical Analysis
3. Results
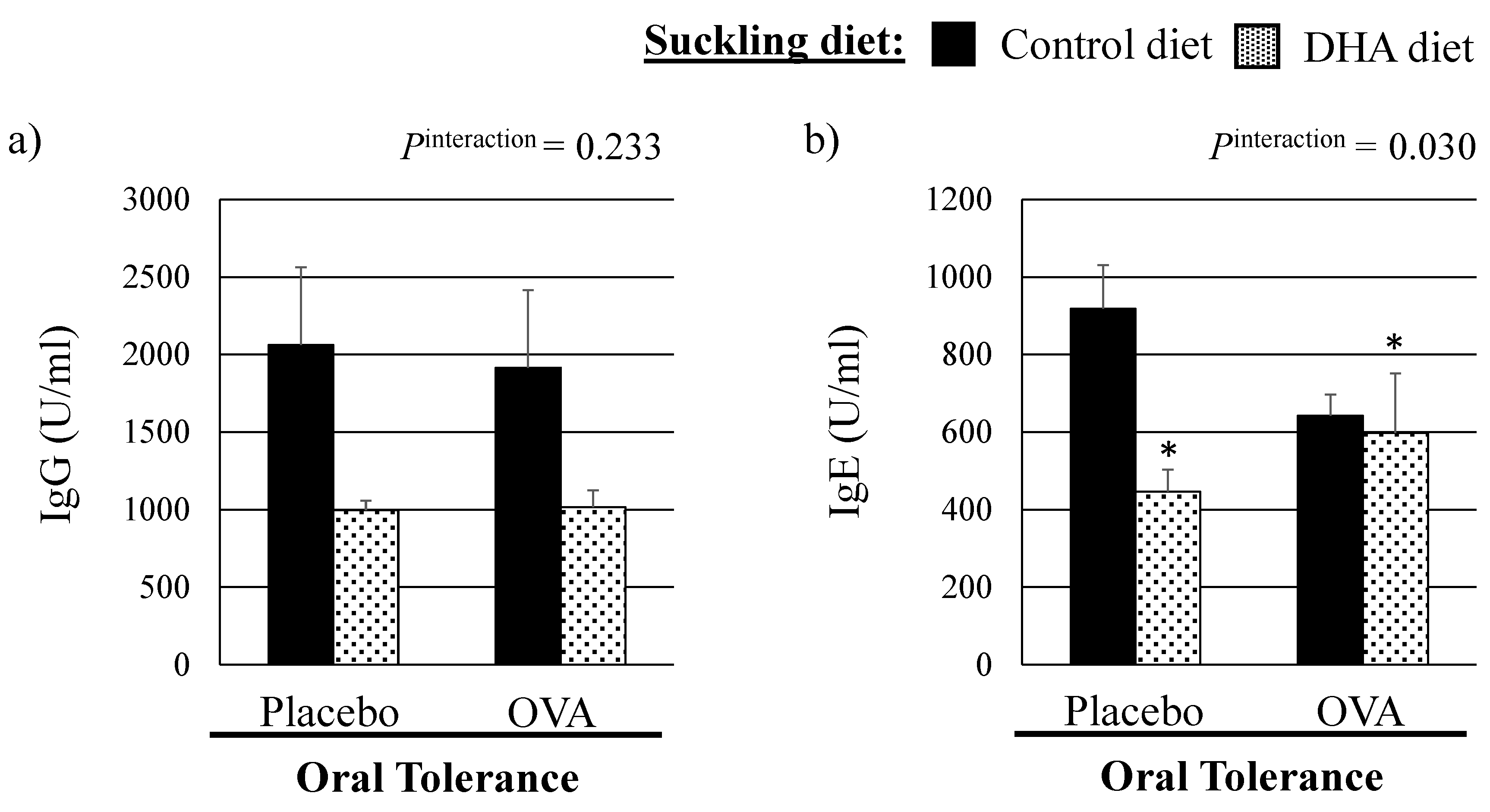
3.1. Growth Parameters and Plasma OVA Specific Immunoglobulin Concentrations
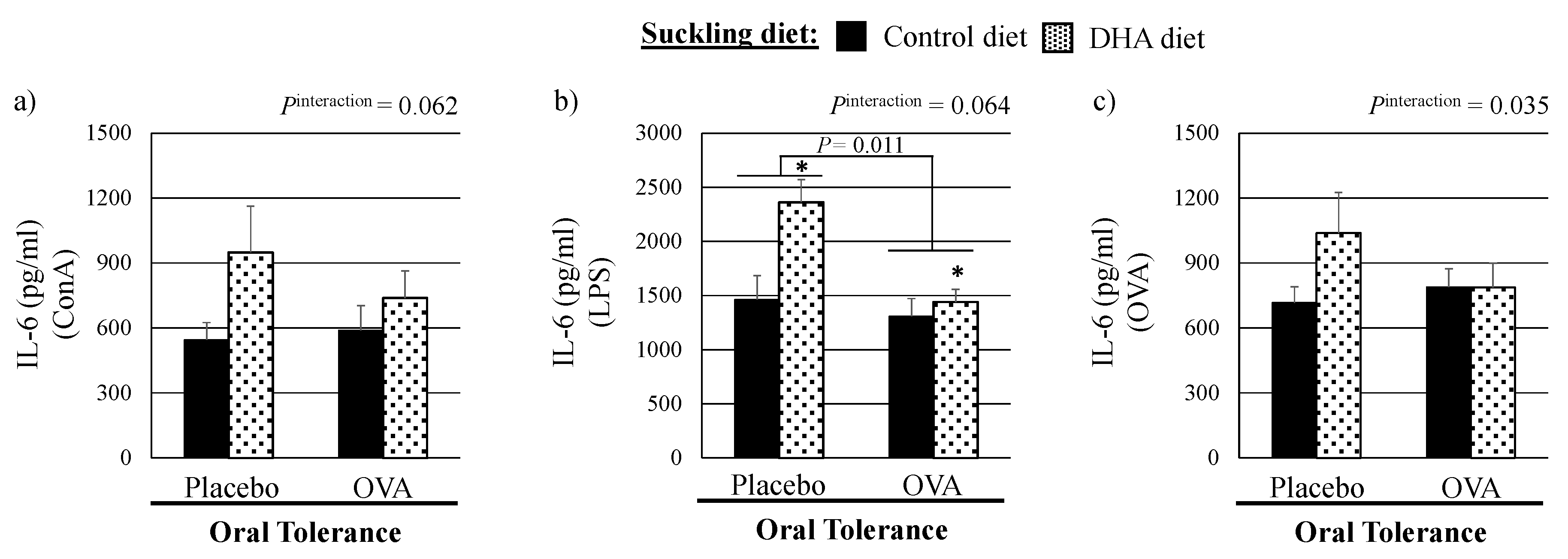
3.2. Ex Vivo Cytokine Production by Mitogen-Stimulated Splenocytes
3.3. Immune Cell Phenotypes in Spleen
3.4. Immune Cell Phenotypes in MLN
4. Discussion
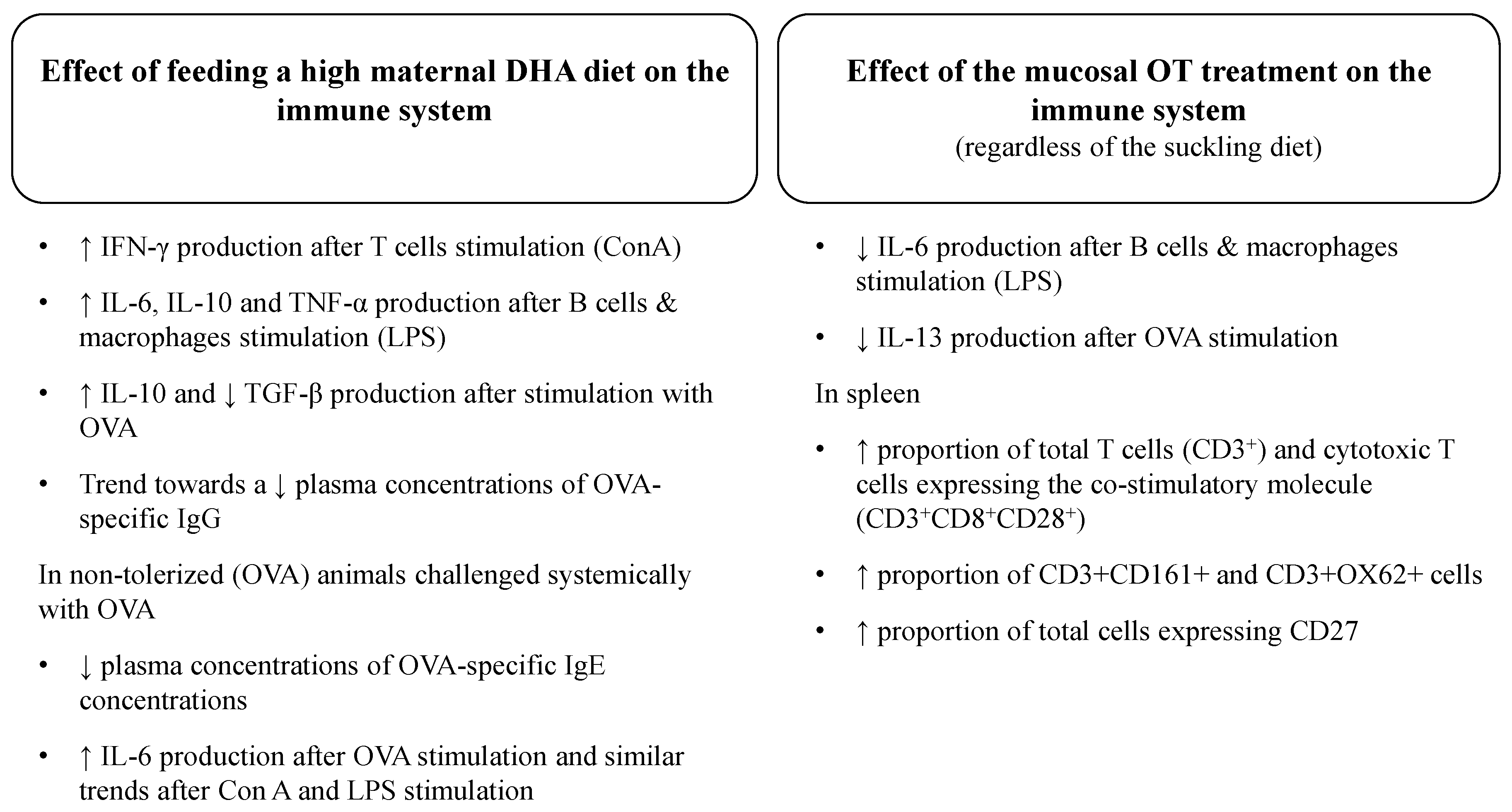
4.1. Effect of a High DHA Maternal Diet on the Ex Vivo Cytokine Response to Mitogens in Suckled Pups
4.2. Effect of the Mucosal Oral Tolerance Treatment on The Immune System
4.3. Effect of a High DHA Maternal Diet on the Establishment of Oral Tolerance in Suckled Pups
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| ALA | α-linolenic acid |
| ConA | Concanavalin A |
| DHA | docosahexaenoic acid |
| DPA | docosapentaenoic acid |
| IFN-γ | interferon-gamma |
| Ig | immunoglobulin |
| IL | interleukin |
| LPS | lipopolysaccharide |
| MUFA | monounsaturated fatty acid |
| n | omega |
| OVA | ovalbumin |
| PUFA | polyunsaturated fatty acid |
| SFA | saturated fatty acid |
| TGF-β | transforming growth factor-beta |
| TNF-α | tumor necrosis factor-alpha |
References
- Garside, P.; Mowat, A.M. Oral tolerance. Semin. Immunol. 2001, 13, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L. Early origins of allergic disease: A review of processes and influences during early immune development. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Kremmyda, L.S.; Vlachava, M.; Noakes, P.S.; Miles, E.A. Is there a role for fatty acids in early life programming of the immune system? Proc. Nutr. Soc. 2010, 69, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K. Fatty acids as modulators of the immune response. Annu. Rev. Nutr. 2006, 26, 45–73. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: A randomized, controlled trial. J. Allergy Clin. Immunol. 2003, 112, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Torrent, M.; Garcia-Esteban, R.; Ferrer, C.; Ribas-Fito, N.; Anto, J.M.; Sunyer, J. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin. Exp. Allergy 2007, 37, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.T.; Li, Y.F.; Langholz, B.; Gilliland, F.D. Maternal fish consumption during pregnancy and risk of early childhood asthma. J. Asthma 2005, 42, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Pabst, O.; Mowat, A.M. Oral tolerance to food protein. Mucosal Immunol. 2012, 5, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Ly, L.H.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. Dietary docosahexaenoic acid suppresses t cell protein kinase c theta lipid raft recruitment and IL-2 production. J. Immunol. 2004, 173, 6151–6160. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Khan, N.A.; McMurray, D.N.; Prior, I.A.; Wang, N.; Chapkin, R.S. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog. Lipid Res. 2010, 49, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kanke, Y.; Kudoh, K.; Misawa, Y.; Shimizu, J.; Takita, T. Effects of dietary docosahexaenoic acid on surface molecules involved in T cell proliferation. Biochim. Biophys. Acta 1999, 1436, 519–530. [Google Scholar] [CrossRef]
- Zeyda, M.; Staffler, G.; Horejsi, V.; Waldhausl, W.; Stulnig, T.M. Lat displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J. Biol. Chem. 2002, 277, 28418–28423. [Google Scholar] [CrossRef] [PubMed]
- Birch, E.E.; Khoury, J.C.; Berseth, C.L.; Castaneda, Y.S.; Couch, J.M.; Bean, J.; Tamer, R.; Harris, C.L.; Mitmesser, S.H.; Scalabrin, D.M. The impact of early nutrition on incidence of allergic manifestations and common respiratory illnesses in children. J. Pediatr. 2010, 156, 902.e1–906.e1. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J.; Thomson, C.A.; Van Aerde, J.E.; Parrott, A.; Euler, A.; Lien, E.; Clandinin, M.T. Lower proportion of CD45r0+ cells and deficient interleukin-10 production by formula-fed infants, compared with human-fed, is corrected with supplementation of long-chain polyunsaturated fatty acids. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Wang, Y.; Forsyth, S.; Brenna, J.T. The European food safety authority recommendation for polyunsaturated fatty acid composition of infant formula overrules breast milk, puts infants at risk, and should be revised. Prostaglandins Leukot. Essent. Fat. Acids 2015, 102, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid quality in infant nutrition: Current knowledge and future opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Carlson, S.E.; van Goudoever, J.B. Should infant formula provide both omega-3 DHA and omega-6 arachidonic acid? Ann. Nutr. Metab. 2015, 66, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Fewtrell, M.; Agostoni, C. Dietary arachidonic acid in perinatal nutrition: A commentary. Pediatr. Res. 2015, 77, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.C.; Lewis, M.E.D.; Field, D.C.J. Evidence for the essentiality of arachidonic and docosahexaenoic acid in the postnatal maternal and infant diet for the development of the infant’s immune system early in life. Appl. Physiol. Nutr. Metab. 2016. [Google Scholar] [CrossRef]
- Ruth, M.R.; Proctor, S.D.; Field, C.J. Feeding long-chain n-3 polyunsaturated fatty acids to obese leptin receptor-deficient JCR:LA-cp rats modifies immune function and lipid-raft fatty acid composition. Br. J. Nutr. 2009, 101, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Lewis, E.D.; Goruk, S.; Field, C.J. The content of docosahexaenoic acid in the maternal diet differentially affects the immune response in lactating dams and suckled offspring. Eur. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Odemuyiwa, S.O.; Ebeling, C.; Duta, V.; Abel, M.; Puttagunta, L.; Cravetchi, O.; Majaesic, C.; Vliagoftis, H.; Moqbel, R. Tryptophan catabolites regulate mucosal sensitization to ovalbumin in respiratory airways. Allergy 2009, 64, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J.; Wu, G.; Metroz-Dayer, M.D.; Montambault, M.; Marliss, E.B. Lactate production is the major metabolic fate of glucose in splenocytes and is altered in spontaneously diabetic BB rats. Biochem. J. 1990, 272, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Blewett, H.J.; Gerdung, C.A.; Ruth, M.R.; Proctor, S.D.; Field, C.J. Vaccenic acid favourably alters immune function in obese JCR:LA-cp rats. Br. J. Nutr. 2009, 102, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J.; Van Aerde, J.E.; Goruk, S.; Clandinin, M.T. Effect of feeding a formula supplemented with long-chain polyunsaturated fatty acids for 14 weeks improves the ex vivo response to a mitogen and reduces the response to a soy protein in infants at low risk for allergy. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J.; Van Aerde, J.E.; Robinson, L.E.; Clandinin, M.T. Effect of providing a formula supplemented with long-chain polyunsaturated fatty acids on immunity in full-term neonates. Br. J. Nutr. 2008, 99, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J.; Van Aerde, J.E.; Robinson, L.E.; Clandinin, M.T. Feeding a formula supplemented with long chain polyunsaturated fatty acids modifies the “ex vivo” cytokine responses to food proteins in infants at low risk for allergy. Pediatr. Res. 2008, 64, 411–417. [Google Scholar] [CrossRef] [PubMed]
- D’Vaz, N.; Meldrum, S.J.; Dunstan, J.A.; Lee-Pullen, T.F.; Metcalfe, J.; Holt, B.J.; Serralha, M.; Tulic, M.K.; Mori, T.A.; Prescott, S.L. Fish oil supplementation in early infancy modulates developing infant immune responses. Clin. Exp. Allergy 2012, 42, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Kjaer, T.M.; Fruekilde, M.B.; Michaelsen, K.F.; Frokiaer, H. Fish oil supplementation of lactating mothers affects cytokine production in 2⅟₂-year-old children. Lipids 2005, 40, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.B.; Kollmann, T.R. Induction of antigen-specific immunity in human neonates and infants. Nestle Nutr. Workshop Ser. Paediatr. Programm. 2008, 61, 183–195. [Google Scholar]
- Sutas, Y.; Hurme, M.; Isolauri, E. Oral cow milk challenge abolishes antigen-specific interferon-gamma production in the peripheral blood of children with atopic dermatitis and cow milk allergy. Clin. Exp. Allergy 1997, 27, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.; Suomalainen, H. Low frequency of CD4+, but not CD8+, t cells expressing interferon-gamma is related to cow’s milk allergy in infancy. Pediatr. Allergy Immunol. 2002, 13, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.R.; Holdaway, M.D.; Flannery, E.M.; Herbison, G.P.; Silva, P.A. Parental and neonatal risk factors for atopy, airway hyper-responsiveness, and asthma. Arch. Dis. Child. 1996, 75, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Dunstan, J.A. Prenatal fatty acid status and immune development: The pathways and the evidence. Lipids 2007, 42, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Benlounes, N.; Candalh, C.; Matarazzo, P.; Dupont, C.; Heyman, M. The time-course of milk antigen-induced tnf-alpha secretion differs according to the clinical symptoms in children with cow’s milk allergy. J. Allergy Clin. Immunol. 1999, 104, 863–869. [Google Scholar] [CrossRef]
- Osterlund, P.; Jarvinen, K.M.; Laine, S.; Suomalainen, H. Defective tumor necrosis factor-alpha production in infants with cow’s milk allergy. Pediatr. Allergy Immunol. 1999, 10, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.; Rincon, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- De Waal-Malefyt, R.; Figdor, C.G.; Huijbens, R.; Mohan-Peterson, S.; Bennett, B.; Culpepper, J.; Dang, W.; Zurawski, G.; de Vries, J.E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J. Immunol. 1993, 151, 6370–6381. [Google Scholar] [PubMed]
- Agematsu, K. Molecules involved in characteristics of naive/memory B cells. Jpn. J. Clin. Immunol. 2004, 27, 309–314. [Google Scholar] [CrossRef]
- Perez-Cano, F.J.; Castellote, C.; Marin-Gallen, S.; Gonzalez-Castro, A.; Franch, A.; Castell, M. Phenotypic and functional characteristics of rat spleen lymphocytes during suckling. Dev. Comp. Immunol. 2007, 31, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Duchamp, M.; Sterlin, D.; Diabate, A.; Uring-Lambert, B.; Guerin-El Khourouj, V.; Le Mauff, B.; Monnier, D.; Malcus, C.; Labalette, M.; Picard, C. B-cell subpopulations in children: National reference values. Immun. Inflamm. Dis. 2014, 2, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.M.; Kosaka, A. Oral tolerance: Intestinal homeostasis and antigen-specific regulatory t cells. Trends Immunol. 2008, 29, 532–540. [Google Scholar] [CrossRef] [PubMed]
- De Waal Malefyt, R.; Yssel, H.; Roncarolo, M.G.; Spits, H.; de Vries, J.E. Interleukin-10. Curr. Opin. Immunol. 1992, 4, 314–320. [Google Scholar] [CrossRef]
- Howard, M.; O’Garra, A. Biological properties of interleukin 10. Immunol. Today 1992, 13, 198–200. [Google Scholar] [CrossRef]
- Van der Velden, V.H.; Laan, M.P.; Baert, M.R.; de Waal Malefyt, R.; Neijens, H.J.; Savelkoul, H.F. Selective development of a strong Th2 cytokine profile in high-risk children who develop atopy: Risk factors and regulatory role of IFN-gamma, IL-4 and IL-10. Clin. Exp. Allergy 2001, 31, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- De Matos, O.G.; Amaral, S.S.; Pereira da Silva, P.E.; Perez, D.A.; Alvarenga, D.M.; Ferreira, A.V.; Alvarez-Leite, J.; Menezes, G.B.; Cara, D.C. Dietary supplementation with omega-3-pufa-rich fish oil reduces signs of food allergy in ovalbumin-sensitized mice. Clin. Dev. Immunol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | Control Diet | DHA Diet |
|---|---|---|
| g/100 g of total fatty acids | ||
| C14:0 | 0.1 ± 0.0 | 0.4 ± 0.0 |
| C16:0 | 6.7 ± 0.3 | 6.2 ± 0.1 |
| C16:1n-7 | 0.2 ± 0.0 | 0.2 ± 0.1 |
| C18:0 | 38.8 ± 1.2 | 40.6 ± 0.2 |
| C18:1n-9 | 29.0 ± 1.7 | 24.8 ± 0.3 |
| C18:2n-6 | 21.2 ± 0.5 | 21.6 ± 0.0 |
| C20:0 | 0.9 ± 0.0 | 0.9 ± 0.0 |
| C18:3n-3 (ALA) | 1.7 ± 0.1 | 3.3 ± 0.1 |
| C20:3n-6 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| C20:4n-6 (AA) | 0.4 ± 0.0 | 0.4 ± 0.0 |
| C22:6n-3 (DHA) | 0 | 0.9 ± 0.1 |
| Other fatty acids b | 0.8 | 0.4 |
| Total SFA | 46.5 ± 0.8 | 48.1 ± 0.3 |
| Total PUFA | 23.6 ± 0.6 | 26.6 ± 0.1 |
| Total n-6 | 21.9 ± 0.5 | 22.3 ± 0.1 |
| Total n-3 | 1.6 ± 0.1 | 4.2 ± 0.1 |
| Total MUFA | 29.1 ± 1.7 | 24.9 ± 0.4 |
| Ratio n-6/n-3 | 13.3 | 5.3 |
| Ratio PUFA/SFA | 0.5 | 0.6 |
| Variable | Control Diet | DHA Diet | P b | P c | P inter | ||
|---|---|---|---|---|---|---|---|
| Placebo Treatment (N = 12) | OVA Treatment (N = 11) | Placebo Treatment (N = 8) | OVA Treatment (N = 8) | ||||
| Con A | |||||||
| IL-2, pg/mL | 4032 ± 240 | 4069 ± 346 | 4294 ± 306 | 3954 ± 260 | 0.782 | 0.576 | 0.539 |
| IL-6, pg/mL | 545 ± 81 | 589 ± 114 | 949 ± 214 | 738 ± 126 | 0.136 | 0.212 | 0.062 |
| IL-10, pg/mL | 517 ± 41 | 484 ± 54 | 654 ± 83 | 542 ± 69 | 0.137 | 0.205 | 0.364 |
| IFN-γ, pg/mL | 311 ± 53 | 354 ± 68 | 769 ± 190 | 653 ± 159 | 0.045 | 0.584 | 0.385 |
| TNF-α, pg/mL | 111 ± 12 | 110 ± 11 | 139 ± 14 | 125 ± 11 | 0.217 | 0.457 | 0.552 |
| LPS | |||||||
| IL-1β, pg/mL | 265 ± 39 | 265 ± 41 | 350 ± 65 | 335 ± 61 | 0.216 | 0.762 | 0.924 |
| IL-6, pg/mL | 1463 ± 221 | 1310 ± 161 | 2362 ± 208 | 1439 ± 118 | 0.043 | 0.011 | 0.064 |
| IL-10, pg/mL | 274 ± 17 | 218 ± 14 | 350 ± 48 | 346 ± 35 b | 0.010 | 0.364 | 0.436 |
| IFN-γ, pg/mL | 73 ± 18 | 43 ± 9 | 81 ± 31 | 105 ± 53 | 0.462 | 0.936 | 0.251 |
| TNF-α, pg/mL | 1433 ± 180 | 1434 ± 201 | 2039 ± 169 | 2027 ± 243 | 0.036 | 0.954 | 0.847 |
| OVA | |||||||
| IL-2, pg/mL | 18 ± 0 | 18 ± 1 | 18 ± 1 | 20 ± 1 | 0.272 | 0.222 | 0.409 |
| IL-6, pg/mL | 716 ± 74 | 788 ± 87 | 1038 ± 188 | 788 ± 111 | 0.254 | 0.170 | 0.035 |
| IL-10, pg/mL | 179 ± 16 | 176 ± 21 | 277 ± 28 | 237 ± 23 | 0.012 | 0.373 | 0.224 |
| IL-13, pg/mL | 34 ± 4 | 19 ± 2 | 32 ± 2 | 18 ± 3 | 0.654 | <0.001 | 0.859 |
| TGF-β, pg/mL | 1262 ± 83 | 1257 ± 51 | 1010 ± 68 | 958 ± 47 | 0.001 | 0.687 | 0.740 |
| Variable | Control Diet | DHA Diet | P b | P c | P inter | ||
|---|---|---|---|---|---|---|---|
| Placebo Treatment (N = 6) | OVA Treatment (N = 6) | Placebo Treatment (N = 6) | OVA Treatment (N = 6) | ||||
| % of total cells | |||||||
| Total CD3+ | 20.9 ± 1.2 | 22.5 ± 1.0 | 22.1 ± 0.5 | 23.2 ± 0.9 | 0.464 | 0.010 | 0.443 |
| CD3+CD4+ (helper T cells) | 10.7 ± 0.7 | 11.6 ± 0.2 | 11.3 ± 0.4 | 11.7 ± 0.4 | 0.587 | 0.186 | 0.578 |
| CD3+CD8+ (cytotoxic T cells) | 6.5 ± 0.2 | 7.3 ± 0.5 | 7.1 ± 0.3 | 8.2 ± 0.4 | 0.118 | 0.003 | 0.800 |
| % of CD3+ cells that also express CD4+ | 51.1 ± 0.5 | 49.7 ± 2.1 | 50.6 ± 0.3 | 45.9 ± 2.1 | 0.194 | 0.068 | 0.298 |
| % of CD3+ cells that also express CD8+ | 30.5 ± 1.1 | 33.0 ± 0.5 | 30.8 ± 0.9 | 34.5 ± 1.0 | 0.354 | 0.004 | 0.510 |
| Ratio CD4/CD8 | 2.1 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.1 | 1.8 ± 0.0 | 0.419 | 0.079 | 0.757 |
| Total CD28+ | 37.1 ± 1.9 | 39.4 ± 1.9 | 38.9 ± 2.4 | 44.4 ± 2.7 | 0.190 | 0.016 | 0.586 |
| % of CD3+ cells that also express CD8+ CD28+ | 31.2 ± 1.3 | 34.1 ± 1.0 | 29.7 ± 0.7 | 34.7 ± 0.9 | 0.696 | 0.002 | 0.323 |
| Total OX62+ | 9.2 ± 0.5 | 10.6 ± 0.3 | 9.0 ± 0.5 | 11.4 ± 1.3 | 0.676 | 0.020 | 0.508 |
| CD3+OX62+ | 4.6 ± 0.4 | 5.8 ± 0.3 | 5.0 ± 0.2 | 5.3 ± 0.2 | 0.879 | 0.004 | 0.128 |
| CD3+CD4+CD25+FoxP3+ (Treg) | 8.9 ± 1.1 | 9.3 ± 0.7 | 8.3 ± 0.7 | 7.8 ± 0.5 | 0.178 | 0.940 | 0.427 |
| Total CD27+ | 42.7 ± 0.8 | 44.1 ± 0.7 | 41.5 ± 1.4 | 42.7 ± 1.2 | 0.299 | 0.003 | 0.104 |
| OX12+CD27+ | 15.0 ± 1.5 | 15.9 ± 1.6 | 13.4 ± 0.9 | 14.6 ± 1.1 | 0.639 | 0.108 | 0.636 |
| OX62+CD74+ (dentritic cells) | 7.3 ± 0.3 | 6.9 ± 0.4 | 6.4 ± 0.2 | 6.7 ± 0.4 | 0.120 | 0.966 | 0.281 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richard, C.; Lewis, E.D.; Goruk, S.; Field, C.J. Feeding a Diet Enriched in Docosahexaenoic Acid to Lactating Dams Improves the Tolerance Response to Egg Protein in Suckled Pups. Nutrients 2016, 8, 103. https://doi.org/10.3390/nu8020103
Richard C, Lewis ED, Goruk S, Field CJ. Feeding a Diet Enriched in Docosahexaenoic Acid to Lactating Dams Improves the Tolerance Response to Egg Protein in Suckled Pups. Nutrients. 2016; 8(2):103. https://doi.org/10.3390/nu8020103
Chicago/Turabian StyleRichard, Caroline, Erin D. Lewis, Susan Goruk, and Catherine J. Field. 2016. "Feeding a Diet Enriched in Docosahexaenoic Acid to Lactating Dams Improves the Tolerance Response to Egg Protein in Suckled Pups" Nutrients 8, no. 2: 103. https://doi.org/10.3390/nu8020103





