Effect of Lactobacillus paracasei Culture Filtrates and Artichoke Polyphenols on Cytokine Production by Dendritic Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Artichoke Phenolic Extract Preparation and Assay
2.2. Phenolic Composition and Concentration Assay
2.3. Chemicals
2.4. Bacterial Strains and Culture Conditions
2.5. Mice
2.6. Isolation and Growth of Bone Marrow-Derived Dendritic Cells
2.7. Microbial Filtrate Challenge
2.8. Fluorescence-Activated Cell Sorting (FACS) Analysis
2.9. Analysis of Cytokine Production
2.10. Statistical Analysis
3. Results
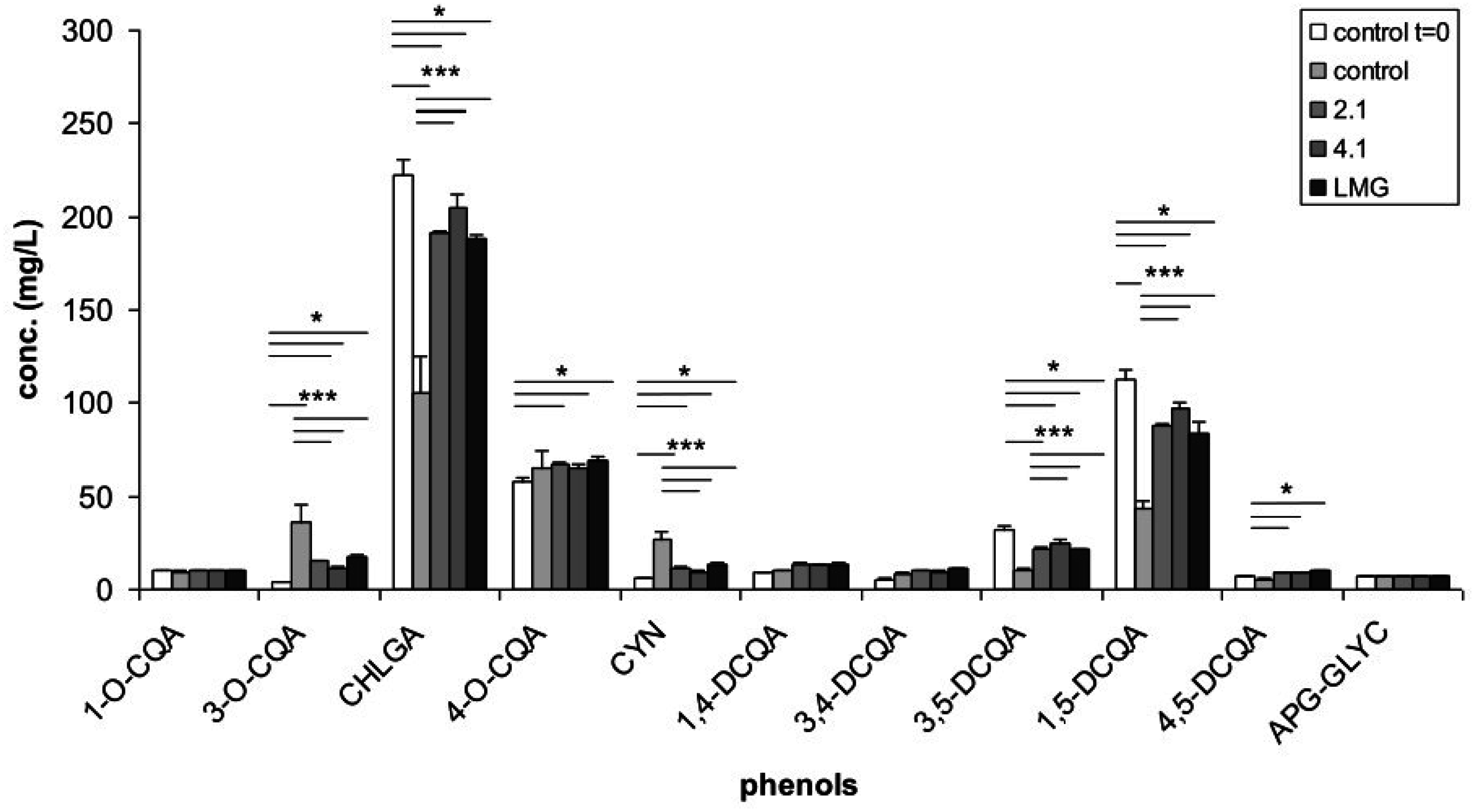
3.1. Phenolic Content of Artichoke Extract and of Bacterial Culture Filtrates
3.2. Growth of L. paracasei Strains in RPMI 1640 and X-Vivo 15 Media
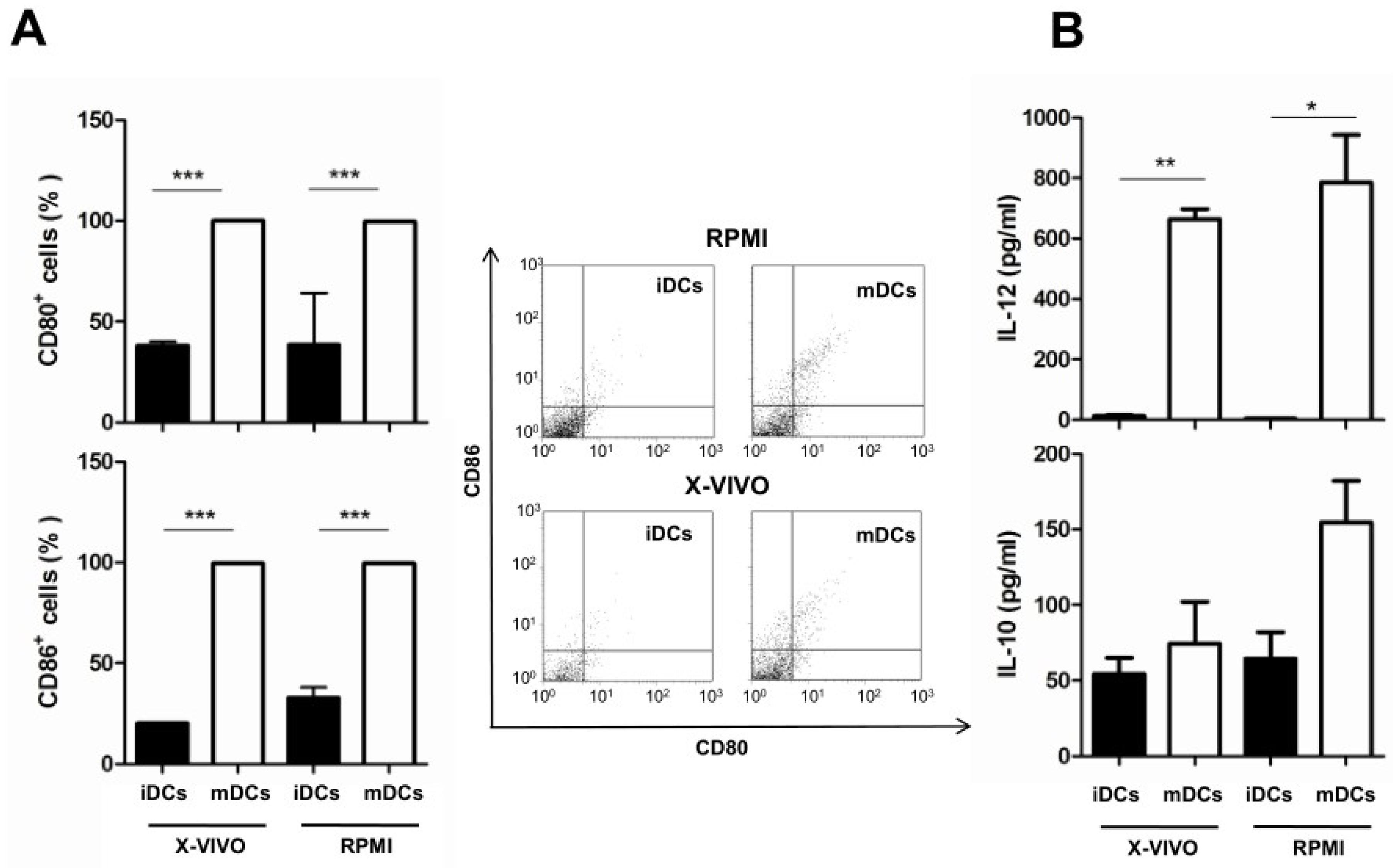
3.3. Effect of Culture Media on the Maturation of Dendritic Cells and Cytokine Production
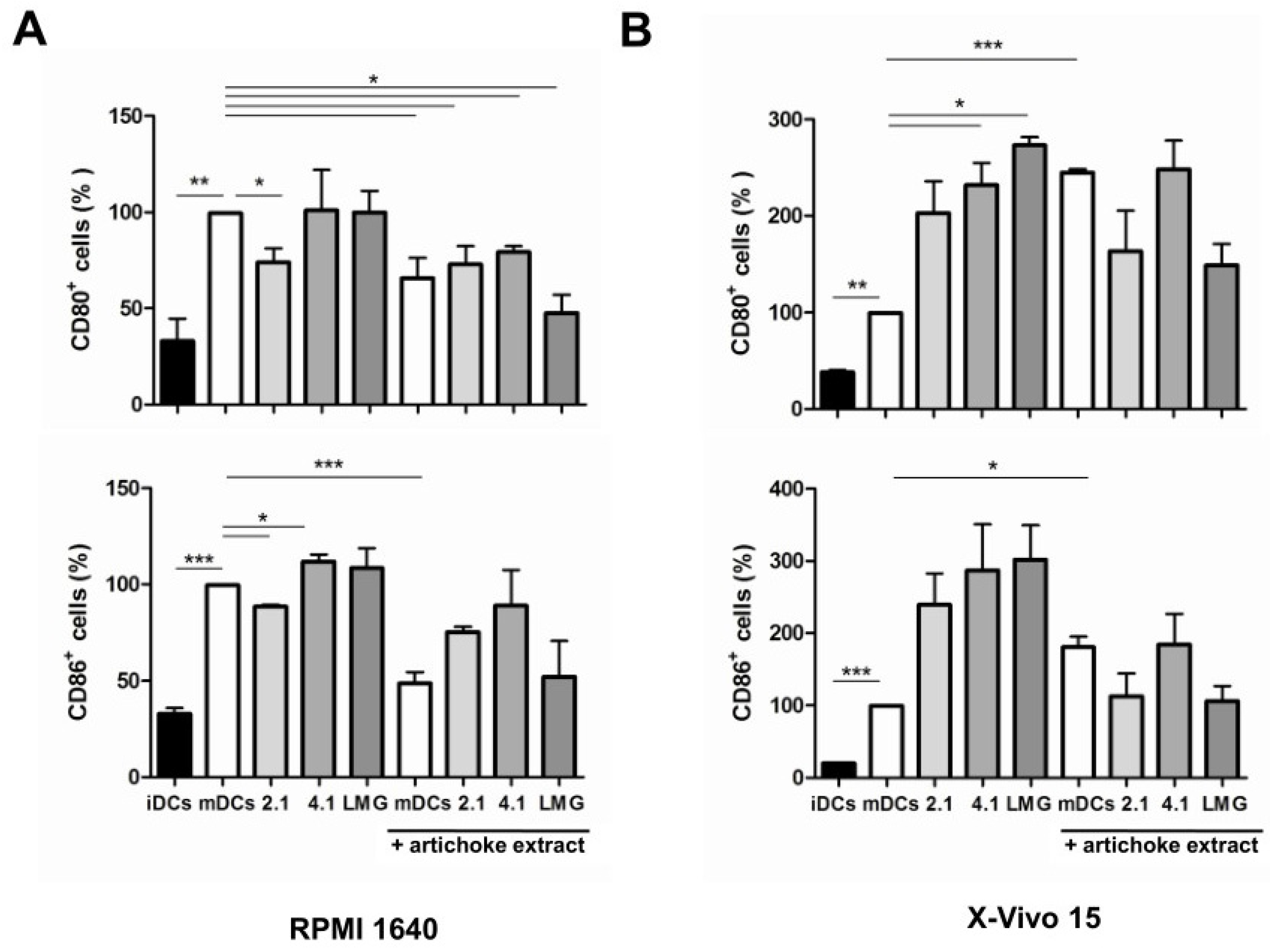
3.4. Effect of Bacterial Culture Filtrates with or without Artichoke Phenolic Extract on the Maturation of Dendritic Cells
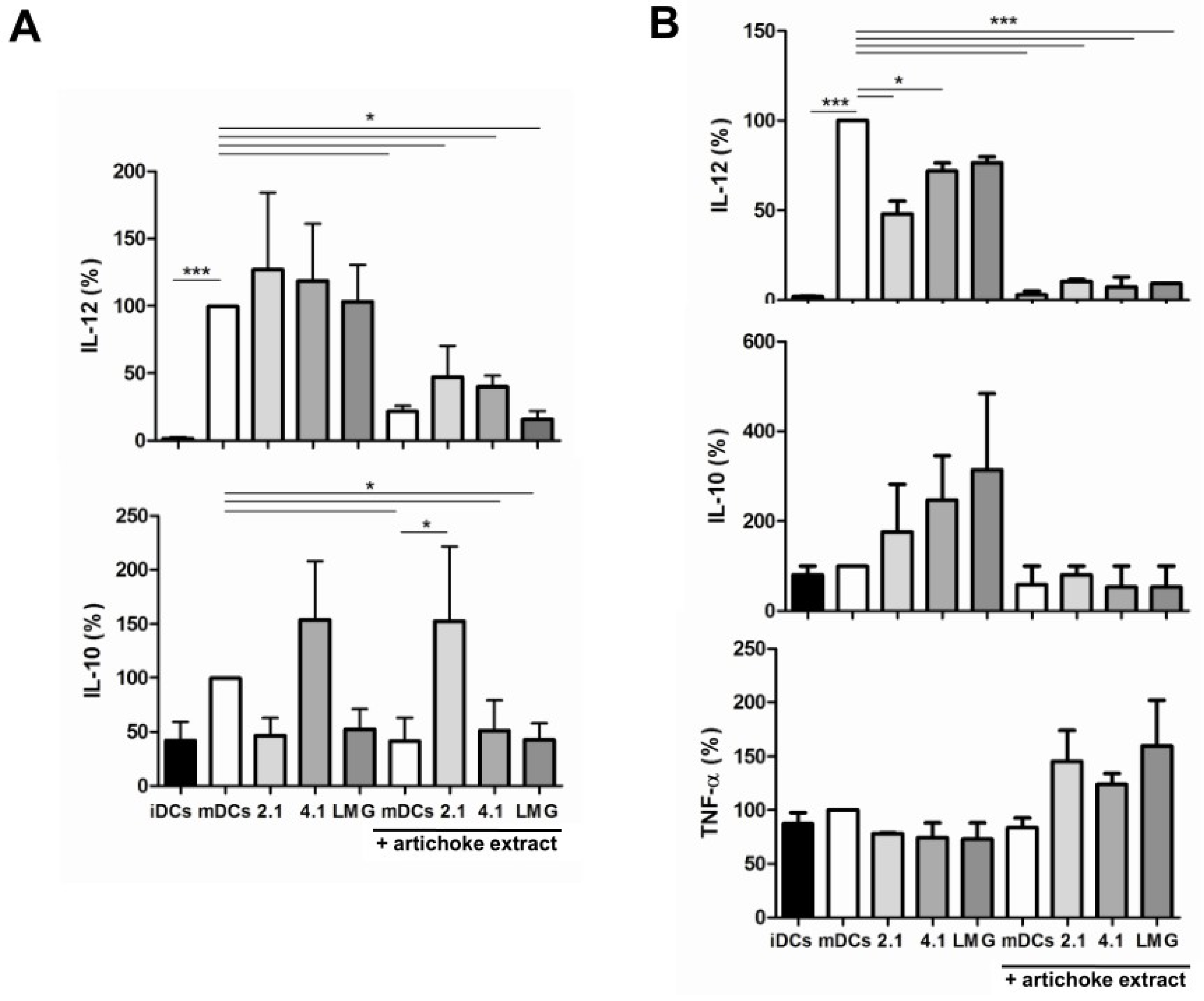
3.5. Effect of Bacterial Culture Filtrates with or without Artichoke Phenolic Extract on Cytokine Production by Dendritic Cells
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barlow, S.; Chesson, A.; Collins, J.D.; Dybing, E.; Flynn, A.; Fruijtier-Pölloth, C.; Hardy, A.; Knaap, A.; Kuiper, H.; Le Neindre, P.; et al. Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. List of taxonomic units proposed for QPS status. EFSA J. 2007, 587, 1–16. [Google Scholar]
- Mogensen, G.; Salminen, S.; O’Brien, J.; Ouwehand, A.C.; Holzapfel, W.; Shortt, C.; Fonden, R.; Miller, G.D.; Donohue, D.; Playne, M.; et al. Inventory of microorganisms with a documented history of safe use in food. Bull. Int. Dairy Fed. 2002, 377, 10–19. [Google Scholar]
- Lavermicocca, P.; Lonigro, S.L.; Visconti, A.; De Angelis, M.; Valerio, F.; Morelli, L. Table Olives Containing Probiotic Microorganisms. European Patent EP1843664, 8 July 2009. [Google Scholar]
- Lavermicocca, P.; Lonigro, S.L.; Valerio, F.; Visconti, A.; Vanadia, S.; Calabrese, N.; Di Venere, D.; Morelli, L. Process for the Preparation of Vegetable Preserves Containing Probiotic Microorganisms. PCT WO2006/037517 A1, 9 March 2009. [Google Scholar]
- Lavermicocca, P.; Valerio, F.; Lonigro, S.L.; De Angelis, M.; Morelli, L.; Callegari, M.L.; Rizzello, C.G.; Visconti, A. Study of adhesion and survival of Lactobacilli and Bifidobacteria on table olives with the aim of formulating a new probiotic food. Appl. Environ. Microbiol. 2005, 71, 4233–4240. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; De Bellis, P.; Lonigro, S.L.; Morelli, L.; Visconti, A.; Lavermicocca, P. In vitro and in vivo survival and transit tolerance of potentially probiotic strains carried by artichokes in the gastrointestinal tract. Appl. Environ. Microbiol. 2006, 72, 3042–3045. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; Lonigro, S.L.; Di Biase, M.; De Candia, S.; Callegari, M.L.; Lavermicocca, P. Bioprotection of ready-to-eat probiotic artichokes processed with Lactobacillus paracasei LMGP22043 against foodborne pathogens. J. Food Sci. 2013, 78, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Di Venere, D.; Linsalata, V.; Calabrese, N.; Pieralice, M.; Bianco, V.V.; Lattanzio, V. Morphological and biochemical changes during development of artichoke buds. Acta Hortic. 2005, 681, 437–443. [Google Scholar] [CrossRef]
- Di Venere, D.; Linsalata, V.; Pace, B.; Bianco, V.V.; Perrino, P. Polyphenol and inulin content in a collection of artichoke. Acta Hortic. 2005, 681, 453–459. [Google Scholar] [CrossRef]
- Sisto, A.; De Bellis, P.; Visconti, A.; Morelli, L.; Lavermicocca, P. Development of a PCR assay for the strain-specific identification of probiotic strain Lactobacillus paracasei IMPC2.1. Int. J. Food Microbiol. 2009, 136, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lavermicocca, P.; Valerio, F.; Lonigro, S.L.; Di Leo, A.; Visconti, A. Antagonistic activity of potential probiotic Lactobacilli against the ureolytic pathogen Yersinia enterocolitica. Curr. Microbiol. 2008, 56, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Refolo, M.G.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC 2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; De Candia, S.; Lonigro, S.L.; Russo, F.; Riezzo, G.; Orlando, A.; De Bellis, P.; Sisto, A.; Lavermicocca, P. Role of the probiotic strain Lactobacillus paracasei LMGP22043 carried by artichokes in influencing faecal bacteria and biochemical parameters in human subjects. J. Appl. Microbiol. 2011, 111, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, G.; Orlando, A.; D’Attoma, B.; Guerra, V.; Valerio, F.; Lavermicocca, P.; De Candia, S.; Russo, F. Randomised clinical trial: Efficacy of Lactobacillus paracasei-enriched artichokes in the treatment of patients with functional constipation—A double-blind, controlled, crossover study. Aliment. Pharmacol. Ther. 2012, 35, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Fragkos, K.C.; Scott, S.M.; Whelan, K. The effect of probiotics on functional constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Selmi, C.; Meyers, F.J.; Keen, C.L.; Gershwin, M.E. Probiotics and immunity. J. Gastroenterol. 2009, 44, 26–46. [Google Scholar] [CrossRef] [PubMed]
- D’Arienzo, R.; Bozzella, G.; Rossi, M.; De Bellis, P.; Lavermicocca, P.; Sisto, A. Distinct immunomodulatory properties of Lactobacillus paracasei strains. J. Appl. Microbiol. 2011, 111, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and molecules of Lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M. Immunomodulatory mechanisms of lactobacilli. Microb. Cell Fact. 2011, 10 (Suppl. S1), S17. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Hong, T.; van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE 2012, 7, e31951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chon, H.; Choi, B.; Jeong, G.; Lee, E.; Lee, S. Suppression of proinflammatory cytokine production by specific metabolites of Lactobacillus plantarum 10hk2 via inhibiting NF-kB and p38 MAPK expressions. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, e41–e49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Cheng, Y.; Liu, X.; Huang, Y. Secreted factors from Bifidobacterium animalis subsp. lactis inhibit NF-_B-mediated interleukin-8 gene expression in Caco-2 Cells. Appl. Environ. Microbiol. 2011, 77, 8171–8174. [Google Scholar]
- Mileti, E.; Matteoli, G.; Iliev, I.D.; Rescigno, M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: Prediction for in vivo efficacy. PLoS ONE 2009, 4, e7056. [Google Scholar] [CrossRef] [PubMed]
- Zagato, E.; Mileti, E.; Massimiliano, L.; Fasano, F.; Budelli, A.; Penna, G.; Rescigno, M. Lactobacillus paracasei CBA L74 metabolic products and fermented milk for infant formula have anti-inflammatory activity on dendritic cells in vitro and protective effects against colitis and an enteric pathogen in vivo. PLoS ONE 2014, 9, e87615. [Google Scholar] [CrossRef] [PubMed]
- Von Schillde, M.-A.; Hörmannsperger, G.; Weiher, M.; Alpert, C.-A.; Hahne, H.; Bӓuerl, C.; van Huynegem, K.; Steidler, L.; Hrncir, T.; Pérez-Martínez, G.; et al. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe 2012, 11, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.A.; Sergio, L.; Ippolito, A.; Di Venere, D. Phenolic extracts from wild edible plants to control postharvest diseases of sweet cherry fruit. Postharvest Biol. Technol. 2016, 120, 180–187. [Google Scholar] [CrossRef]
- Mileo, A.M.; Di Venere, D.; Linsalata, V.; Fraioli, R.; Miccadei, S. Artichoke polyphenols induce apoptosis and decrease the invasive potential of the human breast cancer line MDA-MB231. J. Cell. Physiol. 2012, 227, 3301–3309. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Poiret, S.; Goudercourt, D.; Dennin, V.; Leyer, G.; Pot, B. Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model. Appl. Environ. Microbiol. 2006, 72, 5799–5805. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Kukutsch, N.; Ogilvie, A.L.; Rossner, S.; Koch, F.; Romani, N.; Schuler, G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223, 77–92. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Humblot, C.; Thomas, M.; Guyot, J.P. Lactobacilli as multifaceted probiotics with poorly disclosed molecular mechanisms. Int. J. Food Microbiol. 2010, 143, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, R.D.C.S.; Baines, S.K.; Adams, M.C. Importance of food in probiotic efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Mileo, A.M.; Di Venere, D.; Abbruzzese, C.; Miccadei, S. Long term exposure to polyphenols of artichoke (Cynara scolymus L.) exerts induction of senescence driven growth arrest in the MDA-MB231 human breast cancer cell line. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Miccadei, S.; Di Venere, D.; Cardinali, A.; Romano, F.; Durazzo, A.; Foddai, M.S.; Fraioli, R.; Mobarhan, S.; Maiani, G. Antioxidative and apoptotic properties of polyphenolic extracts from edible part of artichoke (Cynara scolymus L.) on cultured rat hepatocytes and on human hepatoma cells. Nutr. Cancer 2008, 60, 276–283. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisto, A.; Luongo, D.; Treppiccione, L.; De Bellis, P.; Di Venere, D.; Lavermicocca, P.; Rossi, M. Effect of Lactobacillus paracasei Culture Filtrates and Artichoke Polyphenols on Cytokine Production by Dendritic Cells. Nutrients 2016, 8, 635. https://doi.org/10.3390/nu8100635
Sisto A, Luongo D, Treppiccione L, De Bellis P, Di Venere D, Lavermicocca P, Rossi M. Effect of Lactobacillus paracasei Culture Filtrates and Artichoke Polyphenols on Cytokine Production by Dendritic Cells. Nutrients. 2016; 8(10):635. https://doi.org/10.3390/nu8100635
Chicago/Turabian StyleSisto, Angelo, Diomira Luongo, Lucia Treppiccione, Palmira De Bellis, Donato Di Venere, Paola Lavermicocca, and Mauro Rossi. 2016. "Effect of Lactobacillus paracasei Culture Filtrates and Artichoke Polyphenols on Cytokine Production by Dendritic Cells" Nutrients 8, no. 10: 635. https://doi.org/10.3390/nu8100635




