Novel Approaches to Investigate One-Carbon Metabolism and Related B-Vitamins in Blood Pressure
Abstract
:1. Introduction
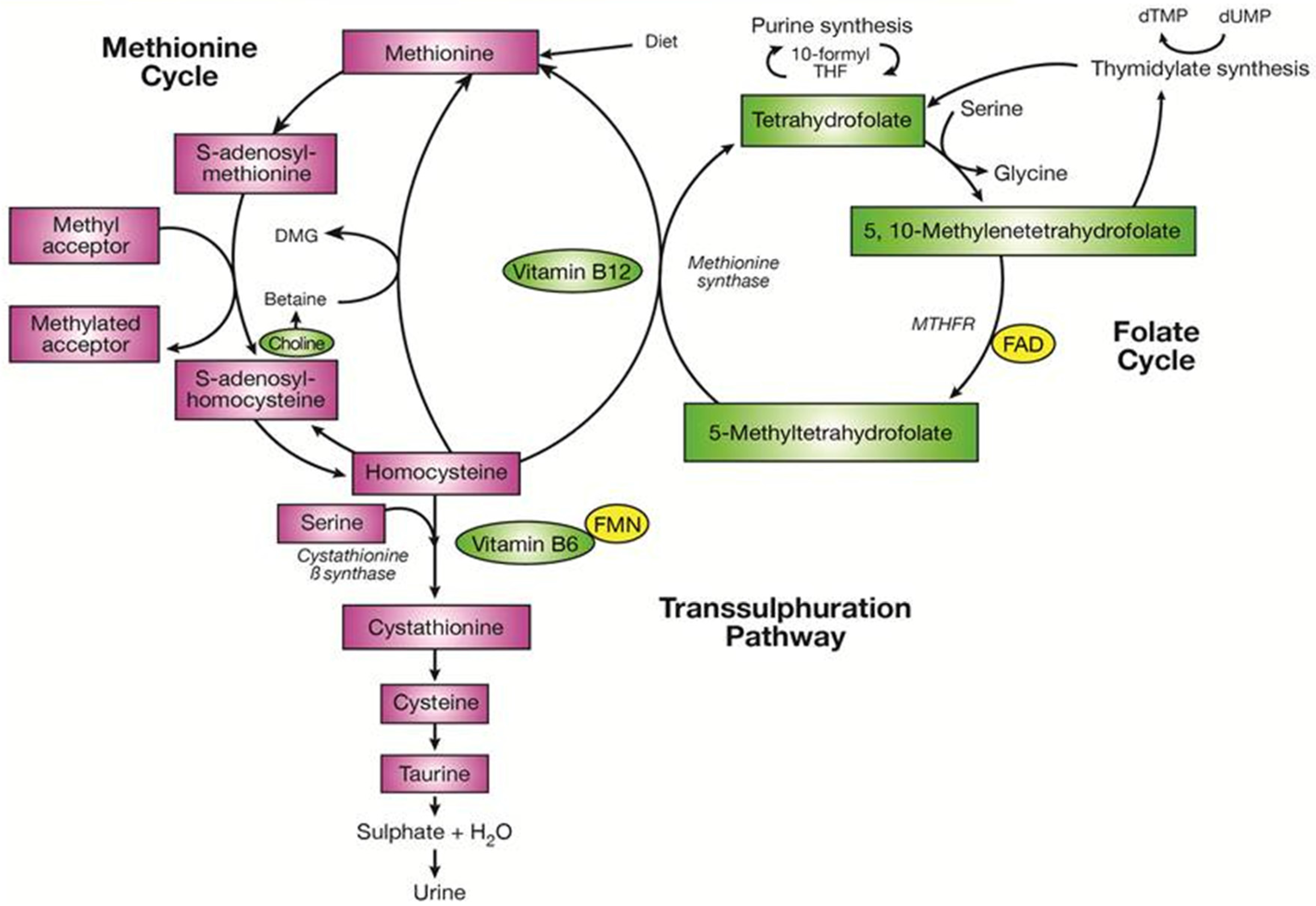
2. One-Carbon Metabolism and Related B-Vitamins
3. B-Vitamins, Cardiovascular Disease and Blood Pressure
4. Biological Mechanisms Linking Blood Pressure with One-Carbon Metabolism
5. Ambulatory Blood Pressure Monitoring
5.1. White-Coat Hypertension
5.2. Circadian Pattern of Blood Pressure
5.3. Nutrition, B-Vitamins and ABPM
5.4. Blood Pressure Lowering by B-Vitamins as Measured by ABPM
6. Conclusions and Future Work
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organisation—Global Health Observatory (GHO) Data for Raised Blood Pressure. Available online: http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence_text/en/ (accessed on 19 September 2016).
- Joffres, M.; Falaschetti, E.; Gillespie, C.; Robitaille, C.; Loustalot, F.; Poulter, N.; McAlister, F.A.; Johansen, H.; Baclic, O.; Campbell, N. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: A cross-sectional study. BMJ Open 2013, 3, e003423. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef]
- National Collaborating Centre for Chronic Conditions and the British Hypertension Society. Hypertension in Adults: Diagnosis and Management Management; Royal College of Physicians: London, UK, 2011. [Google Scholar]
- Wright, J.T.; Williamson, J.D.; Whelton, P.S.K.; Snyder, J.K.; Sink, K.M.; Rocco, M.V.; Reboussin, D.C.E.M.; Rahman, M.; Oparil, S.; Lewis, C.E.; et al. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar] [PubMed]
- Xie, X.; Atkins, E.; Lv, J.; Bennett, A.; Neal, B.; Ninomiya, T.; Woodward, M.; MacMahon, S.; Turnbull, F.; Hillis, G.S.; et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: Updated systematic review and meta-analysis. Lancet 2016, 387, 435–443. [Google Scholar] [CrossRef]
- Williamson, J.D.; Supiano, M.A.; Applegate, W.B.; Berlowitz, D.R.; Campbell, R.C.; Chertow, G.M.; Fine, L.J.; Haley, W.E.; Hawfield, A.T.; Ix, J.H.; et al. Intensive vs. standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years. JAMA 2016, 315, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Newton-Cheh, C.; Johnson, T.; Gateva, V.; Tobin, M.D.; Bochud, M.; Coin, L.; Najjar, S.S.; Zhao, J.H.; Heath, S.C.; Eyheramendy, S.A. Genome-wide association study identifies eight loci associated with blood pressure. Nat. Genet. 2009, 41, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Ehret, G.B.; Rice, K.; Verwoert, G.C.; Launer, L.J.; Dehghan, A.; Glazer, N.L.; Morrison, A.C.; Johnson, A.D.; Aspelund, T. Genome-wide association study of blood pressure and hypertension. Nat. Genet. 2009, 41, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Ehret, G.B.; Munroe, P.B.; Rice, K.M.; Bochud, M.; Johnson, A.D.; Chasman, D.I.; Smith, A.V.; Tobin, M.D.; Verwoert, G.C.; Hwang, S.-J.; et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011, 478, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flister, M.J.; Tsaih, S.W.; O’Meara, C.C.; Endres, B.; Hoffman, M.J.; Geurts, A.M.; Dwinell, M.R.; Lazar, J.; Jacob, H.J.; Moreno, C. Identifying multiple causative genes at a single GWAS locus. Genome Res. 2013, 23, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Lu, Z.; Tan, M.; Liu, H.; Lu, D. A meta-analysis of association between C677T polymorphism in the methylenetetrahydrofolate reductase gene and hypertension. Eur. J. Hum. Genet. 2007, 15, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.-Q.; You, Y.-G.; Qi, Y. Strong association of methylenetetrahydrofolate reductase gene C677T polymorphism with hypertension and hypertension-in-pregnancy in Chinese: A meta-analysis. J. Hum. Hypertens. 2012, 26, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Hu, C.Y.; Lu, S.S.; Gong, F.F.; Feng, F.; Qian, Z.Z.; Ding, X.X.; Yang, H.Y.; Sun, Y.H. Association between methylenetetrahydrofolate reductase (MTHFR) C677T/A1298C polymorphisms and essential hypertension: A systematic review and meta-analysis. Metabolism 2014, 63, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Fan, S.; Zhi, X.; Li, Y.; Liu, Y.; Wang, D.; He, M.; Hou, Y.; Zheng, Q.; Sun, G. Associations of MTHFR gene polymorphisms with hypertension and hypertension in pregnancy: A meta-analysis from 114 studies with 15411 cases and 21970 controls. PLoS ONE 2014, 9, e87497. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-M.; Jia, J.; Mao, L.-N.; Men, C.; Tang, K.-T.; Li, Y.-Y.; Ding, H.-X.; Zhan, Y.-Y. Methylenetetrahydrofolate reductase C677T gene polymorphism and essential hypertension: A meta-analysis of 10,415 subjects. Biomed. Rep. 2014, 2, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Horigan, G.; McNulty, H.; Ward, M.; Strain, J.J.; Purvis, J.; Scott, J.M. Riboflavin lowers blood pressure in cardiovascular disease patients homozygous for the 677C→T polymorphism in MTHFR. J. Hypertens. 2010, 28, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.P.; Ward, M.; McNulty, H.; Strain, J.J.; Trouton, T.G.; Horigan, G.; Purvis, J.; Scott, J.M. Riboflavin offers a targeted strategy for managing hypertension in patients with the MTHFR 677TT genotype: A 4-y follow-up. Am. J. Clin. Nutr. 2012, 95, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.P.; McNulty, H.; Ward, M.; Strain, J.J.; Trouton, T.G.; Hoeft, B.A.; Weber, P.; Roos, F.F.; Horigan, G.; McAnena, L.; et al. Blood pressure in treated hypertensive individuals with the mthfr 677tt genotype is responsive to intervention with riboflavin: Findings of a targeted randomized trial. Hypertension 2013, 61, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Strain, J.; Hughes, C.F.; Mcnulty, H.; Ward, M. Riboflavin Lowers Blood Pressure: A Review of a Novel Gene-nutrient Interaction. Nutr. Food Sci. Res. 2015, 2, 3–6. [Google Scholar]
- Verdecchia, P.; Porcellati, C.; Schillaci, G.; Borgioni, C.; Ciucci, A.; Battistelli, M.; Guerrieri, M.; Gatteschi, C.; Zampi, I.; Santucci, A.; et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension (Dallas, TX, 1979) 1994, 24, 793–801. [Google Scholar] [CrossRef]
- Hinman, A.T.; Engel, B.T.; Bickford, A.F. Portable blood pressure recorder Accuracy and preliminary use in evaluating intradaily variations in pressure. Am. Heart J. 1962, 63, 663–668. [Google Scholar] [CrossRef]
- Bailey, L.B.; Stover, P.J.; Mcnulty, H.; Fenech, M.F.; Iii, J.F.G.; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development—Folate Review. J. Nutr. 2015, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; McPartlin, J.; Goggins, M.; Weir, D.G.; Scott, J.M. Unmetabolized folic acid in serum: Acute studies in subjects consuming fortified food and supplements. Am. J. Clin. Nutr. 1997, 65, 1790–1795. [Google Scholar] [PubMed]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am. J. Clin. Nutr. 2010, 91, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Martienssen, R.; Riggs, A. Epigenetic mechanisms of gene regulation. Cold Spring Harb. Monogr. 1996, 13, 425–437. [Google Scholar]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.; den Heijer, M.; Kluijtmans, L.A.; van den Heuvel, L.P. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Ward, M.; Strain, J.J.; Hoey, L.; Dickey, W.; McNulty, H. B-vitamins and bone in health and disease: The current evidence. Proc. Nutr. Soc. 2014, 73, 330–339. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Strain, J.J.; Pentieva, K.; Ward, M. C1 metabolism and CVD outcomes in older adults. Proc. Nutr. Soc. 2012, 71, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Homocysteine Lowering Trialists. Dose-dependent effects of folic acid on blood concentrations of homocysteine: A meta-analysis of the randomized trials 1–3. Am. J. Clin. Nutr. 2005, 82, 806–812. [Google Scholar]
- Tighe, P.; Ward, M.; Mcnulty, H.; Finnegan, O.; Dunne, A.; Strain, J.J.; Molloy, A.M.; Duffy, M.; Pentieva, K.; et al. A dose-finding trial of the effect of long-term folic acid intervention: Implications for food fortification policy. Am. J. Clin. Nutr. 2011, 93, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Law, M.; Morris, J.K. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202. [Google Scholar] [CrossRef] [PubMed]
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA 2002, 288, 2015–2022. [Google Scholar]
- Wilson, C.P.; McNulty, H.; Scott, J.M.; Strain, J.J.; Ward, M. The MTHFR C677T polymorphism, B-vitamins and blood pressure. Proc. Nutr. Soc. 2010, 69, 156–165. [Google Scholar] [CrossRef] [PubMed]
- The Heart Outcomes Prevention Evaluation Investigators (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar]
- Wang, X.; Qin, X.; Demirtas, H.; Li, J.; Mao, G.; Huo, Y.; Sun, N.; Liu, L.; Xu, X. Efficacy of folic acid supplementation in stroke prevention: A meta-analysis. Lancet 2007, 369, 1876–1882. [Google Scholar] [CrossRef]
- Lee, M.; Hong, K.-S.; Chang, S.-C.; Saver, J.L. Efficacy of homocysteine-lowering therapy with folic Acid in stroke prevention: A meta-analysis. Stroke 2010, 41, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J.; Eikelboom, J.W.; Yi, Q.; Lees, K.R.; Chen, C.; Xavier, D.; Navarro, J.C.; Ranawaka, U.K.; Uddin, W.; Ricci, S.; et al. Antiplatelet therapy and the effects of B vitamins in patients with previous stroke or transient ischaemic attack: A post-hoc subanalysis of VITATOPS, a randomised, placebo-controlled trial. Lancet Neurol. 2012, 11, 512–520. [Google Scholar] [CrossRef]
- Qin, X.; Li, J.; Spence, J.D.; Zhang, Y.; Li, Y.; Wang, X.; Wang, B.; Sun, N.; Chen, F.; Guo, J.; et al. Folic Acid Therapy Reduces the First Stroke Risk Associated With Hypercholesterolemia Among Hypertensive Patients. Stroke 2016, 47, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Toole, J.F.; Malinow, M.R.; Chambless, L.E.; Spence, J.D.; Pettigrew, L.C.; Howard, V.J.; Sides, E.G.; Wang, C.-H.; Stampfer, M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004, 291, 565–575. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.A.; Skeaff, C.M.; Williams, S.M.; Green, T.J. Lowering homocysteine with B vitamins has no effect on blood pressure in older adults. J. Nutr. 2007, 137, 1183–1187. [Google Scholar] [PubMed]
- Van Dijk, R.A.J.M.; Rauwerda, J.A.; Steyn, M.; Twisk, J.W.R.; Stehouwer, C.D.A. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Sherwood, R.A.; Swift, C.G.; Jackson, S.H.D. Folic acid enhances endothelial function and reduces blood pressure in smokers: A randomized controlled trial. J. Intern. Med. 2002, 252, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.P.; Farag, N.H.; Ziegler, A.G.; Mills, P.J. Relationship of systolic blood pressure with plasma homocysteine: Importance of smoking status. J. Hypertens. 2003, 21, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Klerk, M.; Verhoef, P.; Clarke, R.; Blom, H.J.; Kok, F.J.; Schouten, E.G.; MTHFR Studies Collaboration Group. MTHFR 677C→T polymorphism and risk of coronary heart disease: A meta-analysis. JAMA 2002, 288, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.P.; Bautista, L.E.; Smeeth, L.; Sharma, P.; Hingorani, A.D. Homocysteine and stroke: Evidence on a causal link from mendelian randomisation. Lancet 2005, 365, 224–232. [Google Scholar] [CrossRef]
- Lewis, S.J.; Ebrahim, S.; Davey Smith, G. Meta-analysis of MTHFR 677C→T polymorphism and coronary heart disease: Does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ 2005, 331, 1053. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.V.; Newcombe, P.; Hubacek, J.A.; Sofat, R.; Ricketts, S.L.; Cooper, J.; Breteler, M.M.B.; Bautista, L.E.; Sharma, P.; Whittaker, J.C. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: A meta-analysis of genetic studies and randomised trials. Lancet 2011, 378, 584–594. [Google Scholar] [CrossRef] [Green Version]
- Clarke, R.; Bennett, D.A.; Parish, S.; Verhoef, P.; Dötsch-Klerk, M.; Lathrop, M.; Xu, P.; Nordestgaard, B.G.; Holm, H.; Hopewell, J.C.; et al. MTHFR Studies Collaborative Group Homocysteine and coronary heart disease: Meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012, 9, e1001177. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Dowey, L.R.C.; Strain, J.J.; Dunne, A.; Ward, M.; Molloy, A.M.; McAnena, L.B.; Hughes, J.P.; Hannon-Fletcher, M.; Scott, J.M. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C→T polymorphism. Circulation 2006, 113, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R.; Hopkins, S.; Ward, M.; McNulty, H.; McCann, A.; McNulty, B.; Walton, J.; Molloy, A.; Strain, J.J.; Flynn, A.; et al. MTHFR 677TT genotype and related B-vitamins: Novel determinants of hypertension in healthy Irish adults. J. Inherit. Metab. Dis. 2013, 36, 35. [Google Scholar]
- Wilcken, B.; Bamforth, F.; Li, Z.; Zhu, H.; Ritvanen, A.; Renlund, M.; Stoll, C.; Alembik, Y.; Dott, B.; Czeizel, A.E.; et al. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): Findings from over 7000 newborns from 16 areas world wide. J. Med. Genet. 2003, 40, 619–625. [Google Scholar] [CrossRef] [PubMed]
- McAuley, E.; McNulty, H.; Hughes, C.; Strain, J.J.; Ward, M. Riboflavin status, MTHFR genotype and blood pressure: Current evidence and implications for personalised nutrition. Proc. Nutr. Soc. 2016, 75, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Guenther, B.D.; Sheppard, C.A.; Tran, P.; Rozen, R.; Matthews, R.G.; Ludwig, M.L. The structure and properties of methylenetetrahydrofolate reductase from Escherichia coli suggest how folate ameliorates human hyperhomocysteinemia. Nat. Struct. Biol. 1999, 6, 359–365. [Google Scholar] [PubMed]
- Yamada, K.; Chen, Z.; Rozen, R.; Matthews, R.G. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc. Natl. Acad. Sci. USA 2001, 98, 14853–14858. [Google Scholar] [CrossRef] [PubMed]
- Bagley, P.J.; Selhub, J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc. Natl. Acad. Sci. USA 1998, 95, 13217–13220. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Hai, A.; Ahmed, A.; Kizilbash, N.; Alruwaili, J. A structured-based model for the decreased activity of Ala222Val and Glu429Ala methylenetetrahydrofolate reductase (MTHFR) mutants. Bioinformation 2013, 9, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Shirodaria, C.; Warrick, N.; Cai, S.; De Bono, J.; Lee, J.; Leeson, P.; Neubauer, S.; Ratnatunga, C.; Pillai, R.; et al. 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: Effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 2006, 114, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Shirodaria, C.; Leeson, P.; Baarholm, O.A.; Van-Assche, T.; Cunnington, C.; Pillai, R.; Ratnatunga, C.; Tousoulis, D.; Stefanadis, C.; et al. MTHFR 677C>T polymorphism reveals functional importance for 5-methyltetrahydrofolate, not homocysteine, in regulation of vascular redox state and endothelial function in human atherosclerosis. Circulation 2009, 119, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P.; Hoad, G.; Campbell, D.M.; Horgan, G.W.; Piyathilake, C.; McNeill, G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am. J. Clin. Nutr. 2013, 97, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, N.; Leclerc, D.; Gayden, T.; Lazaris, A.; De Jay, N.; Petrillo, S.; Metrakos, P.; Jabado, N.; Rozen, R. Murine diet/tissue and human brain tumorigenesis alter Mthfr/MTHFR 5’-end methylation. Mamm. Genome 2016, 27, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Friso, S.; Choi, S.-W.; Girelli, D.; Mason, J.B.; Dolnikowski, G.G.; Bagley, P.J.; Olivieri, O.; Jacques, P.F.; Rosenberg, I.H.; Corrocher, R.; et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. USA 2002, 99, 5606–5611. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shangguan, S.; Chang, S.; Yu, X.; Wang, Z.; Lu, X.; Wu, L.; Zhang, T. Determining the association between methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and genomic DNA methylation level: A meta-analysis. Birth Defects Res. A Clin. Mol. Teratol. 2016, 106, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lu, Q.; Wang, T. Effect of MTHFR gene polymorphism impact on atherosclerosis via genome-wide methylation. Med. Sci. Monit. 2016, 22, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, J.; Zhang, F.; Diao, B.; Song, Z.F.; Shan, L.L.; Wang, W.; Cao, H.J.; Li, X.Q. Correlation between MTHFR gene methylation and pre-eclampsia, and its clinical significance. Genet. Mol. Res. 2015, 14, 8021–8028. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, S.; Fiorito, G.; Onland-Moret, N.C.; Russo, A.; Agnoli, C.; Allione, A.; Di Gaetano, C.; Mattiello, A.; Ricceri, F.; Chiodini, P. Gene-specific DNA methylation profiles and LINE-1 hypomethylation are associated with myocardial infarction risk. Clin. Epigenet. 2015, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, I.; Herrmann, M.; Kirsch, S.H.; Werner, C.; Hübner, U.; Bodis, M.; Laufs, U.; Wagenpfeil, S.; Geisel, J.; Herrmann, W. Prospective study of telomere length and LINE-1 methylation in peripheral blood cells: The role of B vitamins supplementation. Eur. J. Nutr. 2016, 55, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.L.; De Buyzere, M.L.; De Bacquer, D.A.; de Leeuw, P.W.; Duprez, D.A.; Fagard, R.H.; Gheeraert, P.J.; Missault, L.H.; Braun, J.J.; Six, R.O.; et al. Prognostic Value of Ambulatory Blood-Pressure Recordings in Patients with Treated Hypertension. N. Engl. J. Med. 2003, 348, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Smolensky, M.H.; Ayala, D.E.; Portaluppi, F.; Crespo, J.J.; Fabbian, F.; Haus, E.; Manfredini, R.; Mojón, A.; Moyá, A.; et al. 2013 Ambulatory blood pressure monitoring recommendations for the diagnosis of adult hypertension, assessment of cardiovascular and other hypertension-associated risk, and attainment of therapeutic goals. Chronobiol. Int. 2013, 30, 355–410. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Parati, G.; Stergiou, G.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J. Hypertens. 2013, 31, 1731–1768. [Google Scholar] [CrossRef] [PubMed]
- Solak, Y.; Kario, K.; Covic, A.; Bertelsen, N.; Afsar, B.; Ozkok, A.; Wiecek, A.; Kanbay, M. Clinical value of ambulatory blood pressure: Is it time to recommend for all patients with hypertension? Clin. Exp. Nephrol. 2016, 20, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Stanton, A.V.; Thom, S.; Caulfield, M.; Atkins, N.; McInnes, G.; Collier, D.; Dicker, P.; O’Brien, E. Ambulatory blood pressure monitoring predicts cardiovascular events in treated hypertensive patients—An Anglo-Scandinavian cardiac outcomes trial substudy. J. Hypertens. 2009, 27, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Stanton, A.; Thijs, L.; Hinedi, K.; Atkins, N.; McClory, S.; Den Hond, E.; McCormack, P.; Staessen, J.A.; O’Brien, E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin outcome study. Hypertension 2005, 46, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Stampfer, M.J.; Rosner, B.; Hennekens, C.H.; Gaziano, J.M.; Manson, J.E.; Glynn, R.J. Systolic and Diastolic Blood Pressure, Pulse Pressure, and Mean Arterial Pressure as Predictors of Cardiovascular Disease Risk in Men. Hypertension 2000, 36, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Giles, W.H.; Greenlund, K.J. Blood pressure parameters and risk of fatal stroke, NHANES II mortality study. Am. J. Hypertens. 2007, 20, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Schillaci, G.; Reboldi, G.; Franklin, S.S.; Porcellati, C. Different prognostic impact of 24-hour mean blood pressure and pulse pressure on stroke and coronary artery disease in essential hypertension. Circulation 2001, 103, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the management of arterial hypertension. Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef] [PubMed]
- Morcet, J.F.; Safar, M.; Thomas, F.; Guize, L.; Benetos, A. Associations between heart rate and other risk factors in a large French population. J. Hypertens. 1999, 17, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Perret-Guillaume, C.; Joly, L.; Benetos, A. Heart rate as a risk factor for cardiovascular disease. Prog. Cardiovasc. Dis. 2009, 52, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Rudnichi, A.; Thomas, F.; Safar, M.; Guize, L. Influence of Heart Rate on Mortality in a French Population. Hypertension 1999, 33, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Reule, S.; Drawz, P.E. Heart rate and blood pressure: Any possible implications for management of hypertension? Curr. Hypertens. Rep. 2012, 14, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Pickering, T.G.; James, G.D.; Boddie, C.; Harshfield, G.A.; Blank, S.; Laragh, J.H. How common is white coat hypertension? JAMA 1988, 259, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Thijs, L.; Hansen, T.W.; O’Brien, E.; Staessen, J.A. White-coat hypertension: New insights from recent studies. Hypertension 2013, 62, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Mani, A. White-Coat Hypertension: A True Cardiovascular Risk? J. Clin. Hypertens. 2016, 18, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, S.D.; Cuccurullo, F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: An updated meta analysis. Am. J. Hypertens. 2011, 24, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Tientcheu, D.; Ayers, C.; Das, S.R.; McGuire, D.K.; de Lemos, J.A.; Khera, A.; Kaplan, N.; Victor, R.; Vongpatanasin, W. Target organ complications and cardiovascular events associated with masked hypertension and white-coat hypertension: Analysis from the dallas heart study. J. Am. Coll. Cardiol. 2015, 66, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Gustavsen, P.H.; Høegholm, A.; Bang, L.E.; Kristensen, K.S. White coat hypertension is a cardiovascular risk factor: A 10-year follow-up study. J. Hum. Hypertens. 2003, 17, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Androulakis, E.; Palla, M.; Papageorgiou, N.; Tousoulis, D. White-coat hypertension and cardiovascular events: A meta-analysis. J. Hypertens. 2016, 34, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, W.; Stolarz-Skrzypek, K.; Olszanecka, A.; Klima, Ł.; Gąsowski, J.; Grodzicki, T.; Kawecka-Jaszcz, K.; Czarnecka, D. Subclinical arterial and cardiac damage in white-coat and masked hypertension. Blood Press. 2016, 25, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Morrell, C.H.; Orru’, M.; AlGhatrif, M.; Saba, P.S.; Terracciano, A.; Ferreli, L.A.P.; Loi, F.; Marongiu, M.; Pilia, M.G.; et al. Gender specific profiles of white coat and masked hypertension impacts on arterial structure and function in the SardiNIA study. Int. J. Cardiol. 2016, 217, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ 2016, 354, i4098. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; O’Brien, E.; Thijs, L.; Asayama, K.; Staessen, J.A. Masked hypertension a phenomenon of measurement. Hypertension 2015, 65, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, A.A.; Sarafidis, P.A.; Ruilope, L.M. Ambulatory blood pressure monitoring in the diagnosis, prognosis, and management of resistant hypertension: Still a matter of our resistance? Curr. Hypertens. Rep. 2015, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Sheridan, J.; O’Malley, K. Dippers and non-dippers. Lancet 1988, 332, 397. [Google Scholar] [CrossRef]
- Mezue, K.; Isiguzo, G.; Madu, C.; Nwuruku, G.; Rangaswami, J.; Baugh, D.; Madu, E. Nocturnal non-dipping blood pressure profile in black normotensives is associated with cardiac target organ damage. Ethn. Dis. 2016, 26, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, T.; Hozawa, A.; Yamaguchi, J.; Kikuya, M.; Ohmori, K.; Michimata, M.; Matsubara, M.; Hashimoto, J.; Hoshi, H.; Araki, T.; et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: The Ohasama study. J. Hypertens. 2002, 20, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Angeli, F.; Mazzotta, G.; Garofoli, M.; Ramundo, E.; Gentile, G.; Ambrosio, G.; Reboldi, G. Day-night dip and early-morning surge in blood pressure in hypertension: Prognostic implications. Hypertension 2012, 60, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R.H.; Thijs, L.; Staessen, J.A.; Clement, D.L.; De Buyzere, M.L.; De Bacquer, D.A. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J. Hum. Hypertens. 2009, 23, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Hoshide, S.; Ishikawa, J.; Pickering, T.G.; Schwartz, J.E.; Shimada, K.; Kario, K. Nocturnal nondipping of heart rate predicts cardiovascular events in hypertensive patients. J. Hypertens. 2009, 27, 2265–2270. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Pickering, T.G.; Matsuo, T.; Hoshide, S.; Schwartz, J.E.; Shimada, K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001, 38, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E.; Mojón, A.; Fernández, J.R. Blunted sleep-time relative blood pressure decline increases cardiovascular risk independent of blood pressure level—The “normotensive non-dipper” paradox. Chronobiol. Int. 2013, 30, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.F.; Reboldi, G.; Fagard, R.H.; Cardoso, C.R.L.; Pierdomenico, S.D.; Verdecchia, P.; Eguchi, K.; Kario, K.; Hoshide, S.; Polonia, J.; et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: The ambulatory blood pressure collaboration in patients with hypertension (ABC-H) meta-analysis. Hypertension 2016, 67, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, S.D.; Pierdomenico, A.M.; Di Tommaso, R.; Coccina, F.; Di Carlo, S.; Porreca, E.; Cuccurullo, F. Morning blood pressure surge, dipping, and risk of coronary events in elderly treated hypertensive patients. Am. J. Hypertens. 2016, 29, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.C.; Yan, H.; Zhao, Y.X.; Liu, X.Y. Prognostic value of morning blood pressure surge in clinical events: A meta-analysis of longitudinal studies. J. Stroke Cerebrovasc. Dis. 2015, 24, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.W.; Li, Y.; Boggia, J.; Thijs, L.; Richart, T.; Staessen, J.A. Predictive role of the nighttime blood pressure. Hypertension 2011, 57, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Quarti-Trevano, F.; Dell’Oro, R.; Bombelli, M.; Cuspidi, C.; Facchetti, R.; Bolla, G.; Mancia, G. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension 2008, 52, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, D.; Miceli, G.; Casuccio, A.; Tuttolomondo, A.; Buttà, C.; Zappulla, V.; Schimmenti, C.; Musiari, G.; Pinto, A. Does sympathetic overactivation feature all hypertensives? Differences of sympathovagal balance according to night/day blood pressure ratio in patients with essential hypertension. Hypertens. Res. 2016, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.B.J.; Chen, C.-Y.; Wang, Y.P.; Lan, Y.-Y.; Mak, K.-H.; Lee, G.-S.; Yang, C.C.H. The role of autonomic and baroreceptor reflex control in blood pressure dipping and nondipping in rats. J. Hypertens. 2014, 32, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Wajea-Andreassen, U.; Fromm, A.; Øygarden, H.; Naess, H.; Gerdts, E. Blunted nightly blood pressure reduction is associated with increased arterial stiffness in ischemic stroke patients: A Norwegian stroke in the young study. J. Hypertens. 2015, 33, e41. [Google Scholar] [CrossRef] [PubMed]
- Fontes-Guerra, P.C.A.; Cardoso, C.R.L.; Muxfeldt, E.S.; Salles, G.F. Nitroglycerin-mediated, but not flow-mediated vasodilation, is associated with blunted nocturnal blood pressure fall in patients with resistant hypertension. J. Hypertens. 2015, 33, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Angeli, F.; Borgioni, C.; Gattobigio, R.; Reboldi, G. Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension 2007, 49, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Monte, M.; Cambão, M.; Mesquita Bastos, J.; Polónia, J. Reproducibility of ambulatory blood pressure values and circadian blood pressure patterns in untreated subjects in a 1–11 month interval. Rev. Port. Cardiol. 2015, 34, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Keren, S.; Leibowitz, A.; Grossman, E.; Sharabi, Y. Limited reproducibility of 24-h ambulatory blood pressure monitoring. Clin. Exp. Hypertens. 2015, 37, 599–603. [Google Scholar] [PubMed]
- Hermida, R.C.; Ayala, D.E.; Fernández, J.R.; Calvo, C. Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension 2008, 51, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E.; Mojón, A.; Fernández, J.R. Influence of circadian time of hypertension treatment on cardiovascular risk: Results of the MAPEC study. Chronobiol. Int. 2010, 27, 1629–1651. [Google Scholar] [CrossRef] [PubMed]
- Gould, B.A.; Mann, S.; Davies, A.B.; Altman, D.G.; Raftery, E.B. Does placebo lower blood-pressure? Lancet 1981, 2, 1377–1381. [Google Scholar] [CrossRef]
- Mancia, G.; Omboni, S.; Parati, G.; Ravogli, A.; Villani, A.; Zanchetti, A. Lack of placebo effect on ambulatory blood pressure. Am. J. Hypertens. 1995, 8, 311–315. [Google Scholar] [CrossRef]
- Kotsis, V.; Stabouli, S.; Bouldin, M.; Low, A.; Toumanidis, S.; Zakopoulos, N. Impact of obesity on 24-hour ambulatory blood pressure and hypertension. Hypertension 2005, 45, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Meani, S.; Valerio, C.; Negri, F.; Sala, C.; Maisaidi, M.; Giudici, V.; Zanchetti, A.; Mancia, G. Body mass index, nocturnal fall in blood pressure and organ damage in untreated essential hypertensive patients. Blood Press. Monit. 2008, 13, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Günay, T.; Özmen, G.; Melek, M. Relationship between vitamin D deficiency and nondipper hypertension. Clin. Exp. Hypertens. 2013, 35, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Sen, F.; Ozeke, O.; Temizhan, A.; Topaloglu, S.; Aras, D.; Aydogdu, S. The relationship between vitamin D levels and nondipper hypertension. Blood Press. Monit. 2015, 20, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Isik, B.; Akcay, A.; Ozkara, A.; Karakurt, F.; Turgut, F.; Alkan, R.; Uz, E.; Bavbek, N.; Yigitoglu, R.; et al. Relation between serum calcium, phosphate, parathyroid hormone and “nondipper” circadian blood pressure variability profile in patients with normal renal function. Am. J. Nephrol. 2007, 27, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.J.; Vollmer, W.M.; Appel, L.J.; Sacks, F.M.; Svetkey, L.P.; Vogt, T.M.; Conlin, P.R.; Simons-Morton, D.G.; Carter-Edwards, L.; Harsha, D.W. Effect of dietary patterns on ambulatory blood pressure: Results from the dietary approaches to stop hypertension (DASH) trial. DASH collaborative research group. Hypertension 1999, 34, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; Erlinger, T.P.; Young, D.R.; Jehn, M.; Charleston, J.; Rhodes, D.; Wasan, S.K.; Appel, L.J. Results of the diet, exercise, and weight loss intervention trial (DEW-IT). Hypertension 2002, 40, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Paula, T.P.; Viana, L.V.; Neto, A.T.Z.; Leitão, C.B.; Gross, J.L.; Azevedo, M.J. Effects of the DASH diet and walking on blood pressure in patients with type 2 diabetes and uncontrolled hypertension: A randomized controlled trial. J. Clin. Hypertens. 2015, 17, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Chartier, J.-P.; Gigleux, I.; Tremblay, A.J.; Poirier, L.; Lamarche, B.; Couture, P. Impact of dairy consumption on essential hypertension: A clinical study. Nutr. J. 2014, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Machin, D.R.; Park, W.; Alkatan, M.; Mouton, M.; Tanaka, H. Hypotensive effects of solitary addition of conventional nonfat dairy products to the routine diet: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Goulding, M.G.; Nguyen, A.; Malaver, T.; Walker, C.F.; George, T.W.; Methven, L.; Lovegrove, J.A. Acute ingestion of beetroot bread increases endothelium-independent vasodilation and lowers diastolic blood pressure in healthy men: A randomized controlled trial. J. Nutr. 2013, 143, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Jajja, A.; Sutyarjoko, A.; Lara, J.; Rennie, K.; Brandt, K.; Qadir, O.; Siervo, M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014, 34, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.T.; Clifton, P.M. Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: A randomized, placebo-controlled trial. Nutr. J. 2012, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Mose, F.H.; Bech, J.N.; Hansen, A.B.; Pedersen, E.B. Effect of cholecalciferol supplementation during winter months in patients with hypertension: A randomized, placebo-controlled trial. Am. J. Hypertens. 2012, 25, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grübler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; Ó Hartaigh, B.; Obermayer-Pietsch, B.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 14, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Sauder, K.A.; McCrea, C.E.; Ulbrecht, J.S.; Kris-Etherton, P.M.; West, S.G. Pistachio nut consumption modifies systemic hemodynamics, increases heart rate variability, and reduces ambulatory blood pressure in well-controlled type 2 diabetes: A randomized trial. J. Am. Heart Assoc. 2014, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Cannoletta, M.; Xholli, A.; Piacenti, I.; Palma, F.; Palmieri, B. Folate administration decreases oxidative status and blood pressure in postmenopausal women. Eur. J. Nutr. 2015, 54, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Cannoletta, M.; Volpe, A. High-dose short-term folate administration modifies ambulatory blood pressure in postmenopausal women. A placebo-controlled study. Eur. J. Clin. Nutr. 2009, 63, 1266–1268. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Kingwell, B.A.; Burke, K.; McPherson, J.; Dart, A.M. Folic acid supplementation for 3 weeks reduces pulse pressure and large artery stiffness independent of MTHFR genotype. Am. J. Clin. Nutr. 2005, 82, 26–31. [Google Scholar] [PubMed]
| CLINIC BP | ABPM |
|---|---|
|
|
|
|
|
| Author | Sample Size (n) | Populations | ABPM 24-h Profile | Odds Ratio (95% CI) for Cardiovascular Events |
|---|---|---|---|---|
| Salles et al. [106] | 17,312 hypertensives | South America, Europe and Asia | Non-dippers vs. dippers * | 1.40 (1.20–1.63) |
| Xie et al. [108] | 14,133 hypertensives | Europe, South America and Asia | Morning surge | 1.24 (0.60–2.53) |
| Hansen et al. [109] | 23,856 hypertensives | Europe, South America and Asia | Non-dippers vs. dippers * | 1.25 (1.02–1.52) |
| 9641 general population | 1.15 (1.00–1.33) | |||
| Fagard et al. [102] | 3468 hypertensives | Europe | Reverse dippers vs. other dipping patterns | 1.49 (1.17–1.91) |
| Author | Population | Sample Size | Average Systolic BP Change (mmHg) | Duration of Intervention | ABPM Parameters Reported | |||
|---|---|---|---|---|---|---|---|---|
| Dipping Status | Mean Arterial Pressure | Heart Rate | Pulse Pressure | |||||
| DASH Diet Interventions | ||||||||
| Moore et al. [127] | USA | 354 | −4.5 ≠ | 8 weeks | Yes | No | No | No |
| Miller et al. [128] | USA | 44 | −9.5 ≠ | 9 weeks | No | No | No | No |
| Paula et al. * [129] | Brazil | 40 | −15.0 † | 4 weeks | No | No | Yes | No |
| Dairy Product Interventions | ||||||||
| Drouin-Chartier et al. [130] | Canada | 89 | −2.0 † | 4 weeks | No | No | No | No |
| Machin et al. [131] | USA | 49 | −8.0 ≠ | 4 weeks | No | No | No | Yes |
| Beetroot Interventions | ||||||||
| Hobbs et al. [132] | Europe | 24 | - | Acute | No | Yes | Yes | Yes |
| Jajja et al. [133] | Europe | 24 | - | 3 weeks | No | No | Yes | No |
| Kapil et al. [134] | Europe | 68 | −7.7 ≠ | 4 weeks | No | No | Yes | No |
| Coles et al. [135] | Australia | 30 | −4.6 † | Acute | No | No | Yes | Yes |
| Vitamin D Intervention | ||||||||
| Larsen et al. [136] | Europe | 112 | −3 ≠ | 20 weeks | Yes | No | Yes | No |
| Pilz et al. [137] | Europe | 200 | - | 8 weeks | No | No | No | No |
| Other | ||||||||
| Brull et al. [138] | Europe | 70 | −3.6 ≠ | 6 weeks | Yes | Yes | Yes | No |
| Sauder et al. * [139] | USA | 30 | −3.5 ≠ | 4 weeks | Yes | No | Yes | No |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMahon, A.; McNulty, H.; Hughes, C.F.; Strain, J.J.; Ward, M. Novel Approaches to Investigate One-Carbon Metabolism and Related B-Vitamins in Blood Pressure. Nutrients 2016, 8, 720. https://doi.org/10.3390/nu8110720
McMahon A, McNulty H, Hughes CF, Strain JJ, Ward M. Novel Approaches to Investigate One-Carbon Metabolism and Related B-Vitamins in Blood Pressure. Nutrients. 2016; 8(11):720. https://doi.org/10.3390/nu8110720
Chicago/Turabian StyleMcMahon, Amy, Helene McNulty, Catherine F. Hughes, J. J. Strain, and Mary Ward. 2016. "Novel Approaches to Investigate One-Carbon Metabolism and Related B-Vitamins in Blood Pressure" Nutrients 8, no. 11: 720. https://doi.org/10.3390/nu8110720





