Plasma Free Amino Acid Responses to Intraduodenal Whey Protein, and Relationships with Insulin, Glucagon-Like Peptide-1 and Energy Intake in Lean Healthy Men
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Study Outline
2.3. Intraduodenal Infusions
| AA | Treatment | ||
|---|---|---|---|
| P2.1 | P6.3 | P12.5 | |
| g (mol/L) | |||
| Glutamic acid | 1.3 (36.3) | 3.8 (108.9) | 7.7 (217.8) |
| Leucine | 0.9 (29.7) | 2.8 (89.1) | 5.6 (178.2) |
| Aspartic acid | 0.8 (26.5) | 2.5 (79.3) | 5.1 (158.7) |
| Lysine | 0.8 (23.4) | 2.5 (70.3) | 5.0 (140.5) |
| Alanine | 0.4 (20.8) | 1.3 (62.4) | 2.7 (124.8) |
| Valine | 0.4 (14.7) | 1.2 (44.2) | 2.5 (88.4) |
| Proline | 0.4 (14.5) | 1.2 (43.4) | 2.4 (86.9) |
| Isoleucine | 0.4 (14.1) | 1.3 (42.4) | 2.7 (84.8) |
| Threonine | 0.4 (13.2) | 1.1 (39.5) | 2.3 (79.1) |
| Serine | 0.3 (9.9) | 0.8 (29.6) | 1.5 (59.2) |
| Phenylalanine | 0.3 (7.8) | 0.9 (23.4) | 1.9 (46.7) |
| Tyrosine | 0.3 (7.3) | 0.9 (21.9) | 1.9 (43.7) |
| Arginine | 0.3 (5.9) | 0.8 (17.8) | 1.5 (35.7) |
| Glycine | 0.2 (10.5) | 0.6 (31.4) | 1.1 (62.8) |
| Cysteine | 0.2 (6.5) | 0.6 (19.5) | 1.1 (38.9) |
| Methionine | 0.2 (4.9) | 0.5 (14.5) | 1.0 (29.1) |
| Histidine | 0.2 (4.7) | 0.5 (14.0) | 1.0 (28.0) |
| Tryptophan | 0.2 (3.9) | 0.6 (11.6) | 1.1 (23.1) |
| Glutamine | Not reported | ||
| Asparagine | Not reported | ||
| Total | 8.0 (254) | 24.0 (763) | 48.0 (1526) |
2.4. Measurements
2.5. Data and Statistical Analyses
3. Results
3.1. Plasma AA Concentrations
| AA | C | Treatment | p Value c | ||
|---|---|---|---|---|---|
| P2.1 | P6.3 | P12.5 | |||
| µmol/L | |||||
| Glutamine | 520 | 530 | 540 | 540 | >0.05 |
| Alanine | 240 | 240 | 240 | 270 | >0.05 |
| Valine | 190 | 190 | 200 | 190 | >0.05 |
| Glycine | 180 | 180 | 190 | 190 | >0.05 |
| Proline | 150 | 150 | 150 | 160 | >0.05 |
| Lysine | 150 | 150 | 150 | 150 | >0.05 |
| Threonine | 200 | 100 | 100 | 100 | >0.05 |
| Leucine | 100 | 100 | 100 | 100 | >0.05 |
| Serine | 90 | 90 | 90 | 90 | >0.05 |
| Glutamic acid | 80 | 90 | 90 | 90 | >0.05 |
| Arginine | 80 | 80 | 80 | 80 | >0.05 |
| Histidine | 60 | 70 | 70 | 70 | >0.05 |
| Isoleucine | 50 | 50 | 50 | 50 | >0.05 |
| Tyrosine | 50 | 50 | 50 | 50 | >0.05 |
| Asparagine | 40 | 50 | 50 | 50 | >0.05 |
| Phenylalanine | 40 | 40 | 40 | 40 | >0.05 |
| Methionine | 020 | 20 | 20 | 20 | >0.05 |
| Aspartic acid | 3 | 3 | 3 | 3 | >0.05 |
| Tryptophan | 3 | 3 | 3 | 3 | >0.05 |
| Cysteine | 2 | 2 | 2 | 2 | >0.05 |
| Total | 2130 | 2200 | 2200 | 2230 | >0.05 |
| AA | C | Treatment | p Value c | ||
|---|---|---|---|---|---|
| P2.1 | P6.3 | P12.5 | |||
| µmol.60 min/L | |||||
| Aspartic acid | 200 | 300 d,e | 500 d | 7000 d | <0.001 |
| Isoleucine | 3200 | 6200 d,e | 8600 d,e | 12,100 d | <0.001 |
| Leucine | 6100 | 11,700 d,e | 15,900 d,e | 21,900 d | <0.001 |
| Lysine | 9000 | 14,000 d,e | 18,000 d,e | 22,800 d | <0.001 |
| Methionine | 1100 | 1700 d,e | 2100 d,e | 2900 d | <0.001 |
| Tryptophan | 2000 | 300 e | 300 d,e | 4000 d | <0.001 |
| Tyrosine | 2800 | 3800 d,e | 4500 d,e | 5800 d | <0.001 |
| Valine | 11,300 | 14,600 d,e | 17,500 d,e | 20,800 d | <0.001 |
| Phenylalanine | 2500 | 3200 d,e | 3600 d,e | 4500 d | <0.001 |
| Threonine | 5800 | 7600 d,e | 9100 d | 10,500 d | <0.001 |
| Asparagine | 2700 | 3500 d,e | 4200 d | 4800 d | <0.001 |
| Proline | 8800 | 10,700 d,e | 12,100 d,e | 14,800 d | <0.001 |
| Alanine | 14,500 | 17,000 e | 19,200 d | 23,100 d | <0.001 |
| Arginine | 4600 | 6100 d,e | 700 d,e | 8400 d | <0.001 |
| Serine | 5200 | 6500 d,e | 7400 d | 8000 d | <0.001 |
| Glutamic acid | 4900 | 6100 d | 6200 d | 7100 d | <0.001 |
| Histidine | 3900 | 4500 d,e | 4800 d,e | 5100 d | <0.001 |
| Glutamine | 31,900 | 35,100 d,e | 36,700 d | 38,100 d | <0.001 |
| Glycine | 10,800 | 11,600 | 12,300 d | 12,800 d | <0.001 |
| Cysteine | 100 | 100 | 200 | 100 | >0.05 |
| Total | 129,000 | 165,000 d,e | 190,000 d,e | 225,000 d | <0.001 |
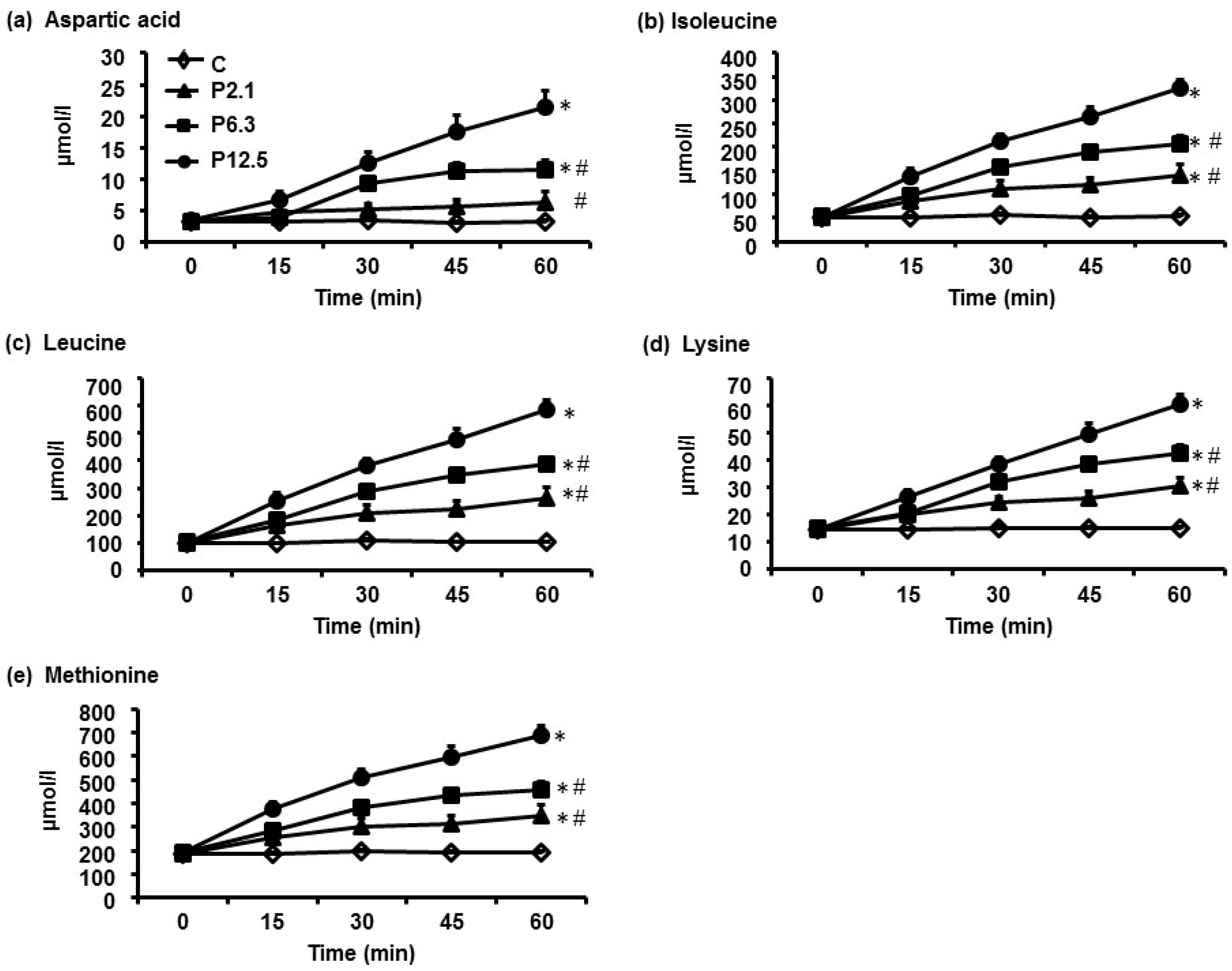
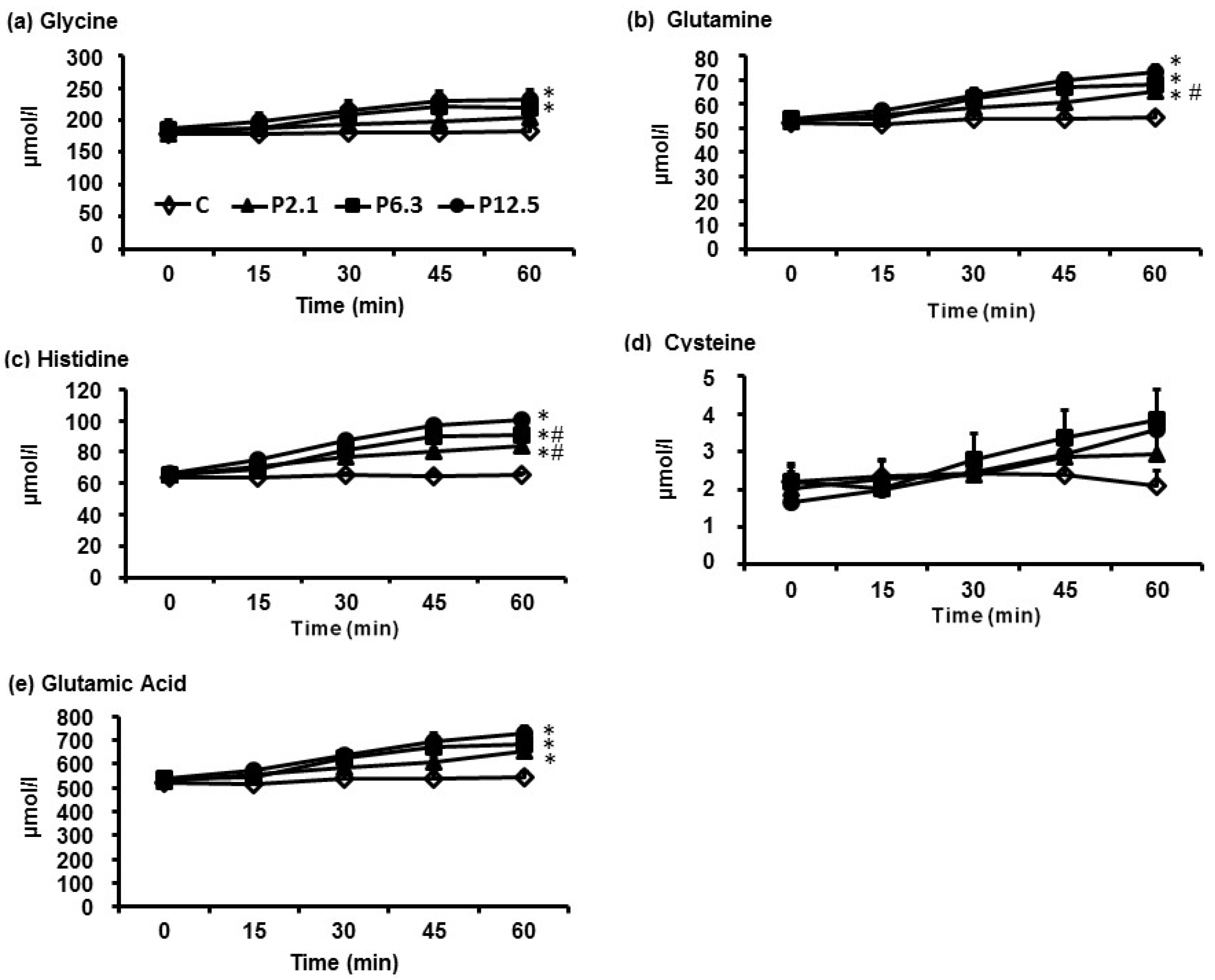
3.2. Serum Insulin and Plasma GLP-1 Concentrations, and Energy Intake and Amount of Food Consumed at the Buffet Meal
| Hormone and Food Intake Response | C | P2.1 | P6.3 | P12.5 | p Value c |
|---|---|---|---|---|---|
| Insulin (mU.60min/L) | 150 ± 19 | 354 ± 94 d | 721 ± 99 d | 1087 ± 145 d,e | <0.05 |
| GLP-1 ( mmol.60min/L) | 1489±113 | 1698 ± 142 | 2053 ± 159 d,f | 2147 ± 171 d,f | <0.05 |
| Energy intake (buffet; kJ) | 5173 ± 464 | 4981 ± 473 | 4504 ± 523 g | 3814 ± 502 d,e,f | <0.05 |
| Amount of food eaten (g) | 1261 ± 102 | 1196 ± 104 | 1109 ± 118 | 1009 ± 107 | 0.08 |
3.3. Relations between Insulin GLP-1 and Energy Intake with Plasma AA Concentrations
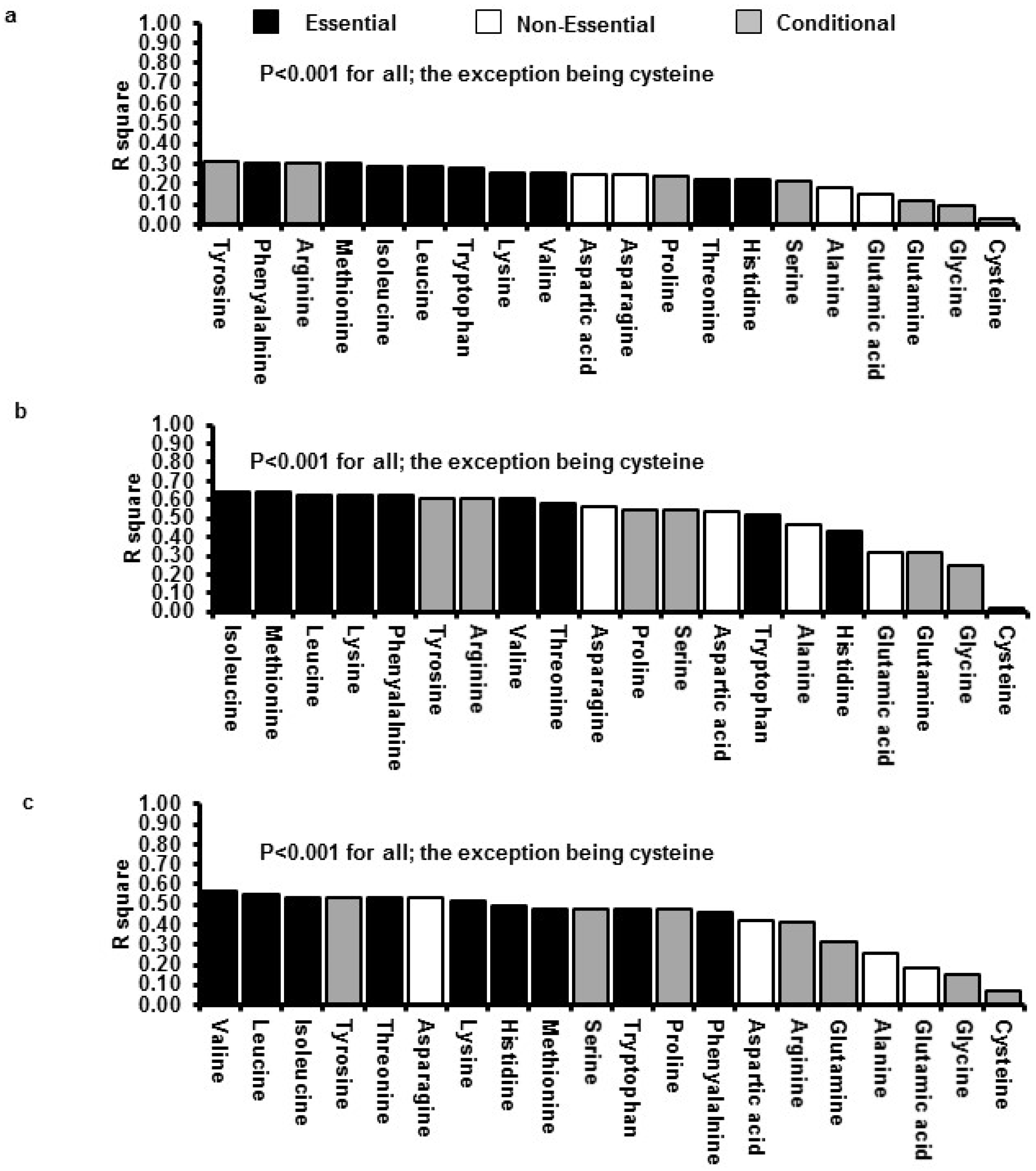
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ID | intraduodenalGI: Gastrointestinal |
| GLP-1 | glucagon-like peptide-1 |
| CCK | cholecystokinin |
| GIP | glucose-dependent insulinotropic polypeptide |
| PYY | peptide YY |
| AA | area amino acids |
| AUC0–60 min | area under the curve from 0 to 60 min |
| P2.1 | protein load 2.1 kJ/min |
| P6.3 | protein load 6.3 kJ/min |
| P12.5 | protein load 12.5 kJ/min |
| C | saline control |
| APD | antropyloroduodenal motility |
| VAS | visual analog scale questionnaire |
References
- Luscombe-Marsh, N.D.; Noakes, M.; Wittert, G.A.; Keogh, J.B.; Foster, P.; Clifton, P.M. Carbohydrate-restricted diets high in either monounsaturated fat or protein are equally effective at promoting fat loss and improving blood lipids. Am. J. Clin. Nutr. 2005, 81, 762–772. [Google Scholar] [PubMed]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [PubMed]
- Clifton, P.M.; Bastiaans, K.; Keogh, J.B. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.; Noakes, M.; Luscombe, N.; Clifton, P. Effect of a high-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes. Diabetes Care 2002, 25, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Pilichiewicz, A.N.; Papadopoulos, P.; Brennan, I.M.; Little, T.J.; Meyer, J.H.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Load-dependent effects of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2170–R2178. [Google Scholar] [CrossRef] [PubMed]
- Pilichiewicz, A.N.; Chaikomin, R.; Brennan, I.M.; Wishart, J.M.; Rayner, C.K.; Jones, K.L.; Smout, A.J.; Horowitz, M.; Feinle-Bisset, C. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E743–E753. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.T.; Feinle-Bisset, C.; Kallas, A.; Wishart, J.M.; Clifton, P.M.; Horowitz, M.; Luscombe-Marsh, N.D. Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am. J. Clin. Nutr. 2012, 96, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Read, N.W.; Heddle, R.; Horowitz, M.; Collins, P.J.; Chatterton, B.; Dent, J. Relationship of the motor activity of the antrum, pylorus, and duodenum to gastric emptying of a solid-liquid mixed meal. Gastroenterology 1988, 94, 1285–1291. [Google Scholar] [PubMed]
- Horowitz, M.; Edelbroek, M.A.L.; Wishart, J.M.; Straathof, J.W. Relationship between oral glucose-tolerance and gastric-emptying in normal healthy-subjects. Diabetologia 1993, 36, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.T.; Luscombe-Marsh, N.D.; Saies, A.A.; Little, T.J.; Standfield, S.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am. J. Clin. Nutr. 2013, 98, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Floyd, J.C., Jr.; Fajans, S.S.; Conn, J.W.; Knopf, R.F.; Rull, J. Insulin secretion in response to protein ingestion. J. Clin. Investig. 1966, 45, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Veldhorst, M.A.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; Westerterp, K.R.; Engelen, M.P.; Brummer, R.J.; Deutz, N.E.; Westerterp-Plantenga, M.S. Effects of complete whey-protein breakfasts versus whey without GMP-breakfasts on energy intake and satiety. Appetite 2009, 52, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Holst, J.J.; Bjorck, I.M. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: Studies using glucose-equivalent drinks. Am. J. Clin. Nutr. 2007, 85, 996–1004. [Google Scholar] [PubMed]
- Fromentin, G.; Darcel, N.; Chaumontet, C.; Marsset-Baglieri, A.; Nadkarni, N.; Tome, D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr. Res. Rev. 2012, 25, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.H.; Tecimer, S.N.; Shah, D.; Zafar, T.A. Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J. Nutr. 2004, 134, 3011–3015. [Google Scholar] [PubMed]
- Pal, S.; Ellis, V. The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br. J. Nutr. 2010, 104, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Silk, D.B.; Grimble, G.K.; Rees, R.G. Protein digestion and amino acid and peptide absorption. Proc. Nutr. Soc. 1985, 44, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Stanton, C.; Fitz Gerald, R.J.; Donnelly, W.J.; O’Connor, P. Development of technologies for separation and functional improvement of individual milk protein fractions. Available online: http://www.teagasc.ie/research/reports/dairyproduction/4216/eopr-4216.asp (accessed on 30 November 2015).
- Nair, N.S.; Brennan, I.M.; Little, T.J.; Gentilcore, D.; Hausken, T.; Jones, K.L.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Reproducibility of energy intake, gastric emptying, blood glucose, plasma insulin and cholecystokinin responses in healthy young males. Br. J. Nutr. 2009, 101, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A. Amino Acid Analysis Using Precolumn Derivatisation with 6-Aminoquino lyl-n-Hydroxysuccinimidyl Carbamate; Humana Press: Totowa, NJ, USA, 2001; pp. 39–47. [Google Scholar]
- Shoveller, A.K.; Stoll, B.; Ball, R.O.; Burrin, D.G. Nutritional and functional importance of intestinal sulfur amino acid metabolism. J. Nutr. 2005, 135, 1609–1612. [Google Scholar] [PubMed]
- Adibi, S.A.; Gray, S.J. Intestinal absorption of essential amino acids in man. Gastroenterology 1967, 52, 837–845. [Google Scholar] [PubMed]
- Hegarty, J.E.; Fairclough, P.D.; Moriarty, K.J.; Clark, M.L.; Kelly, M.J.; Dawson, A.M. Comparison of plasma and intraluminal amino acid profiles in man after meals containing a protein hydrolysate and equivalent amino acid mixture. Gut 1982, 23, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Frame, E.G. The levels of individual free amino acids in the plasma of normal man at various intervals after a high-protein meal. J. Clin. Investig. 1958, 37, 1710–1723. [Google Scholar] [CrossRef] [PubMed]
- Yearick, E.S.; Nadeau, R.G. Serum amino acid response to isocaloric test meals. Am. J. Clin. Nutr. 1967, 20, 338–344. [Google Scholar] [PubMed]
- Longenecker, J.B.; Hause, N.L. Relationship between plasma amino acids and composition of the ingested protein. II. A shortened procedure to determine plasma amino acid (PAA) ratios. Am. J. Clin. Nutr. 1961, 9, 356–362. [Google Scholar] [PubMed]
- Calbet, J.A.; MacLean, D.A. Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans. J. Nutr. 2002, 132, 2174–2182. [Google Scholar] [PubMed]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.M. Intestinal absorption of amino acids and peptides. Proc. Nutr. Soc. 1972, 31, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Uneyama, H.; Niijima, A.; San Gabriel, A.; Torii, K. Luminal amino acid sensing in the rat gastric mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G1163–G1170. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.D.; Reed, S.D.; Henagan, T.M. Homeostatic regulation of protein intake: In search of a mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R917–R928. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.R.; Farooqi, I.S.; Keogh, J.M.; Henning, E.; Habib, A.M.; Blackwood, A.; Reimann, F.; Holst, J.J.; Gribble, F.M. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am. J. Clin. Nutr. 2009, 89, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Kim-Harris, S.; Fischman, M.W.; Foltin, R.W.; Moran, T.H.; Stoner, S.A. Satiety after preloads with different amounts of fat and carbohydrate: Implications for obesity. Am. J. Clin. Nutr. 1994, 60, 476–487. [Google Scholar] [PubMed]
- Brennan, I.M.; Feltrin, K.L.; Nair, N.S.; Hausken, T.; Little, T.J.; Gentilcore, D.; Wishart, J.M.; Jones, K.L.; Horowitz, M.; Feinle-Bisset, C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G602–G610. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luscombe-Marsh, N.D.; Hutchison, A.T.; Soenen, S.; Steinert, R.E.; Clifton, P.M.; Horowitz, M.; Feinle-Bisset, C. Plasma Free Amino Acid Responses to Intraduodenal Whey Protein, and Relationships with Insulin, Glucagon-Like Peptide-1 and Energy Intake in Lean Healthy Men. Nutrients 2016, 8, 4. https://doi.org/10.3390/nu8010004
Luscombe-Marsh ND, Hutchison AT, Soenen S, Steinert RE, Clifton PM, Horowitz M, Feinle-Bisset C. Plasma Free Amino Acid Responses to Intraduodenal Whey Protein, and Relationships with Insulin, Glucagon-Like Peptide-1 and Energy Intake in Lean Healthy Men. Nutrients. 2016; 8(1):4. https://doi.org/10.3390/nu8010004
Chicago/Turabian StyleLuscombe-Marsh, Natalie D., Amy T. Hutchison, Stijn Soenen, Robert E. Steinert, Peter M. Clifton, Michael Horowitz, and Christine Feinle-Bisset. 2016. "Plasma Free Amino Acid Responses to Intraduodenal Whey Protein, and Relationships with Insulin, Glucagon-Like Peptide-1 and Energy Intake in Lean Healthy Men" Nutrients 8, no. 1: 4. https://doi.org/10.3390/nu8010004





