Nutrient Transporter Expression in the Jejunum in Relation to Body Mass Index in Patients Undergoing Bariatric Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Participant Demographics and Clinical Characteristics
2.3. Jejunal Samples
2.4. Glucose, Insulin, and HbA1c
2.5. Serum Amino Acid and Short-Chain Fatty Acid Analyses
2.6. Statistical Analyses
3. Results
3.1. Jejunal Amino Acid Transporters
3.2. Jejunal Glucose and Short-Chain Fatty Acid/Monocarboxylate Transporters
3.3. Pre-Operative Glucose, Insulin, HOMA-IR, and HbA1c
3.4. Pre-Operative Serum Concentrations of Branched-Chain Amino Acids (BCAA) and Aromatic Amino Acids (AAA)
3.5. Pre-Operative Serum Concentrations of Short-Chain Fatty Acids
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Shah, S.H.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.; Slentz, C.A.; Tanner, C.J.; Kuchibhatla, M.; Houmard, J.A.; Newgard, C.B.; et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009, 32, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Spanier, B.; Lasch, K.; Marsch, S.; Benner, J.; Liao, W.; Hu, H.; Kienberger, H.; Eisenreich, W.; Daniel, H. How the intestinal peptide transporter pept-1 contributes to an obesity phenotype in caenorhabditits elegans. PLoS ONE 2009, 4, e6279. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.S.; Meirelles, K.; Meng, Q.; Pan, M.; Cooney, R.N. Roux-en-y gastric bypass alters small intestine glutamine transport in the obese zucker rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G594–G601. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, V.; Berkenstadt, H.; Goitein, D.; Dickman, R.; Bernstine, H.; Rubin, M. Gastric emptying is not prolonged in obese patients. Surg. Obes. Relat. Dis. 2013, 9, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Camilleri, M.; Forstrom, L.A.; Zinsmeister, A.R. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin. Endocrinol. 2009, 70, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Laway, B.A.; Malik, T.S.; Khan, S.H.; Rather, T.A. Prevalence of abnormal gastric emptying in asymptomatic women with newly detected diabetes and its reversibility after glycemic control-a prospective case control study. J. Diabetes Complicat. 2013, 27, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Vinnakota, R.R. Intestinal nutrient transport in genetically obese mice. Am. J. Clin. Nutr. 1995, 62, 540–546. [Google Scholar] [PubMed]
- Still, C.D.; Benotti, P.; Wood, G.C.; Gerhard, G.S.; Petrick, A.; Reed, M.; Strodel, W. Outcomes of preoperative weight loss in high-risk patients undergoing gastric bypass surgery. Arch. Surg. 2007, 142, 994–998; discussion 999. [Google Scholar] [CrossRef] [PubMed]
- Nih conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann. Intern. Med. 1991, 115, 956–961.
- Ganapathy, V. Protein Digestion and Absorption. In Physiology of the Gastrointestinal Tract; Johnson, L.R., Ed.; Elsevier: New York, NY, USA, 2012; pp. 1595–1623. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Irving, B.A.; Carter, R.E.; Soop, M.; Weymiller, A.; Syed, H.; Karakelides, H.; Bhagra, S.; Short, K.R.; Tatpati, L.; Barazzoni, R.; et al. Effect of insulin sensitizer therapy on amino acids and their metabolites. Metabolism 2015, 64, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Moreau, N.M.; Goupry, S.M.; Antignac, J.P.; Monteau, F.J.; Le Bizec, B.J.; Champ, M.M.; Martin, L.J.; Dumon, H.J. Simultaneous measurement of plasma concentrations and 13c-enrichment of short-chain fatty acids, lactic acid and ketone bodies by gas chromatography coupled to mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 784, 395–403. [Google Scholar] [CrossRef]
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Fanjul, C.; Barrenetxe, J.; Inigo, C.; Sakar, Y.; Ducroc, R.; Barber, A.; Lostao, M.P. Leptin regulates sugar and amino acids transport in the human intestinal cell line caco-2. Acta Physiol. (Oxf.) 2012, 205, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, A.; Anbazhagan, A.N.; Kumar, A.; Raheja, G.; Singh, V.; Ramaswamy, K.; Dudeja, P.K. The probiotic lactobacillus plantarum counteracts tnf-{alpha}-induced downregulation of smct1 expression and function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G928–G934. [Google Scholar] [CrossRef] [PubMed]
- Rahat-Rozenbloom, S.; Fernandes, J.; Gloor, G.B.; Wolever, T.M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. 2014, 38, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Segain, J.P.; Raingeard de la Bletiere, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottiere, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through nfkappab inhibition: Implications for crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.; Collado, M.C.; Ferreira, C.L.; Bressan, J.; Peluzio Mdo, C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr. Res. 2012, 32, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Casselbrant, A.; Elias, E.; Fandriks, L.; Wallenius, V. Expression of tight-junction proteins in human proximal small intestinal mucosa before and after roux-en-y gastric bypass surgery. Surg. Obes. Relat. Dis. 2015, 11, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, H.Y.; Rhoads, D.B.; Ashley, S.W.; Tavakkolizadeh, A. Topographic expression of intestinal sugar sensors and transporters in obesity: Tissue analysis of patients undergoing gastric bypass surgery. Gastroenterology 2011, 140, S-1045. [Google Scholar]
- Nguyen, N.Q.; Debreceni, T.L.; Bambrick, J.E.; Chia, B.; Deane, A.M.; Wittert, G.; Rayner, C.K.; Horowitz, M.; Young, R.L. Upregulation of intestinal glucose transporters after roux-en-y gastric bypass to prevent carbohydrate malabsorption. Obesity 2014, 22, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.Q.; Debreceni, T.L.; Bambrick, J.E.; Chia, B.; Wishart, J.; Deane, A.M.; Rayner, C.K.; Horowitz, M.; Young, R.L. Accelerated intestinal glucose absorption in morbidly obese humans: Relationship to glucose transporters, incretin hormones, and glycemia. J. Clin. Endocrinol. Metab. 2015, 100, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Ait-Omar, A.; Monteiro-Sepulveda, M.; Poitou, C.; Le Gall, M.; Cotillard, A.; Gilet, J.; Garbin, K.; Houllier, A.; Chateau, D.; Lacombe, A.; et al. GLUT2 accumulation in enterocyte apical and intracellular membranes: A study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes 2011, 60, 2598–2607. [Google Scholar] [PubMed]
| Female, % | 90% (n = 38) |
|---|---|
| White, % | 98% (n = 41) |
| Age, years | 42.6 (11.9) [20, 64] |
| Body Mass Index, kg/m2 | 55.5 (11.3) [38.1, 78.4] |
| Diabetes | 29% (n = 12) |
| Glucose, mg/dL | 100.1 (33.2) [72, 264] |
| Insulin, µU/mL | 22.8 (13.1) [5, 58.9] |
| HbA1c, % | 5.9 (0.7) [5.1, 8.3] |
| HOMA-IR, | 6.04 (4.03) [1.16, 18.68] |
| Waist circumference, cm | 143.8 (19.7) [103.5, 182.9] |
| Total cholesterol, mg/dL | 173.7 ± 38.1 [93, 266] |
| LDL cholesterol, mg/dL | 101.5 ± 30.0 [39, 178] |
| HDL cholesterol, mg/dL | 49.0 ± 12.0 [30, 86] |
| Triglycerides, mg/dL | 116.0 ± 55.9 [44, 350] |
| Systolic Blood Pressure, mmHg | 131.9 ± 17.4 [108, 198] |
| Diastolic Blood Pressure, mmHg | 76.1 ± 8.8 [60, 100] |
| NT mRNA Abundance (40-ΔCT Values) by BMI Quartile | Pearson’s Correlation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall n = 42 | 35–44 n = 10 | 45–54 n = 11 | 55–64 n = 10 | 65+ n = 11 | p-value1 | p-value2 | r | p-value | |
| GLUT2 | 22.7 (2.8) | 23.2 (3.3) | 21.5 (3.7) | 23.8 (1.5) | 22.4 (2) | 0.957 | 0.856 | –0.082 | 0.604 |
| SGLT1 | 25.2 (3.3) | 25.1 (4.6) | 24.4 (3.3) | 26.9 (1.7) | 24.6 (2.9) | 0.834 | 0.927 | –0.066 | 0.676 |
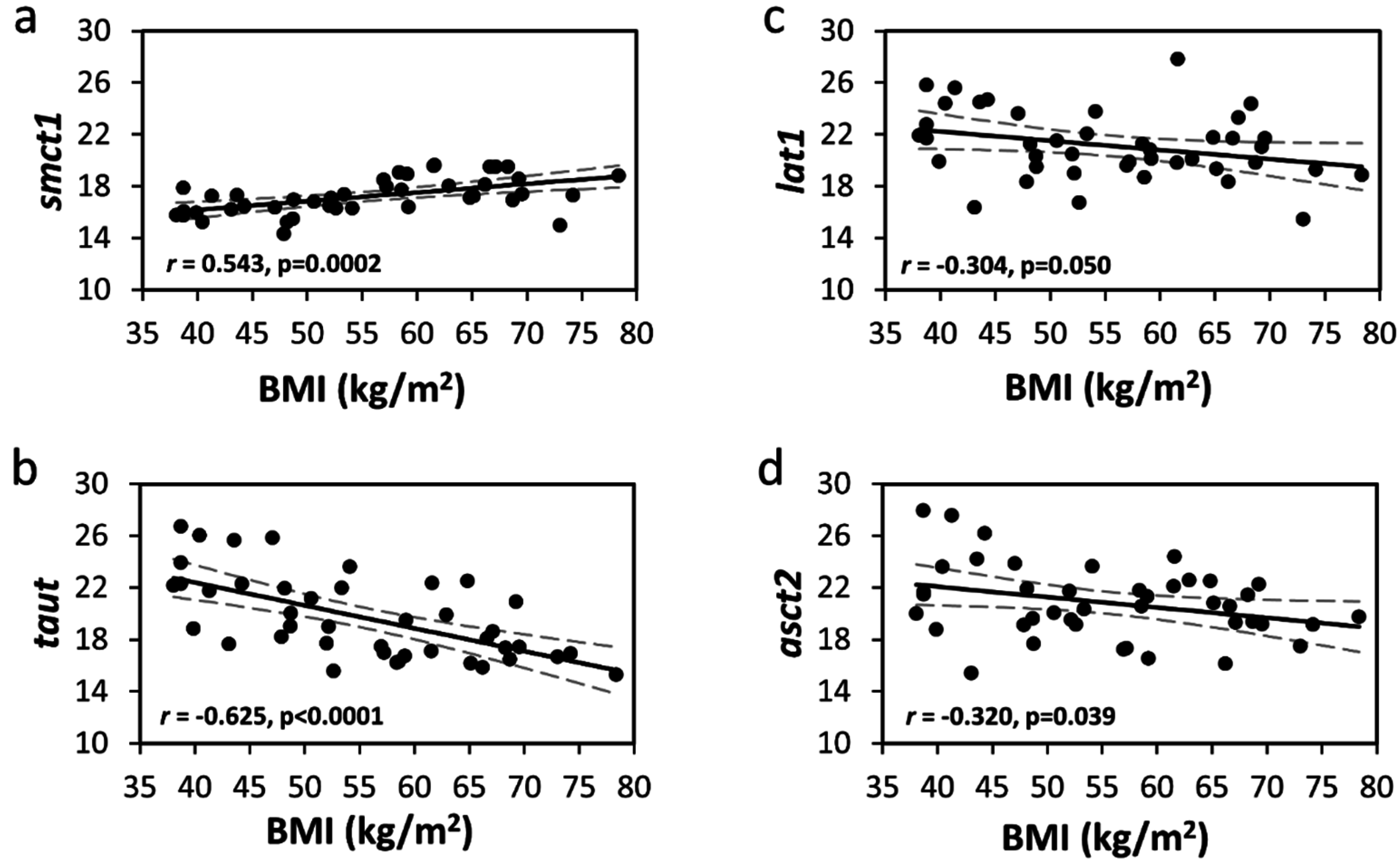
| SMCT1 | 17.2 (1.4) | 16.4 (0.8) | 16.2 (0.9) | 18.3 (1.1) | 18.0 (1.4) | <0.0001 | <0.0001 | 0.543 | 0.0002 |
| SMCT2 | 21.2 (3.1) | 21.6 (3.7) | 20.0 (3.4) | 22.6 (2.8) | 20.6 (2.3) | 0.908 | 0.791 | –0.080 | 0.616 |
| TauT | 19.7 (3.2) | 22.7 (3.0) | 20.4 (2.9) | 18.5 (2.4) | 17.3 (1.5) | <0.0001 | <0.0001 | –0.625 | <0.0001 |
| ATB0,+ | 19.1 (2.3) | 18.4 (2.9) | 18.4 (1.8) | 20.0 (2.4) | 19.4 (2.0) | 0.175 | 0.204 | 0.183 | 0.245 |
| B0AT1 | 23.7 (3.4) | 23.4 (5.1) | 22.9 (3.4) | 25.4 (1.6) | 23.2 (2.6) | 0.695 | 0.785 | –0.043 | 0.786 |
| SNAT2 | 21.7 (2.6) | 22.8 (3.0) | 20.5 (2.8) | 22.8 (1.3) | 20.7 (2.2) | 0.244 | 0.229 | –0.229 | 0.144 |
| LAT1 | 21.1 (2.6) | 22.8 (2.9) | 20.6 (2.2) | 21.0 (2.6) | 20.3 (2.5) | 0.052 | 0.046 | –0.304 | 0.050 |
| LAT2 | 21.9 (2.6) | 23.2 (2.8) | 20.9 (2.9) | 23.0 (1.6) | 20.8 (2.4) | 0.161 | 0.134 | –0.278 | 0.074 |
| xCT | 18.7 (2.9) | 19.8 (3.4) | 17.1 (3.3) | 19.3 (2.3) | 18.9 (2.2) | 0.881 | 0.846 | –0.050 | 0.749 |
| CAT1 | 19.7 (2.7) | 21.0 (3.1) | 18.6 (3.1) | 20.6 (1.8) | 19.0 (2.0) | 0.256 | 0.217 | –0.212 | 0.178 |
| EAAT3 | 21.4 (2.8) | 22.2 (3.1) | 20.2 (3.1) | 22.6 (1.8) | 20.6 (2.6) | 0.522 | 0.434 | –0.172 | 0.275 |
| ASCT2 | 20.8 (2.8) | 22.7 (4.0) | 20.6 (2.0) | 20.6 (2.7) | 19.6 (1.7) | 0.018 | 0.016 | –0.320 | 0.039 |
| Glucose | Insulin | HbA1c | HOMA-IR | Waist Circumference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | |
| GLUT2 | 0.193 | 0.234 | 0.160 | 0.330 | 0.188 | 0.257 | 0.212 | 0.196 | –0.018 | 0.910 |
| SGLT1 | 0.174 | 0.284 | 0.173 | 0.292 | 0.154 | 0.354 | 0.227 | 0.165 | –0.013 | 0.935 |
| SMCT1 | –0.126 | 0.440 | –0.239 | 0.143 | –0.232 | 0.162 | –0.296 | 0.068 | 0.476 | 0.0017 |
| SMCT2 | 0.137 | 0.399 | 0.145 | 0.377 | 0.177 | 0.289 | 0.198 | 0.227 | –0.021 | 0.895 |
| TauT | 0.238 | 0.139 | 0.079 | 0.633 | 0.219 | 0.186 | 0.206 | 0.208 | –0.509 | 0.0007 |
| ATB0,+ | –0.002 | 0.989 | 0.163 | 0.320 | 0.019 | 0.909 | 0.157 | 0.339 | 0.210 | 0.187 |
| B0AT1 | 0.166 | 0.307 | 0.184 | 0.261 | 0.188 | 0.259 | 0.234 | 0.152 | 0.026 | 0.870 |
| SNAT2 | 0.071 | 0.665 | 0.187 | 0.255 | 0.020 | 0.905 | 0.224 | 0.171 | –0.237 | 0.135 |
| LAT1 | 0.080 | 0.624 | 0.043 | 0.795 | 0.046 | 0.785 | –0.006 | 0.969 | –0.241 | 0.130 |
| LAT2 | 0.145 | 0.371 | 0.171 | 0.299 | 0.086 | 0.608 | 0.219 | 0.181 | –0.204 | 0.200 |
| xCT | 0.052 | 0.749 | 0.148 | 0.367 | 0.030 | 0.858 | 0.143 | 0.384 | –0.113 | 0.481 |
| CAT1 | 0.121 | 0.455 | 0.150 | 0.362 | 0.046 | 0.786 | 0.164 | 0.318 | –0.226 | 0.155 |
| EAAT3 | 0.181 | 0.265 | 0.192 | 0.243 | 0.140 | 0.402 | 0.234 | 0.152 | –0.147 | 0.358 |
| ASCT2 | 0.030 | 0.856 | 0.028 | 0.866 | –0.024 | 0.888 | –0.006 | 0.970 | –0.335 | 0.032 |
| Mean Serum Metabolite Concentrations by BMI Quartile | Pearson’s Correlation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall n = 30 | 35–44 n = 7 | 45–54 n = 10 | 55–64 n = 7 | 65+ n = 6 | p-value1 | p-value2 | r | p-value | |
| Leucine, µmol/L | 129.8 (24.3) | 117.2 (25.1) | 141.2 (27.4) | 131.7 (17.1) | 123.4 (20.9) | 0.824 | 0.828 | 0.008 | 0.965 |
| Isoleucine, µmol/L | 61.7 (14.5) | 58.1 (14.8) | 66.7 (18.9) | 63.3 (7.2) | 55.7 (11.5) | 0.677 | 0.678 | –0.125 | 0.511 |
| Valine, µmol/L | 247.6 (45.2) | 226.2 (56.1) | 265.8 (44.1) | 241.4 (25.3) | 249.3 (49.4) | 0.570 | 0.573 | 0.073 | 0.700 |
| Phenylalanine, µmol/L | 83.3 (15.9) | 71.1 (4.6) | 90.3 (19.2) | 82.4 (17.9) | 86.9 (7.7) | 0.137 | 0.145 | 0.257 | 0.171 |
| Tyrosine, µmol/L | 66.2 (19.2) | 64.0 (19.7) | 75.5 (23.1) | 54.9 (10.0) | 66.5 (15.6) | 0.692 | 0.692 | –0.107 | 0.575 |
| Butyric Acid, µmol/L | 0.55 (0.21) | 0.46 (0.10) | 0.50 (0.24) | 0.53 (0.13) | 0.74 (0.23) | 0.016 | 0.018 | 0.446 | 0.013 |
| Isovaleric Acid, µmol/L | 0.34 (0.24) | 0.35 (0.22) | 0.27 (0.11) | 0.24 (0.10) | 0.56 (0.41) | 0.136 | 0.142 | 0.252 | 0.179 |
| Valeric Acid, µmol/L | 0.34 (0.13) | 0.27 (0.07) | 0.30 (0.12) | 0.39 (0.16) | 0.42 (0.12) | 0.014 | 0.012 | 0.439 | 0.015 |
| Isocaproic Acid, µmol/L | 0.04 (0.04) | 0.04 (0.03) | 0.03 (0.02) | 0.03 (0.02) | 0.07 (0.06) | 0.124 | 0.119 | 0.364 | 0.048 |
| Hexanoic Acid, µmol/L | 0.87 (0.34) | 0.69 (0.15) | 0.83 (0.26) | 0.99 (0.57) | 1.00 (0.22) | 0.073 | 0.072 | 0.292 | 0.118 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irving, B.A.; Wood, G.C.; Bennotti, P.N.; Babu, E.; Deshpande, A.; Lent, M.R.; Petrick, A.; Gabrielsen, J.; Strodel, W.; Gerhard, G.S.; et al. Nutrient Transporter Expression in the Jejunum in Relation to Body Mass Index in Patients Undergoing Bariatric Surgery. Nutrients 2016, 8, 683. https://doi.org/10.3390/nu8110683
Irving BA, Wood GC, Bennotti PN, Babu E, Deshpande A, Lent MR, Petrick A, Gabrielsen J, Strodel W, Gerhard GS, et al. Nutrient Transporter Expression in the Jejunum in Relation to Body Mass Index in Patients Undergoing Bariatric Surgery. Nutrients. 2016; 8(11):683. https://doi.org/10.3390/nu8110683
Chicago/Turabian StyleIrving, Brian A., G. Craig Wood, Peter N. Bennotti, Ellappan Babu, Abhishek Deshpande, Michelle R. Lent, Anthony Petrick, Jon Gabrielsen, William Strodel, Glenn S. Gerhard, and et al. 2016. "Nutrient Transporter Expression in the Jejunum in Relation to Body Mass Index in Patients Undergoing Bariatric Surgery" Nutrients 8, no. 11: 683. https://doi.org/10.3390/nu8110683





