Altered Preconception Fatty Acid Intake Is Associated with Improved Pregnancy Rates in Overweight and Obese Women Undertaking in Vitro Fertilisation
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Intervention
2.3. Outcomes
2.4. Statistical Analysis
3. Results
| Intervention n = 18 | Control n = 20 | p | |
|---|---|---|---|
| Age (years) | 33.8 ± 3.5 | 32.5 ± 3.3 | 0.25 |
| Infertility type | 6/18: Female | 4/20: Female | 0.43 |
| 7/18: Male | 10/20: Male | ||
| 2/18: Combined | 4/20: Combined | ||
| 2/18: Unexplained | 0/20: Unexplained | ||
| Infertility duration (years) | 4.2 ± 2.2 | 5.2 ± 2.6 | 0.27 |
| OCP use prior to trying for conception (years) | 4.3 ± 5.5 | 3.0 ± 4.9 | 0.46 |
| Menstrual cycle length (days) | 30.8 ± 5.7 | 30.0 ± 3.8 | 0.63 |
| Smoking (female) | 1/13 | 6/17 | 0.10 |
| Smoking (male) | 2/12 | 5/16 | 0.66 |
| Weight (kg) | 93.0 ± 16.0 | 92.1 ± 13.8 | 0.86 |
| Waist circumference (cm) | 103.9 ± 10.9 | 106.8 ± 8.8 | 0.39 |
| BMI (kg/m2) | 34.0 ± 4.5 | 33.9 ± 4.4 | 0.93 |
| Nutrient | Pregnant | Non-Pregnant | p | Logistic Regression |
|---|---|---|---|---|
| Energy (kJ) | 7969.1 ± 2666.1 | 6839.0 ± 3174.1 | 0.254 | OR = 1.00, 95% CI (1.00, 1.0007) p = 0.34 |
| % Protein | 20.5 ± 1.8 | 21.5 ± 4.4 | 0.369 | OR = 0.80, 95% CI (0.57, 1.12) p = 0.19 |
| % Carbohydrate | 40.9 ± 5.5 | 39.8 ± 7.1 | 0.585 | OR = 1.00, 95% CI (0.84, 1.19) p = 0.98 |
| % Fat | 37.4 ± 5.3 | 36.7 ± 5.5 | 0.693 | OR = 1.11, 95% CI (0.92, 1.31) p = 0.31 |
| % saturated fat | 14.7 ± 2.5 | 15.5 ± 2.8 | 0.409 | OR = 0.88, 95% CI (0.64, 1.19) p = 0.40 |
| % monounsaturated fat | 13.8 ± 2.2 | 13.2 ± 2.2 | 0.502 | OR = 1.54, 95% CI (0.90, 2.62) p = 0.11 |
| % polyunsaturated fat | 5.7 ± 1.8 | 4.7 ± 1.1 | 0.057 | OR = 2.30, 95% CI (1.11, 4.8) p = 0.03 |
| Total n3 fatty acids (g) | 1.7 ± 1.0 | 1.3 ± 0.9 | 0.167 | OR = 5.38, 95% CI (0.91, 31.78) p = 0.06 |
| Total n6 fatty acids (g) | 10.4 ± 5.0 | 7.6 ± 5.0 | 0.109 | OR = 1.27, 95% CI (1.01, 1.61) p = 0.045 |
| n6:n3 | 6.5 ± 2.3 | 6.3 ± 1.5 | 0.734 | OR = 1.28, 95% CI (0.77, 2.11) p = 0.34 |
| GL | 101.2 ± 31.5 | 80.4 ± 32.6 | 0.061 | OR = 1.02, 95% CI (0.98, 1.05) p = 0.32 |
| GI | 52.1 ± 3.6 | 49.9 ± 4.1 | 0.091 | OR = 1.32, 95% CI (0.96, 1.80) p = 0.09 |
| Fibre (g) | 20.4 ± 5.8 | 17.2 ± 7.1 | 0.141 | OR = 1.08, 95% CI (0.92, 1.26) p = 0.33 |
| Cholesterol (mg) | 292.0 ± 116.2 | 290.6 ± 190.7 | 0.979 | OR = 1.00, 95% CI (0.99, 1.01) p = 0.77 |
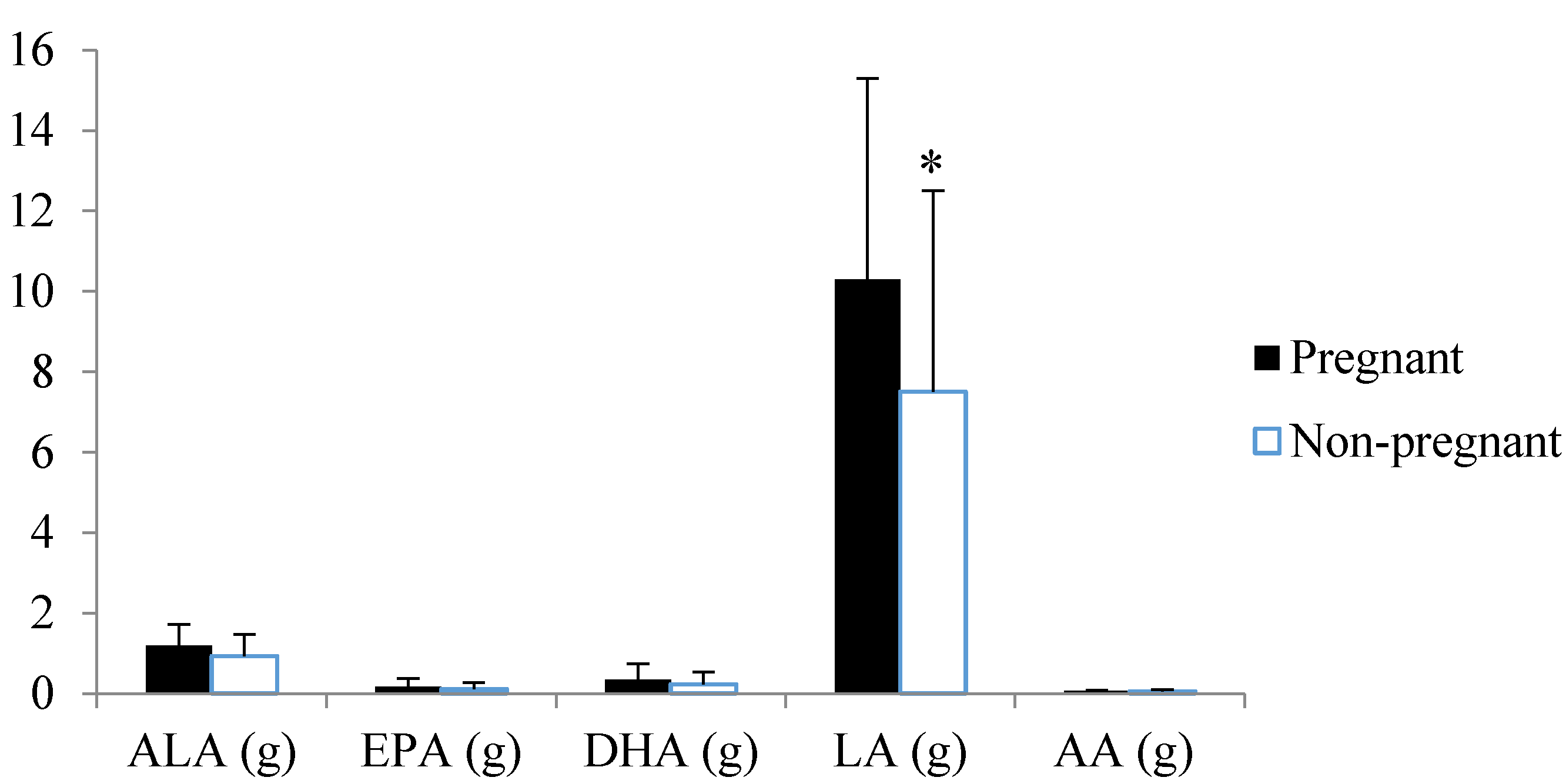
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur. J. Clin. Nutr. 2007, 19, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Protein intake and ovulatory infertility. Am. J. Obstet. Gynecol. 2008, 198, 210.e1–217.e1. [Google Scholar] [CrossRef] [PubMed]
- Mmbaga, N.; Luk, J. The impact of preconceptual diet on the outcome of reproductive treatments. Curr. Opin. Obstet. Gynecol. 2012, 24, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Shaaker, M.; Rahimipour, A.; Nouri, M.; Khanaki, K.; Darabi, M.; Farzadi, L.; Shahnazi, V.; Mehdizadeh, A. Fatty acid composition of human follicular fluid phospholipids and fertilization rate in assisted reproductive techniques. Iran. Biomed. J. 2012, 16, 162–168. [Google Scholar] [PubMed]
- Hammiche, F.; Vujkovic, M.; Wijburg, W.; de Vries, J.H.; Macklon, N.S.; Laven, J.S.; Steegers-Theunissen, R.P. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertil. Steril. 2011, 95, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Kaipia, A.; Chun, S.Y.; Eisenhauer, K.; Hsueh, A.J. Tumor necrosis factor-alpha and its second messenger, ceramide, stimulate apoptosis in cultured ovarian follicles. Endocrinology 1996, 137, 4864–4870. [Google Scholar] [PubMed]
- Ou, X.H.; Li, S.; Wang, Z.B.; Li, M.; Quan, S.; Xing, F.; Guo, L.; Chao, S.B.; Chen, Z.; Liang, X.W.; et al. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum. Reprod. 2012, 27, 2130–2145. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, E.S.; Frolova, A.I.; Jiang, H.; Riley, J.K. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J. Clin. Endocrinol. Metab. 2013, 98, E1364–E1368. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.; Tsagareli, V.; Norman, R.; Noakes, M. Diet and IVF pilot study: Short-term weight loss improves pregnancy rates in overweight/obese women undertaking IVF. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Cleanthous, X.; Noakes, M.; Brinkworth, G.D.; Keogh, J.B.; Williams, G.; Clifton, P.M. A pilot comprehensive lifestyle intervention program (CLIP)—Comparison with qualitative lifestyle advice and simvastatin on cardiovascular risk factors in overweight hypercholesterolaemic individuals. Nutr. Metab. Cardiovasc. Dis. 2010, 21, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Patterson, A.J.; Brown, W.J.; Ireland, P.; Giles, G. The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public Health 2000, 24, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Dietary fatty acid intakes and the risk of ovulatory infertility. Am. J. Clin. Nutr. 2007, 85, 231–237. [Google Scholar] [PubMed]
- Jungheim, E.S.; Macones, G.A.; Odem, R.R.; Patterson, B.W.; Moley, K.H. Elevated serum alpha-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil. Steril. 2011, 96, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, S.L.; Lane, M.; Schulz, S.J.; Hebart, M.L.; Thompson, J.G.; Mitchell, M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E425–E434. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lim, H.; Paria, B.C.; Matsumoto, H.; Swift, L.L.; Morrow, J.; Bonventre, J.V.; Dey, S.K. Cytosolic phospholipase A2alpha is crucial [correction of A2alpha deficiency is crucial] for “on-time” embryo implantation that directs subsequent development. Development 2002, 129, 2879–2889. [Google Scholar] [PubMed]
- Marei, W.F.; Wathes, D.C.; Fouladi-Nashta, A.A. Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction 2010, 139, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Lopez-del Burgo, C.; Ruiz-Zambrana, A.; Donazar, M.; Navarro-Blasco, I.; Martinez-Gonzalez, M.A.; de Irala, J. Dietary patterns and difficulty conceiving: A nested case-control study. Fertil. Steril. 2011, 96, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic, M.; de Vries, J.H.; Lindemans, J.; Macklon, N.S.; van der Spek, P.J.; Steegers, E.A.; Steegers-Theunissen, R.P. The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertil. Steril. 2010, 94, 2096–2101. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moran, L.J.; Tsagareli, V.; Noakes, M.; Norman, R. Altered Preconception Fatty Acid Intake Is Associated with Improved Pregnancy Rates in Overweight and Obese Women Undertaking in Vitro Fertilisation. Nutrients 2016, 8, 10. https://doi.org/10.3390/nu8010010
Moran LJ, Tsagareli V, Noakes M, Norman R. Altered Preconception Fatty Acid Intake Is Associated with Improved Pregnancy Rates in Overweight and Obese Women Undertaking in Vitro Fertilisation. Nutrients. 2016; 8(1):10. https://doi.org/10.3390/nu8010010
Chicago/Turabian StyleMoran, Lisa J., Victoria Tsagareli, Manny Noakes, and Robert Norman. 2016. "Altered Preconception Fatty Acid Intake Is Associated with Improved Pregnancy Rates in Overweight and Obese Women Undertaking in Vitro Fertilisation" Nutrients 8, no. 1: 10. https://doi.org/10.3390/nu8010010




