Amino Acid Composition of Breast Milk from Urban Chinese Mothers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
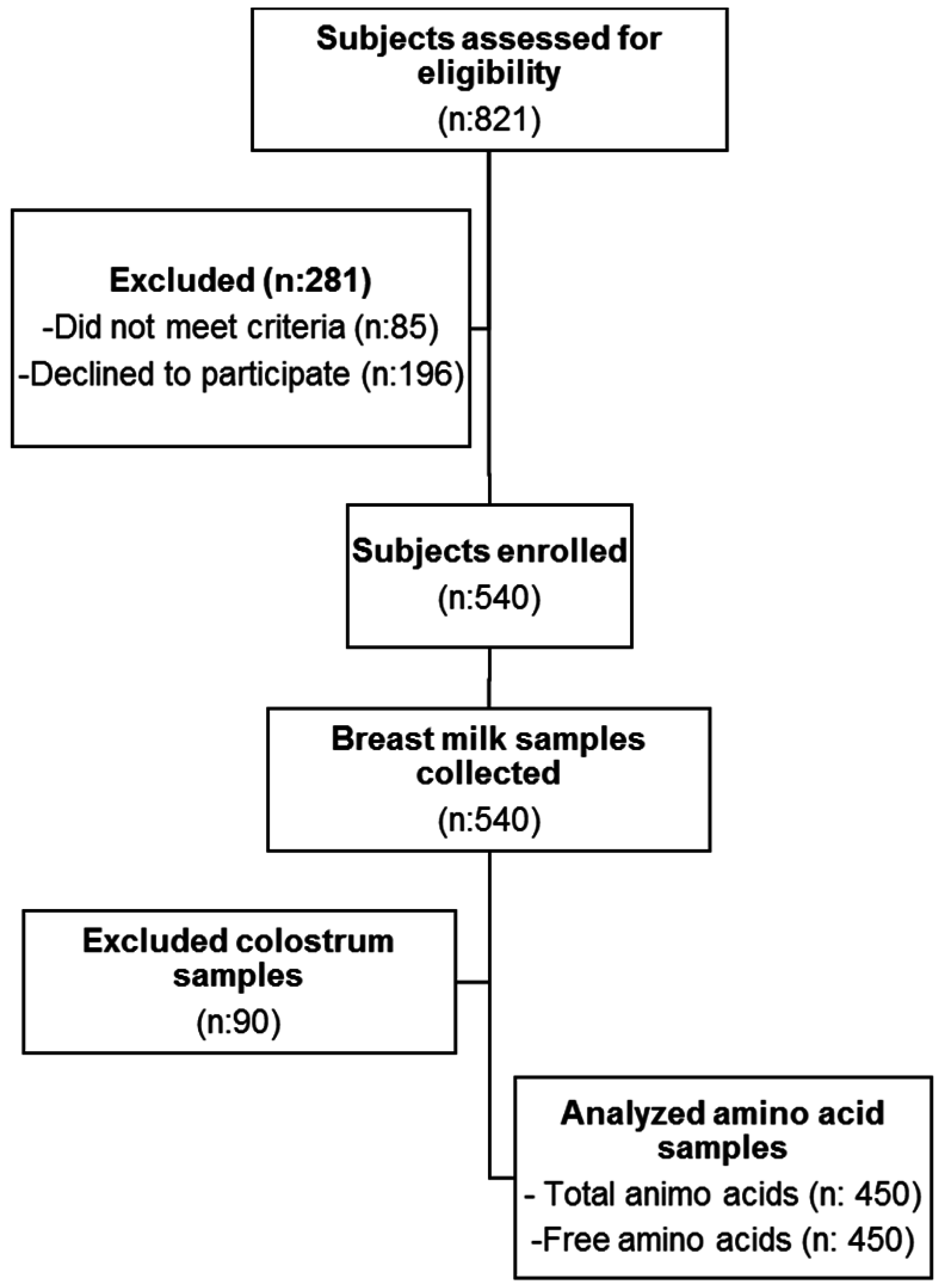
2.2. Data Collection
2.3. Sample Collection
2.4. Amino Acid Analysis
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
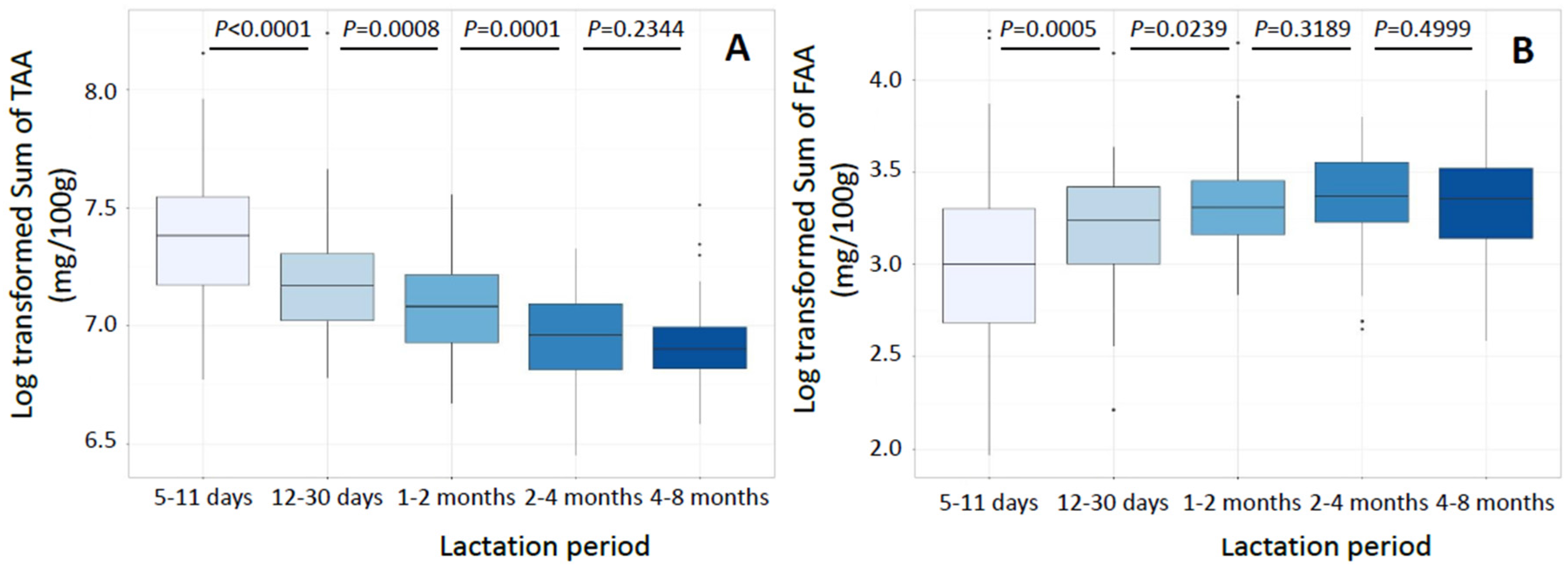
3.2. Total Amino Acids
3.3. Free Amino Acids
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stam, J.; Sauer, P.J.; Boehm, G. Can we define an infant's need from the composition of human milk? Am. J. Clin. Nutr. 2013, 98, 521S–528S. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F.; Greer, F.R. Protein needs early in life and long-term health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization of the United Nations; United Nations University. Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition; WHO technical report series; WHO Press: Geneva, Switzerland, 2007; Volume 935, pp. 1–265. [Google Scholar]
- Da Costa, T.H.; Haisma, H.; Wells, J.C.; Mander, A.P.; Whitehead, R.G.; Bluck, L.J. How much human milk do infants consume? Data from 12 countries using a standardized stable isotope methodology. J. Nutr. 2010, 140, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Svanberg, U.; Gebre-Medhin, M.; Ljungqvist, B.; Olsson, M. Breast milk composition in Ethiopian and Swedish mothers. III. Amino acids and other nitrogenous substances. Am. J. Clin. Nutr. 1977, 30, 499–507. [Google Scholar] [PubMed]
- Carratu, B.; Boniglia, C.; Scalise, F.; Ambruzzib, A.M.; Sanzinia, E. Nitrogenous components of human milk: Non-protein nitrogen, true protein and free amino acids. Food Chem. 2003, 81, 357–362. [Google Scholar] [CrossRef]
- Ventura, A.K.; Beauchamp, G.K.; Mennella, J.A. Infant regulation of intake: The effect of free glutamate content in infant formulas. Am. J. Clin. Nutr. 2012, 95, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Adelman, A.S.; Rai, D.; Boettcher, J.; Lőnnerdal, B. Amino acid profiles in term and preterm human milk through lactation: A systematic review. Nutrients 2013, 5, 4800–4821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, Z.; Wang, Y.; Sun, Y. Studies of the relation between the nutritional status of lactating mothers and milk composition as well as the milk intake and growth of their infants in Beijing. Pt. 4. The protein and amino acid content of breast milk. Acta Nutr. Sin. 1989, 11, 227–232. [Google Scholar]
- Ding, M.; Li, W.; Zhang, Y.; Wang, X.; Zhao, A.; Zhao, X.; Wang, P.; Sheng, Q.H. Amino acid composition of lactating mothers' milk and confinement diet in rural North China. Asia Pac. J. Clin. Nutr. 2010, 19, 344–349. [Google Scholar] [PubMed]
- Yang, T.; Zhang, Y.; Ning, Y.; You, L.; Ma, D.; Zheng, Y.; Yang, X.; Li, W.; Wang, J.; Wang, P. Breast milk macronutrient composition and the associated factors in urban Chinese mothers. Chin. Med. J. (Engl.) 2014, 127, 1721–1725. [Google Scholar] [PubMed]
- Blankenship, D.T.; Krivanek, M.A.; Ackermann, B.L.; Cardin, A.D. High-sensitivity amino acid analysis by derivatization with O-phthalaldehyde and 9-fluorenylmethyl chloroformate using fluorescence detection: Applications in protein structure determination. Anal. Biochem. 1989, 178, 227–232. [Google Scholar] [CrossRef]
- Lonnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [PubMed]
- Dupont, C. Protein requirements during the first year of life. Am. J. Clin. Nutr. 2003, 77, 1544S–1549S. [Google Scholar] [PubMed]
- Hassiotou, F.; Geddes, D.T. Programming of appetite control during breastfeeding as a preventative strategy against the obesity epidemic. J. Hum. Lac. 2014, 30, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Peace, R.W.; Gilani, G.S. Chromatographic determination of amino acids in foods. J. AOAC Int. 2005, 88, 877–887. [Google Scholar] [PubMed]
- Affolter, M.; Garcia-Rodenas, C.L.; Vinyes-Pares, G.; Jenni, R.; Roggero, I.; Avanti-Nigro, O.; de Castro, C.A.; Zhao, A.; Zhang, Y.; Wang, P.; et al. Temporal Changes of Protein Composition in Breast Milk of Chinese Urban Mothers and Impact of Caesarean Section Delivery. Nutrients 2016, 8, E504. [Google Scholar] [CrossRef] [PubMed]
- Sindayikengera, S.; Xia, W.S. Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex. J. Zhejiang Univ. Sci. B 2006, 7, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Carratu, B.; Boniglia, C.; Lammardo, A.M.; Riva, E.; Sanzini, E. Free glutamine and glutamic acid increase in human milk through a three-month lactation period. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Reeds, P.J. Dispensable and indispensable amino acids for humans. J. Nutr. 2000, 130, 1835S–1840S. [Google Scholar] [PubMed]
- Reeds, P.J.; Burrin, D.G. Glutamine and the bowel. J. Nutr. 2001, 131, 2505S–2508S. [Google Scholar] [PubMed]
- Rezaei, R.; Wang, W.; Wu, Z.; Dai, Z.; Wang, J.; Wu, G. Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J. Anim. Sci. Biotechnol. 2013, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Van der Hulst, R.R.; van Kreel, B.K.; von Meyenfeldt, M.F.; Brummer, R.J.; Arends, J.W.; Deutz, N.E.; Soeters, P.B. Glutamine and the preservation of gut integrity. Lancet 1993, 341, 1363–1365. [Google Scholar] [CrossRef]
- Roig, J.C.; Meetze, W.H.; Auestad, N.; Jasionowski, T.; Veerman, M.; McMurray, C.A.; Neu, J. Enteral glutamine supplementation for the very low birthweight infant: Plasma amino acid concentrations. J. Nutr. 1996, 126, 1115S–1120S. [Google Scholar] [PubMed]
- Burrin, D.G.; Stoll, B. Key nutrients and growth factors for the neonatal gastrointestinal tract. Clin. Perinatol. 2002, 29, 65–96. [Google Scholar] [CrossRef]
- Sánchez, C.L.; Cubero, J.; Sánchez, J.; Franco, L.; Rodríguez, A.B.; Rivero, M.; Barriga, C. Evolution of the circadian profile of human milk amino acids during breastfeeding. J. Appl. Biomed. 2013, 11, 59–70. [Google Scholar] [CrossRef]
| Lactation Period | ||||||
|---|---|---|---|---|---|---|
| 5–11 Days | 12–30 Days | 1–2 Months | 2–4 Months | 4–8 Months | p Value | |
| (n = 90) | (n = 90) | (n = 90) | (n = 90) | (n = 90) | ||
| MOTHER | ||||||
| Age (years), Mean (SD) | 27 (4) | 27 (3) | 28 (4) | 27 (4) | 26 (4) | 0.005 |
| Height (cm), Mean (SD) | 160 (4) | 160 (5) | 161 (5) | 161 (5) | 159 (5) | 0.102 |
| Weight (kg), Mean (SD) | 60.7 (8.7) | 60.8 (7.9) | 61.9 (8.9) | 58.4 (8.3) | 56.2 (8.1) | <0.001 |
| BMI (kg/m2), Mean (SD) | 23.7 (3.2) | 23.7 (3.0) | 23.9 (3.1) | 22.5 (2.9) | 22.2 (3.1) | <0.001 |
| Gestational weight gain (kg), Mean (SD) | 16.7 (7.4) | 16.2 (6.0) | 15.9 (5.7) | 15.9 (5.9) | 14.9 (7.6) | 0.419 |
| Postpartum weight loss (kg), Mean (SD) | 9.1 (6.1) | 8.6 (5.3) | 9.8 (4.0) | 10.0 (6.2) | 10.6 (5.9) | 0.119 |
| Non-Smoker, n (%) | 90 (100) | 89 (99) | 90 (100) | 86 (98) | 89 (100) | 0.176 |
| Cesarean delivery, n (%) | 39 (42) | 43 (48) | 53 (59) | 35 (39) | 35 (38) | 0.004 |
| Household income (RMB/month) | ||||||
| <2000 RMB, n (%) | 20 (22) | 17 (19) | 24 (27) | 26 (29) | 31 (34) | |
| 2000–4000 RMB, n (%) | 37 (41) | 45 (50) | 41 (46) | 40 (44) | 41 (46) | |
| >4000 RMB, n (%) | 30 (33) | 22 (24) | 23 (26) | 22 (24) | 18 (20) | |
| Unknown, n (%) | 1 (1) | 6 (7) | 2 (2) | 0 (0) | 0 (0) | 0.206 |
| INFANT | ||||||
| Males, n (%) | 51 (57) | 48 (53) | 48 (53) | 54 (60) | 43 (48) | 0.865 |
| Gestational age at birth (weeks), Mean (SD) | 39.3 (1.2) | 39.2 (1.3) | 39.2 (1.6) | 39.4 (1.3) | 39.5 (1.5) | 0.684 |
| Lactation Period | |||||
|---|---|---|---|---|---|
| 5–11 Days | 12–30 Days | 1–2 Months | 2–4 Months | 4–8 Months | |
| IAA † | |||||
| Histidine | 51.2 (19.9) | 44.5 § (14.1) | 36.5 § (12.6) | 34.9 § (7.2) | 25.0 § (6.8) |
| Isoleucine | 81.0 (23.4) | 71.6 § (15.4) | 64.6 § (16.8) | 54.0 § (11.6) | 53.8 (10.7) |
| Leucine | 153.7 (63.2) | 133.7 § (35.1) | 130.3 (33.5) | 108.1 § (24.9) | 122.6 § (38.8) |
| Lysine | 112.0 (31.0) | 93.8 § (23.1) | 78.8 § (18.9) | 63.4 § (13.1) | 67.9 § (13.1) |
| Methionine | 21.8 (11.7) | 16.7 § (6.6) | 13.0 § (9.0) | 9.2 § (6.1) | 11.8 § (7.1) |
| Phenylalanine | 64.4 (35.9) | 52.4 § (18.3) | 40.4 § (13.6) | 37.6 § (10.8) | 28.4 § (9.0) |
| Threonine | 85.1 (28.1) | 66.9 § (14.6) | 58.0 § (13.3) | 50.0 § (8.7) | 48.6 (11.3) |
| Valine | 97.9 (34.3) | 81.1 § (16.7) | 72.1 § (21.0) | 59.7 § (16.0) | 60.9 (12.7) |
| DAA † | |||||
| Alanine | 70.9 (23.0) | 55.9 § (14.3) | 45.9 § (15.7) | 38.7 § (10.9) | 38.6 (9.1) |
| Arginine | 106.5 (36.6) | 90.8 § (22.8) | 77.0 § (24.5) | 64.6 § (21.3) | 65.3 (16.7) |
| Asx ‡ | 132.9 (84.1) | 115.5 § (54.4) | 106.9 (40.0) | 97.2 (56.8) | 83.8 § (24.6) |
| Cystine | 25.4 (12.5) | 17.7 § (6.3) | 12.5 § (5.2) | 12.3 (3.4) | 9.9 § (5.5) |
| Glx ‡ | 248.1 (193.7) | 220.1 § (92.4) | 216.3 (59.3) | 188.6 (105.2) | 182.8 § (30.8) |
| Glycine | 46.3 (15.2) | 34.5 § (9.7) | 27.6 § (10.5) | 23.6 § (7.0) | 23.5 (6.8) |
| Proline | 140.2 (42.4) | 117.7 § (26.5) | 110.6 § (25.4) | 95.3 § (20.9) | 94.5 (17.2) |
| Serine | 77.8 (27.0) | 59.0 § (14.1) | 47.9 § (9.8) | 42.9 § (8.1) | 41.7 (8.0) |
| Tyrosine | 72.5 (30.4) | 57.7 § (14.1) | 44.1 § (19.5) | 41.4 (13.9) | 37.1 § (10.3) |
| SUM | 1608.3 (589.5) | 1296.5 § (368.4) | 1188.1 § (341.7) | 1053.2 § (291.9) | 992.4 (175.9) |
| Lactation Period | |||||
|---|---|---|---|---|---|
| 5–11 Days | 12–30 Days | 1–2 Months | 2–4 Months | 4–8 Months | |
| IAA † | |||||
| Histidine | 0.29 (0.21) | 0.42 § (0.23) | 0.33 § (0.15) | 0.33 (0.19) | 0.28 (0.10) |
| Isoleucine | 0.17 (0.11) | 0.19 (0.13) | 0.13 § (0.10) | 0.13 (0.07) | 0.15 § (0.07) |
| Leucine | 0.33 (0.20) | 0.4 (0.2) | 0.34 § (0.14) | 0.33 (0.14) | 0.34 (0.15) |
| Lysine | 0.61 (0.51) | 0.56 § (0.28) | 0.46 § (0.20) | 0.42 § (0.23) | 0.54 § (0.28) |
| Methionine | 0.11 (0.07) | 0.13 (0.13) | 0.10 § (0.07) | 0.07 § (0.06) | 0.12 § (0.05) |
| Phenylalanine | 0.31 (0.17) | 0.40 (0.17) | 0.32 § (0.17) | 0.33 (0.17) | 0.30 (0.12) |
| Threonine | 0.69 (0.38) | 0.69 (0.36) | 0.70 (0.36) | 0.78 (0.34) | 0.85 (0.38) |
| Valine | 0.58 (0.27) | 0.70 § (0.30) | 0.61 § (0.21) | 0.59 (0.21) | 0.59 (0.18) |
| DAA † | |||||
| Alanine | 1.26 (0.81) | 1.75 § (0.79) | 2.07 § (0.67) | 1.93 (0.68) | 1.85 (0.46) |
| Arginine | 0.46 (0.49) | 0.42 § (0.28) | 0.25 § (0.22) | 0.25 (0.19) | 0.25 (0.13) |
| Asx ‡ | 0.47 (0.36) | 0.52 (0.30) | 0.54 (0.35) | 0.55 (0.38) | 0.58 (0.40) |
| Cystine | 0.32 (0.13) | 0.49 § (0.21) | 0.46 (0.17) | 0.49 (0.21) | 0.50 (0.15) |
| Glx ‡ | 10.89 (9.89) | 15.09 § (8.74) | 18.03 § (7.17) | 20.22 § (7.28) | 19.36 (8.07) |
| Glycine | 0.51 (0.29) | 0.62 § (0.23) | 0.68 § (0.25) | 0.64 (0.28) | 0.76 § (0.28) |
| Proline | 0.56 (0.33) | 0.40 § (0.28) | 0.54 (0.29) | 0.40 (0.31) | 0.45 § (0.44) |
| Serine | 0.72 (0.43) | 0.85 § (0.36) | 0.91 § (0.40) | 1.11 § (0.63) | 1.11 (0.43) |
| Taurine | 2.26 (2.65) | 1.91 (1.78) | 1.94 (1.31) | 1.87 (1.42) | 2.03 (1.12) |
| Tyrosine | 0.38 (0.26) | 0.40 (0.20) | 0.28 § (0.17) | 0.25 (0.14) | 0.28 § (0.13) |
| SUM | 20.1 (12.5) | 25.5 § (10.4) | 27.4 § (8.0) | 29.0 (9.7) | 28.6 (10.7) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Rodenas, C.L.; Affolter, M.; Vinyes-Pares, G.; De Castro, C.A.; Karagounis, L.G.; Zhang, Y.; Wang, P.; Thakkar, S.K. Amino Acid Composition of Breast Milk from Urban Chinese Mothers. Nutrients 2016, 8, 606. https://doi.org/10.3390/nu8100606
Garcia-Rodenas CL, Affolter M, Vinyes-Pares G, De Castro CA, Karagounis LG, Zhang Y, Wang P, Thakkar SK. Amino Acid Composition of Breast Milk from Urban Chinese Mothers. Nutrients. 2016; 8(10):606. https://doi.org/10.3390/nu8100606
Chicago/Turabian StyleGarcia-Rodenas, Clara L., Michael Affolter, Gerard Vinyes-Pares, Carlos A. De Castro, Leonidas G. Karagounis, Yumei Zhang, Peiyu Wang, and Sagar K. Thakkar. 2016. "Amino Acid Composition of Breast Milk from Urban Chinese Mothers" Nutrients 8, no. 10: 606. https://doi.org/10.3390/nu8100606





