Leptin Levels Are Higher in Whole Compared to Skim Human Milk, Supporting a Cellular Contribution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Measurement of Leptin in Whole and Skim Human Milk by an Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Measurement of Fat Content in Human Milk
2.4. Statistical Analyses
3. Results
3.1. Participants
3.2. Leptin Optimisation
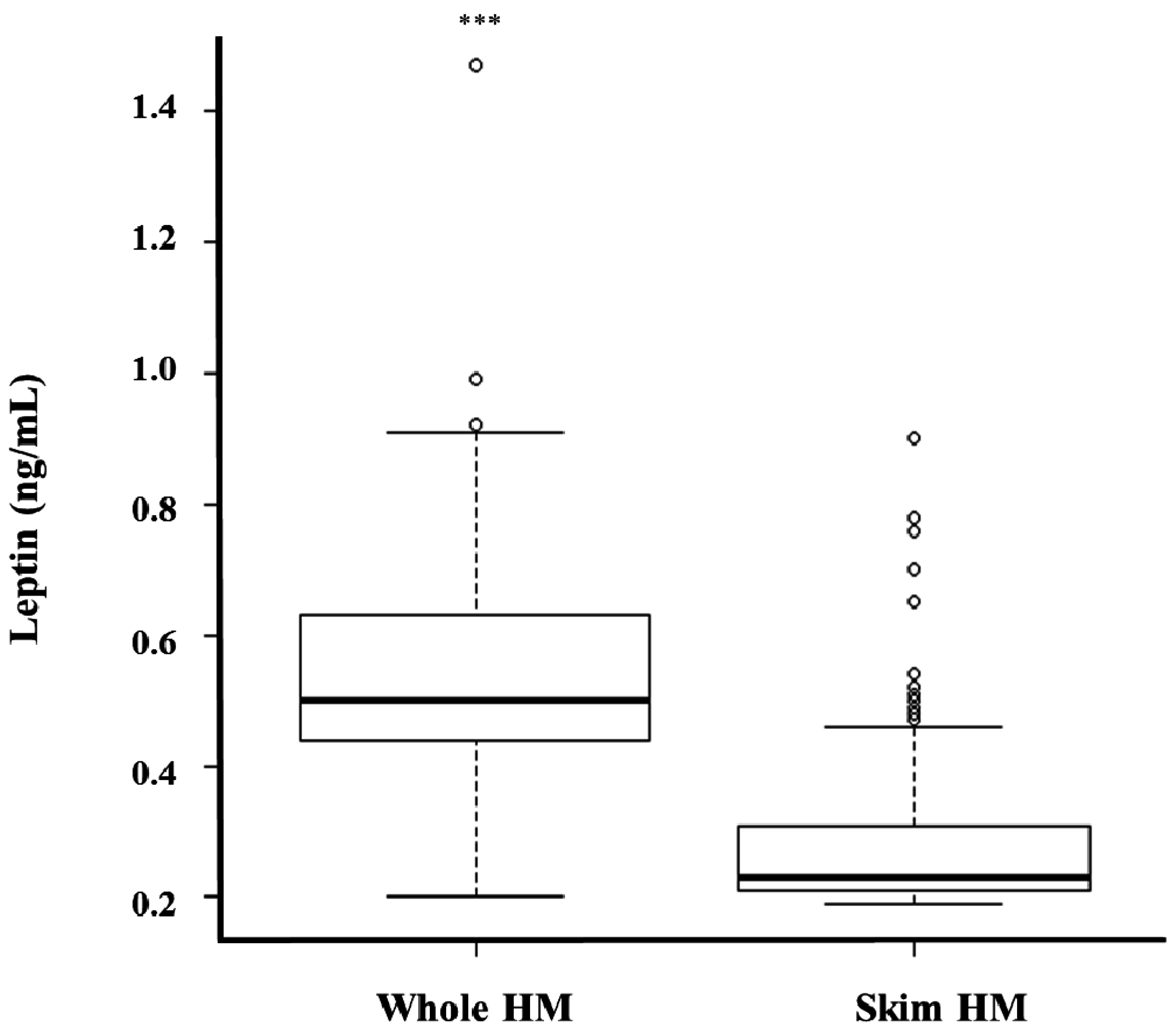
3.3. Whole and Skim Human Milk Leptin
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HM | Human milk |
| ELISA | Enzyme linked immunosorbent assay |
| RIA | Radioimmunoassay |
| PBS | Phosphate-buffered solution |
| BMI | Body mass index |
| SD | Standard deviation |
| SEM | Standard error of the mean |
References
- Hassiotou, F.; Geddes, D.T.; Hartmann, P.E. Cells in Human Milk: State of the Science. J. Hum. Lact. 2013, 29, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, M. Bioactive Factors in Human Milk. Pediatr. Clin. N. Am. 2001, 48, 69–86. [Google Scholar] [CrossRef]
- Hassiotou, F.; Geddes, D.T. Programming of Appetite Control during Breastfeeding as a Preventative Strategy against the Obesity Epidemic. J. Hum. Lact. 2014, 30, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Von Kries, R.; Koletzko, B.; Sauerwald, T.; von Mutius, E.; Barnert, D.; Grunert, V.; von Voss, H. Breast feeding and obesity: cross sectional study. BMJ 1999, 319, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W.; Rifas-Shiman, S.L.; Camargo, C.A., Jr.; Berkey, C.S.; Frazier, A.L.; Rockett, H.R.; Field, A.E.; Colditz, G.A. Risk of overweight among adolescents who were breastfed as infants. JAMA 2001, 285, 2461–2467. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Reilly, J.J. Breastfeeding and lowering the risk of childhood obesity. Lancet 2002, 359, 2003–4. [Google Scholar] [CrossRef]
- Bouret, S.G.; Bates, S.H.; Chen, S.; Myers, M.G.; Simerly, R.B. Distinct Roles for Specific Leptin Receptor Signals in the Development of Hypothalamic Feeding Circuits. J. Neurosci. 2012, 32, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Pico, C.; Oliver, P.; Sanchez, J.; Miralles, O.; Caimari, A.; Priego, T.; Palou, A. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. Int. J. Obes. 2007, 31, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Liguori, S.A.; Petrucci, E.; Lupica, M.M.; Fissore, M.F.; Oggero, R.; Silvestro, L. Evaluation of leptin in breast milk, lactating mothers and their infants. Eur. J. Clin. Nutr. 2010, 64, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Lupica, M.M.; Benetti, S.; Petrucci, E.; Liguori, S.A.; Cordero Di Montezemolo, L. Adiponectin in breast milk: Relation to serum adiponectin concentration in lactating mothers and their infants. Acta Paediatr. 2012, 101, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Sorrenti, M.; Benetti, S.; Lupica, M.M.; Liguori, S.A.; Oggero, R. Resistin and leptin in breast milk and infants in early life. Early Hum. Dev. 2012, 88, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M. Proteins in human milk. Breastfeed. Rev. 2007, 15, 5–16. [Google Scholar]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Bassi, M.; Furuya, W.I.; Zoccal, D.B.; Menani, J.V.; Colombari, E.; Hall, J.E.; do Carmo, J.M.; Colombari, D.S. Control of respiratory and cardiovascular functions by leptin. Life Sci. 2015, 125, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Magariños, M.P.; Sánchez-Margalet, V.; Kotler, M.; Calvo, J.C.; Varone, C.L. Leptin Promotes Cell Proliferation and Survival of Trophoblastic Cells. Biol. Reprod. 2007, 76, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Coppack, S.W.; Mohamed-Ali, V.; Landt, M. Adipose Tissue Leptin Production and Plasma Leptin Kinetics in Humans. Diabetes 1996, 45, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog. Horm. Res. 2004, 59, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Mix, H.; Widjaja, A.; Jandl, O.; Cornberg, M.; Kaul, A.; Goke, M.; Beil, W.; Kuske, M.; Brabant, G.; Manns, M.P.; et al. Expression of leptin and leptin receptor isoforms in the human stomach. Gut 2000, 47, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Smith-Kirwin, S.M.; O’Connor, D.M.; De Johnston, J.; Lancey, E.D.; Hassink, S.G.; Funanage, V.L. Leptin expression in human mammary epithelial cells and breast milk. J. Clin. Endocrinol. Metab. 1998, 83, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Savigni, D.; Hartmann, P.; Geddes, D. Mammary cells synthesize appetite hormones that may contribute to breastmilk. In Proceedings of the Bi-annual Conference of International Society for Research in Human Milk and Lactation, Charleston, SC, USA, 27 October 2014.
- Oliver, P.; Pico, C.; De Matteis, R.; Cinti, S.; Palou, A. Perinatal expression of leptin in rat stomach. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2002, 223, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Proulx, K.; Richard, D.; Walker, C.D. Leptin regulates appetite-related neuropeptides in the hypothalamus of developing rats without affecting food intake. Endocrinology 2002, 143, 4683–4692. [Google Scholar] [CrossRef] [PubMed]
- Miralles, O.; Sanchez, J.; Palou, A.; Pico, C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver Spring, MD) 2006, 14, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Weyermann, M.; Beermann, C.; Brenner, H.; Dietrich, R. Adiponectin and Leptin in Maternal Serum, Cord Blood, and Breast Milk. Clin. Chem. 2006, 52, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Hechler, C.; Gebauer, C.; Kiess, W.; Kratzsch, J. Leptin in maternal serum and breast milk: association with infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatr. Res. 2011, 70, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Kline, A.D.; Becker, G.W.; Churgay, L.M.; Landen, B.E.; Martin, D.K.; Muth, W.L.; Rathnachalam, R.; Richardson, J.M.; Schoner, B.; Ulmer, M.; et al. Leptin is a four-helix bundle: Secondary structure by NMR. FEBS Lett. 1997, 407, 239–242. [Google Scholar] [CrossRef]
- Xie, X.; Steiner, S.H.; Bickel, M.H. Kinetics of distribution and adipose tissue storage as a function of lipophilicity and chemical structure. II. Benzodiazepines. Drug Metab. Dispos. 1991, 19, 15–19. [Google Scholar] [PubMed]
- Hassiotou, F.; Hartmann, P.E. At the Dawn of a New Discovery: The Potential of Breast Milk Stem Cells. Adv. Nutr. Int. Rev. J. 2014, 5, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Houseknecht, K.L.; McGuire, M.K.; Portocarrero, C.P.; McGuire, M.A.; Beerman, K. Leptin Is Present in Human Milk and Is Related to Maternal Plasma Leptin Concentration and Adiposity. Biochem. Biophys. Res. Commun. 1997, 240, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Resto, M.; O’Connor, D.; Leef, K.; Funanage, V.; Spear, M.; Locke, R. Leptin Levels in Preterm Human Breast Milk and Infant Formula. Pediatrics 2001, 108, e15. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Mok, H.Y.I.; Zech, L.; Steinberg, D.; Berman, M. Transport of Very Low Density Lipoprotein Triglycerides in Varying Degrees of Obesity and Hypertriglyceridemia. J. Clin. Investig. 1979, 63, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Beltran, A.; Chetwynd, E.; Stuebe, A.M.; Twigger, A.J.; Metzger, P.; Trengove, N.; Lai, C.T.; Filgueira, L.; Blancafort, P.; et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells 2012, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.; Kakulas, F.; Hepworth, A.; Lai, C.; Hartmann, P.; Geddes, D. The Effects of Leptin on Breastfeeding Behaviour. Int. J. Environ. Res. Public Health 2015, 12, 12340. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in Fat, Lactose, and Protein Composition in Breast Milk over 24 Hours: Associations with Infant Feeding Patterns. J. Hum. Lact. 2013, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Simmer, K.; Hartmann, P.E. A method for standardizing the fat content of human milk for use in the neonatal intensive care unit. Int. Breastfeed. J. 2009, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Hepworth, A.R.; Williams, T.M.; Twigger, A.-J.; Perrella, S.; Lai, C.T.; Filgueira, L.; Geddes, D.T.; Hartmann, P.E. Breastmilk Cell and Fat Contents Respond Similarly to Removal of Breastmilk by the Infant. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Linear and Nonlinear Mixed Effects Models; Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- World Health Organization. Birth to 24 Months: Boys Head Circumference-for-Age and Weight-for-Length Percentiles; Centres for Disease Control and Prevention: Atlanta, GA, USA, 2009. [Google Scholar]
- World Health Organization. Birth to 24 Months: Girls Head Circumference-for-Age and Weight-for-Length Percentiles; Centres for Disease Control and Prevention: Atlanta, GA, USA, 2009. [Google Scholar]
- Bouret, S.G.; Simerly, R.B. Developmental programming of hypothalamic feeding circuits. Clin. Genet. 2006, 70, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [PubMed]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Bloom, S.R.; Lechler, R.I. Leptin inhibits the anti-CD3-driven proliferation of peripheral blood T cells but enhances the production of proinflammatory cytokines. J. Leukoc. Biol. 2002, 72, 330–338. [Google Scholar] [PubMed]
- Ho, F.C.; Wong, R.L.; Lawton, J.W. Human colostral and breast milk cells. A light and electron microscopic study. Acta Paediatr. Scand. 1979, 68, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Boutinaud, M.; Jammes, H. Potential uses of milk epithelial cells: A review. Reprod. Nutr. Dev. 2002, 42, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Bronsky, J.; Mitrova, K.; Karpisek, M.; Mazoch, J.; Durilova, M.; Fisarkova, B.; Stechova, K.; Prusa, R.; Nevoral, J. Adiponectin, AFABP, and leptin in human breast milk during 12 months of lactation. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Schueler, J.; Alexander, B.; Hart, A.M.; Austin, K.; Larson-Meyer, D.E. Presence and dynamics of leptin, GLP-1, and PYY in human breast milk at early postpartum. Obesity (Silver Spring, MD) 2013, 21, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Bielicki, J.; Huch, R.; von Mandach, U. Time-Course of leptin levels in term and preterm human milk. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2004, 151, 271–276. [Google Scholar] [CrossRef]
- King, B.L.; Crisi, G.M.; Tsai, S.C.; Haffty, B.G.; Phillips, R.F.; Rimm, D.L. Immunocytochemical analysis of breast cells obtained by ductal lavage. Cancer Cytopathol. 2002, 96, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Geddes, D.T. Immune cell-mediated protection of the mammary gland and the infant during breastfeeding. Adv. Nutr. (Bethesda, MD) 2015, 6, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Weiler, I.J.; Hickler, W.; Sprenger, R. Demonstration That Milk Cells Invade the Suckling Neonatal Mouse. Am. J. Reprod. Immunol. 1983, 4, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, K.; Numata, Y.; Katsuura, G.; Sakaguchi, I.; Morita, A.; Kikuoka, S.; Matumoto, Y.; Tsuji, T.; Tamaki, M.; Sasakura, K.; et al. Structure-Function Studies of Human Leptin. J. Biol. Chem. 1998, 273, 35245–35249. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, F.W. Solutions of the electronegative elements in liquid ammonia; the actions of selenium, tellurium, arsenic acid and a solution of sulfur in liquid ammonia upon cyanides. J. Am. Chem. Soc. 1926, 48, 2319–2327. [Google Scholar] [CrossRef]




| Stage of Lactation (Month) | 2 | 5 | 9 | 12 |
|---|---|---|---|---|
| Maternal age (years) | 32.9 ± 4.21 (28–40) | 33.4 ± 4.27 (24–40) | 34.0 ± 4.57 (25–43) | 34.1 ± 4.35 (26–44) |
| Maternal BMI | 27.1 ± 7.15 (20.1–38.5) | 23.5 ± 4.46 (18.0–35.2) | 24.0 ± 5.15 (18.7–37.2) | 24.8 ± 5.6 (18.2–34.6) |
| Parity | 2.10 ± 0.75 (1–4) | 2.13 ± 0.85 (1–4) | 1.96 ± 0.94 (1–4) | 2.05 ± 0.98 (1–4) |
| Infant sex (Male/Female) | 12/9 | 16/16 | 17/13 | 12/12 |
| Infant birth weight (kg) | 3.58 ± 0.64 (2.66–4.23) | 3.49 ± 0.45 (2.66–4.46) | 3.49 ± 0.46 (2.82–4.46) | 3.59 ± 0.46 (2.80–4.46) |
| Infant body length (cm) | 57.6 ± 2.17 (54.2–61.3) | 64.5 ± 2.09 (61.5–69.5) | 70.9 ± 2.11 (68.0–74.5) | 73.9 ± 2.38 (71.5–78.5) |
| Dilution Factor | Skim Human Milk Leptin (%) | Whole Human Milk Leptin (%) |
|---|---|---|
| 1 | 61.5 ± 2.09 | 17.1 ± 2.9 |
| 2 | 179.0 ± 0.82 | 14.0 ± 2.7 |
| 5 | 96.3 ± 1.2 | 14.0 ± 1.4 |
| 10 | 71.3 ± 1.6 | 97.1 ± 9.1 |
| Month of Lactation | Whole Human Milk Leptin (ng/mL) | Skim Human Milk Leptin (ng/mL) | p-Value * |
|---|---|---|---|
| 2 | 0.50 ± 0.16 | 0.32 ± 0.16 | p < 0.0001 |
| 5 | 0.48 ± 0.16 | 0.26 ± 0.07 | p < 0.0001 |
| 9 | 0.56 ± 0.11 | 0.22 ± 0.03 | p < 0.0001 |
| 12 | 0.54 ± 0.14 | 0.21 ± 0.02 | p < 0.0001 |
| Month of Lactation | N (Samples) | R2 | p-Value * |
|---|---|---|---|
| 2 | 66 | 0.0013 | 0.782 |
| 5 | 72 | 0.018 | 0.686 |
| 9 | 83 | 0.069 | 0.577 |
| 12 | 66 | 0.153 | 0.889 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kugananthan, S.; Lai, C.T.; Gridneva, Z.; Mark, P.J.; Geddes, D.T.; Kakulas, F. Leptin Levels Are Higher in Whole Compared to Skim Human Milk, Supporting a Cellular Contribution. Nutrients 2016, 8, 711. https://doi.org/10.3390/nu8110711
Kugananthan S, Lai CT, Gridneva Z, Mark PJ, Geddes DT, Kakulas F. Leptin Levels Are Higher in Whole Compared to Skim Human Milk, Supporting a Cellular Contribution. Nutrients. 2016; 8(11):711. https://doi.org/10.3390/nu8110711
Chicago/Turabian StyleKugananthan, Sambavi, Ching Tat Lai, Zoya Gridneva, Peter J. Mark, Donna T. Geddes, and Foteini Kakulas. 2016. "Leptin Levels Are Higher in Whole Compared to Skim Human Milk, Supporting a Cellular Contribution" Nutrients 8, no. 11: 711. https://doi.org/10.3390/nu8110711





