Is It the Appropriate Time to Stop Applying Selenium Enriched Salt in Kashin-Beck Disease Areas in China?
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design and Subjects

2.2. Data Collection
2.2.1. Anthropometric Measurements
2.2.2. Questionnaire Investigation
| Information Categories | In Details |
|---|---|
| Including 17 items of non-dietary items | |
| Basic information | Serial number, date, contact number, name, gender, age, nationality, school, address, residence type |
| Parents | Occupation, education |
| Birth data | Cesarean, full-term birth or not, ate colostrums or not, feeding methods within six months after birth |
| Family history of KBD | Number of KBD patients and relationship between them and the children |
| Dietary items including the intakes, frequencies and sources | |
| Seasonings | 3 kinds of table salts, 3 kinds of oils, soy sauce, vinegar and other kinds of seasonings |
| Grains | Wheat, rice and corn |
| Vegetables | 4 kinds of greens, 4 kinds of cabbage, 6 kinds of tuber vegetables, 6 kinds of cucurbits, 3 kinds of solanaceous vegetables, 5 kinds of root vegetables, 7 kinds of bulb vegetables, 3 kinds of mushrooms, 2 kinds of aquatic vegetables and 2 kinds of flower vegetables |
| Beans | 8 kinds of beans and their products |
| Egg | Egg white only, yolk only, and whole egg |
| Meat | 3 kinds of livestock meat and products and 2 kinds of haslet, 2 kinds of poultry meat and products and 2 kinds of fish and seafood |
| Dairy products | 4 kinds of dairy products |
| Nuts | Peanut, sunflower seed, walnut |
| Fruits | 2 kinds of kernel fruit, 5 kinds of stone fruit, 4 kinds of citrus fruit, 3 kinds of melon, 5 kinds of grape and berry, 3 kinds of tropical fruit |
| Non-staple food | 7 kinds of fried-popping food, 16 kinds of confectionery and 4 kinds of soft drinks |
| Nutritious supplements | Yes or no, if yes then recorded in detail |
| Taste preferences | Normal, salty, sweat, spicy, sour, greasy |
| Water sources | Hole water, ditch water, well water, tap water, mineral water |
| Drinking habit | Unboiled water, boiled water, tea |

2.2.3. Sample Collection and Se Content Determination
2.3. Statistical Analyses
3. Results
3.1. General Situation
| County | Area | Male (n) | Age (years) X ± SD | Female (n) | Age (years) X ± SD | Total (n) | Age (years) X ± SD |
|---|---|---|---|---|---|---|---|
| KBD (Se+) | |||||||
| Linyou | Mild | 10 | 9.3 ± 3.7 | 20 | 8.5 ± 3.5 | 30 | 8.8 ± 3.5 |
| Medium | 15 | 8.2 ± 3.1 | 15 | 8.7 ± 2.5 | 30 | 8.4 ± 2.8 | |
| Severe | 15 | 9.0 ± 3.4 | 18 | 7.9 ± 2.9 | 33 | 8.4 ± 3.1 | |
| Total | 40 | 8.8 ± 3.3 | 53 | 8.4 ± 3.0 | 93 | 8.5 ± 3.1 | |
| Bin | Mild | 23 | 7.7 ± 3.3 | 13 | 9.6 ± 2.7 | 36 | 8.4 ± 3.2 |
| Medium | 15 | 7.8 ± 3.2 | 17 | 9.3 ± 2.8 | 32 | 8.6 ± 3.1 | |
| Severe | 20 | 8.0 ± 3.2 | 10 | 10.1 ± 4.3 | 30 | 8.7 ± 3.6 | |
| Total | 58 | 7.8 ± 3.2 | 40 | 9.6 ± 3.2 | 98 | 8.6 ± 3.3 | |
| KBD (Se−) | |||||||
| Ningshan | Mild | 14 | 10.6 ± 3.1 | 16 | 10.6 ± 1.4 | 30 | 10.6 ± 2.3 |
| Medium | 9 | 9.4 ± 2.3 | 21 | 10.0 ± 2.7 | 30 | 9.8 ± 2.6 | |
| Severe | 12 | 8.8 ± 3.0 | 15 | 8.7 ± 2.8 | 27 | 8.8 ± 2.9 | |
| Total | 35 | 9.7 ± 2.9 | 52 | 9.8 ± 2.5 | 87 | 9.8 ± 2.7 | |
| Non-KBD | |||||||
| Liquan | Total | 51 | 8.1 ± 3.2 | 39 | 8.7 ± 3.1 | 90 | 8.4 ± 3.1 |
| Total | 184 | 8.5 ± 3.2 | 184 | 9.1 ± 3.0 | 368 | 8.8 ± 3.1 | |
3.2. Comparison of Hair Se Content
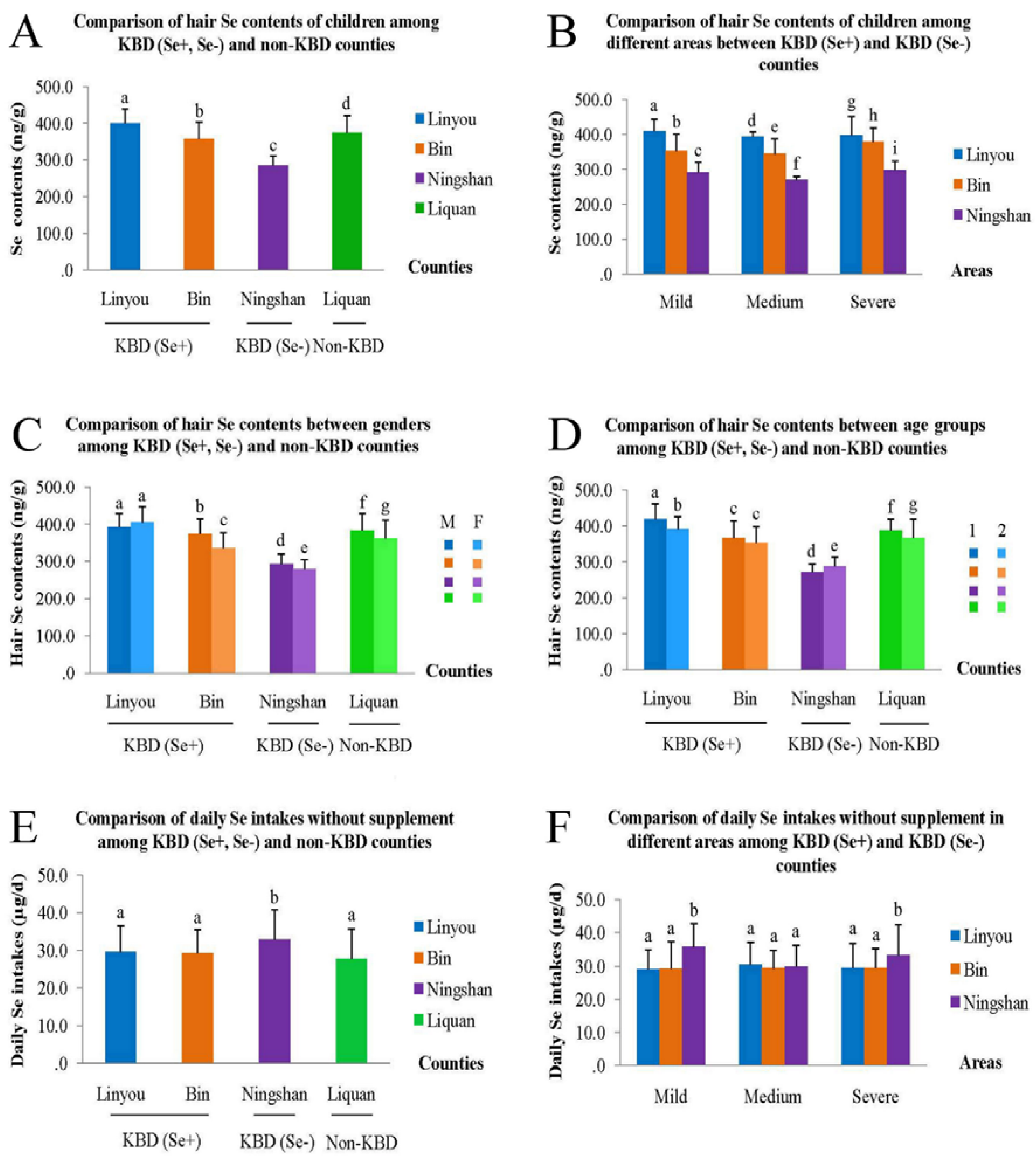
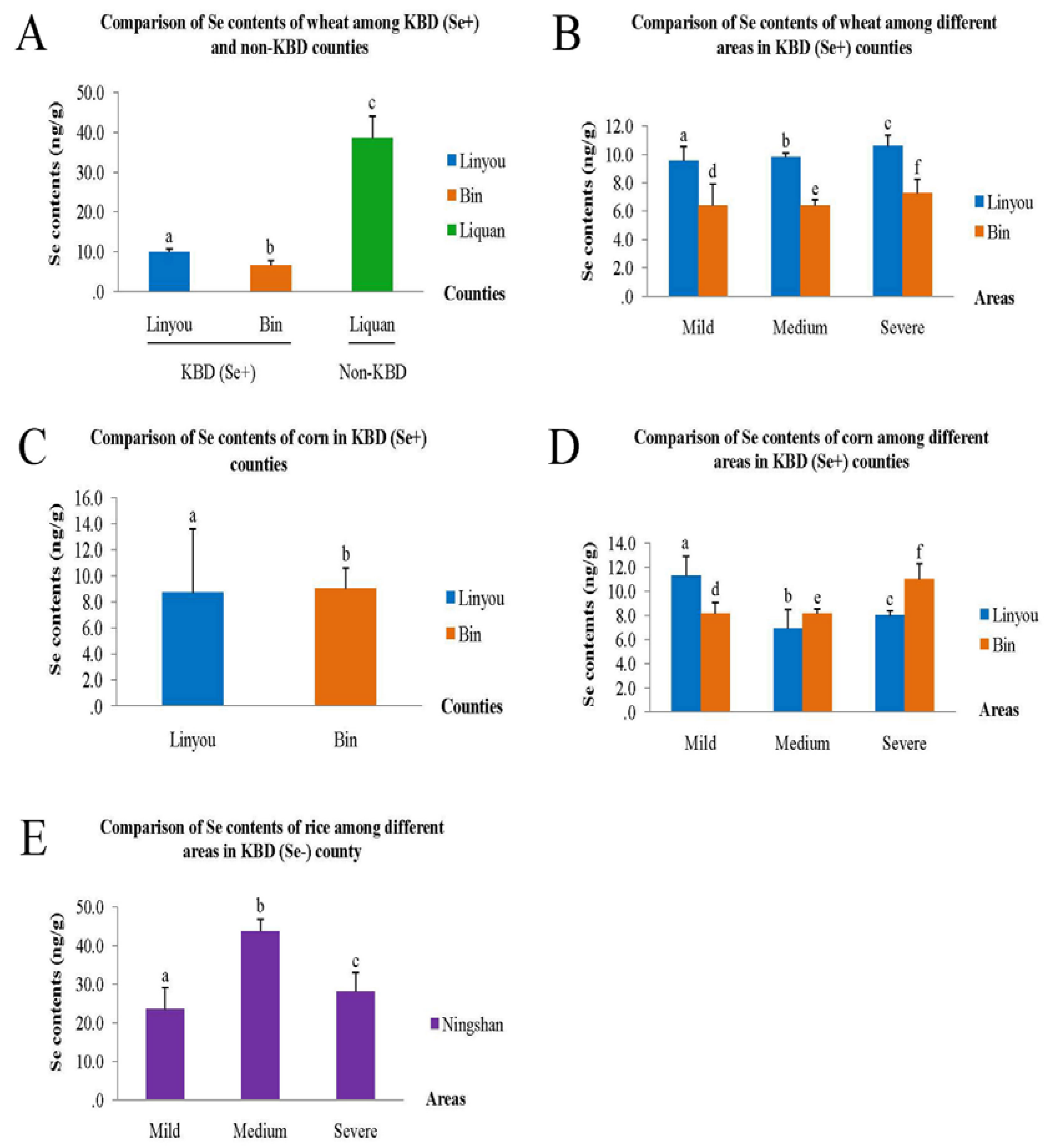
3.3. Comparison of Se Content of Food Samples
3.4. Relationship between Hair Se Content and Dietary and Non-Dietary Factors
3.5. Comparison of Daily Se Intake among Counties and the Contributing Food Categories
| Factors | B | 95.0% CI for B | t | P | R | |
|---|---|---|---|---|---|---|
| Lower bond | Upper bond | |||||
| Univariate regression | ||||||
| Non-dietary factors | ||||||
| Gender* | −20.12 | −31.90 | −8.35 | −3.36 | 0.001 | 0.17 |
| (−13.36) | (−22.14) | (−4.58) | (−2.99) | (0.003) | - | |
| Age (year) * | −4.58 | −6.46 | −2.71 | −4.80 | <0.01 | |
| (−2.46) | (−4.04) | (−0.87) | (−3.05) | (0.002) | ||
| BMI | 7.25 | 4.33 | 10.17 | 4.88 | <0.01 | 0.25 |
| Living area * | −25.14 | −38.80 | −11.47 | −3.62 | <0.01 | 0.19 |
| (−89.69) | (−106.01) | (−73.37) | (−10.81) | <0.01 | - | |
| Education level of father | 28.48 | 16.32 | 40.64 | 4.61 | <0.01 | 0.23 |
| Drank colostrum or not | −41.23 | −57.72 | −24.74 | −4.92 | <0.01 | 0.25 |
| Dietary factors | - | |||||
| Se-enriched salt or not * | −48.43 | −59.31 | −37.56 | −8.76 | <0.01 | 0.42 |
| (−103.03) | (−120.60) | (−85.46) | (−11.53) | <0.01 | - | |
| Oil source * | 27.41 | 15.59 | 39.24 | 4.56 | <0.01 | 0.23 |
| (15.25) | (4.12) | (26.37) | (2.70) | (0.007) | - | |
| Type of drinking Water | 58.56 | 47.17 | 69.96 | 10.11 | <0.01 | 0.47 |
| Daily Se intake (without Se-salt) * | 2.17 | 0.37 | 3.98 | 4.89 | 0.004 | 0.35 |
| (14.36) | (2.62) | (26.11) | (2.41) | (0.017) | - | |
| Daily energy intake | 0.02 | 0.01 | 0.04 | 2.91 | 0.004 | 0.15 |
| Daily protein intake * | 0.73 | 0.35 | 1.11 | 3.74 | <0.01 | 0.19 |
| 0.79 | 0.14 | 1.44 | 2.39 | 0.017 | - | |
| Daily carbohydrate intake | −0.15 | −0.26 | −0.04 | −2.64 | 0.009 | 0.14 |
| Daily fat intake | −1.70 | −2.18 | −1.22 | −6.93 | <0.01 | 0.34 |
| Daily vitamin C intake | 0.70 | 0.43 | 0.97 | 5.15 | <0.01 | 0.26 |
| Daily Ca intake | 0.08 | 0.05 | 0.11 | 5.84 | <0.01 | 0.29 |
| Daily Fe intake | 0.72 | 0.29 | 1.16 | 3.26 | 0.001 | 0.17 |
| Daily Zn intake | 7.05 | 5.65 | 8.46 | 9.86 | <0.01 | 0.46 |
| Multivariate regression * | ||||||
| Total | - | - | - | - | - | 0.77 |
| Factors | KBD (Se+) | KBD (Se−) | Non-KBD | Total | ||||
|---|---|---|---|---|---|---|---|---|
| P | R | P | R | P | R | P | R | |
| Wheat * | <0.01 | † 0.24 | 0.08 | 0.19 | <0.01 | † 0.48 | 0.01 | 0.22 |
| Rice * | 0.22 | 0.09 | 0.01 | † 0.28 | 0.31 | 0.11 | <0.01 | 0.24 |
| Greens * | 0.04 | 0.15 | 0.13 | 0.16 | 0.12 | 0.17 | 0.01 | 0.14 |
| Tuber vegetable * | 0.01 | † 0.23 | 0.03 | 0.23 | 0.06 | 0.20 | <0.01 | 0.22 |
| Solanaceous * | 0.01 | † 0.18 | 0.18 | 0.14 | 0.01 | 0.29 | <0.01 | 0.24 |
| Bulb vegetable * | 0.02 | † 0.17 | 0.11 | 0.17 | 0.06 | 0.20 | <0.01 | 0.20 |
| Aquatic vegetable | 0.02 | † 0.17 | 0.16 | 0.15 | 0.47 | 0.08 | <0.01 | 0.22 |
| Livestock meat * | 0.03 | † 0.16 | 0.04 | † 0.22 | 0.04 | † 0.21 | <0.01 | 0.20 |
| Poultry meat * | 0.12 | 0.11 | 0.50 | 0.07 | 0.23 | 0.13 | 0.04 | 0.11 |
| Milk * | <0.01 | † 0.18 | 0.04 | † 0.21 | 0.04 | † 0.22 | <0.01 | 0.21 |
| Nuts * | 0.16 | 0.10 | 0.01 | 0.27 | 0.19 | 0.14 | 0.01 | 0.14 |
| Kernel fruit | 0.06 | 0.14 | 0.18 | 0.14 | 0.04 | † 0.21 | 0.75 | 0.02 |
| Beverage * | 0.23 | 0.09 | <0.01 | † 0.36 | 0.25 | 0.12 | <0.01 | 0.20 |
| Total | <0.01 | 0.53 | <0.01 | 0.51 | <0.01 | 0.57 | <0.01 | 0.69 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Underwood, E.J.; Suttle, N.F. Selenium—Mineral Nutrition of Live Stock; CAB International: Midlothian, UK, 1999; Chapter 15. [Google Scholar]
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor-3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Loscalzo, J. Keshan disease, selenium deficiency, and the selenoproteome. N. Engl. J. Med. 2014, 370, 1756–1760. [Google Scholar] [PubMed]
- Hasunuma, N.; Umebayashi, Y.; Manabe, M. True leukonychia in crohn disease induced by selenium deficiency. JAMA Dermatol. 2014, 150, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.W.; Singh, K.; Motley, A.K.; Lintel, M.K.; Matafonova, E.; Bradley, A.M.; Ning, W.; Poindexter, S.V.; Parang, B.; Reddy, V.K.; et al. Dietary selenium deficiency exacerbates dss-induced epithelial injury and aom/dss-induced tumorigenesis. PLoS ONE 2013, 8, e67845. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.B.; Knudsen, N.; Carle, A.; Schomburg, L.; Kohrle, J.; Jorgensen, T.; Rasmussen, L.B.; Ovesen, L.; Laurberg, P. Serum selenium is low in newly diagnosed graves’ disease: A population-based study. Clin. Endocrinol. 2013, 79, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Javid, P.J.; Horslen, S. Selenium deficiency in pediatric patients with intestinal failure as a consequence of drug shortage. J. Parenter. Enteral Nutr. 2014, 38, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Streeter, R.M.; Divers, T.J.; Mittel, L.; Korn, A.E.; Wakshlag, J.J. Selenium deficiency associations with gender, breed, serum vitamin e and creatine kinase, clinical signs and diagnoses in horses of different age groups: A retrospective examination 1996–2011. Equine Vet. J. Suppl. 2012, 44, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-Moe, M.; Wisloff, H.; Bernhoft, A. Selenium deficiency associated porcine and human cardiomyopathies. J. Trace Elem. Med. Biol. 2014, 31, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.A.; Zhu, W.Y.; Wang, W.Y.; Li, R.B.; Hou, S.F.; Wang, D.C.; Yang, L.S. Selenium in soil and endemic diseases in china. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef]
- Mo, D.; Ding, D.; Wang, Z.; Zhang, J.; Bai, C. Study on relationship between selenium and kashin-beck disease in 20 years. Chin. J. Ctrl. Endem. Dis. 1997, 12, 18–21. [Google Scholar]
- Guo, X.; Zhang, S.; Mo, D. The role of selenium deficiency in the etiology of kashin-beck disease. Chin. J. Ctrl. Endem. Dis. 1993, 8, 82–84. [Google Scholar]
- Xiong, G. Diagnostic, clinical and radiological characteristics of kashin-beck disease in shaanxi province, PR China. Int. Orthop. 2001, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Liu, G.; Wu, T.; Du, L. Selenium for preventing kashin-beck osteoarthropathy in children: A meta-analysis. Osteoarthr. Cartil. 2009, 17, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Jirong, Y.; Huiyun, P.; Zhongzhe, Y.; Birong, D.; Weimin, L.; Ming, Y.; Yi, S. Sodium selenite for treatment of kashin-beck disease in children: A systematic review of randomised controlled trials. Osteoarthr. Cartil. 2012, 20, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhu, G. Summary of prevention of kashin-beck disease with selenium enriched salt. Chin. J. Endemiol. 1987, 6, 375–376. [Google Scholar]
- Li, Q.; Zhao, Z.; Wen, H. Effective observation on children kbd control with three different methods of selenium supplement in guide endemic area, qinghai. Endemic Dis. Bull. 2004, 19, 62–64. [Google Scholar]
- Ning, Y.J.; Wang, X.; Ren, L.; Guo, X. Effects of dietary factors on selenium levels of children to prevent kashin-beck disease during a high-prevalence period in an endemic area: A cohort study. Biol. Trace Elem. Res. 2013, 153, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Ochi, A.; Ishimura, E.; Tsujimoto, Y.; Kakiya, R.; Tabata, T.; Mori, K.; Tahara, H.; Shoji, T.; Yasuda, H.; Nishizawa, Y.; et al. Elemental concentrations in scalp hair, nutritional status and health-related quality of life in hemodialysis patients. Ther. Apher. Dial. 2012, 16, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Tamburo, E.; Varrica, D.; Dongarra, G.; Grimaldi, L.M.E. Trace elements in scalp hair samples from patients with relapsing-remitting multiple sclerosis. PLoS ONE 2015, 10, e0122142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younge, J.O.; Wester, V.L.; van Rossum, E.F.; Gotink, R.A.; Wery, M.F.; Utens, E.M.; Hunink, M.G.; Roos-Hesselink, J.W. Cortisol levels in scalp hair of patients with structural heart disease. Int. J. Cardiol. 2015, 184, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Zheng, F.Y.; Cai, S.J.; Cai, T.S. Determination of mercury and selenium in herbal medicines and hair by using a nanometer tio2-coated quartz tube atomizer and hydride generation atomic absorption spectrometry. J. Hazard. Mater. 2011, 189, 609–613. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.A.; Dillon, C.F.; Osterloh, J.; Bolger, P.M.; Pellizzari, E.; Fernando, R.; Montes de Oca, R.; Schober, S.E.; Sinks, T.; Jones, R.L.; et al. Hair mercury levels in U.S. Children and women of childbearing age: Reference range data from nhanes 1999–2000. Environ. Health Perspect. 2004, 112, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Yang, L.S.; Wang, W.Y.; Li, H.R.; Lv, J.M.; Zou, X.Y. Trace element concentrations in hair of healthy chinese centenarians. Sci. Total Environ. 2011, 409, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, S.; Wang, Z.; Zhou, Y.; Wang, P.; Wang, G.; Zhang, L.; Li, H. Effect of 24-year comprehensive method conducted to control kashin-beck disease in xianyang county. Chin. J. Ctrl. Endem. Dis. 2005, 20, 227–229. [Google Scholar]
- Guo, X.; Ding, D.; Wang, Z.; Lv, S.; Zhang, J.; Tan, X.; Geng, D.; Yin, P.; Ma, C.; Chen, W.; et al. A study on the reparative action of x-ray lesions in metaphyses and distal end of bone in children’s fingers with kashin-beck disease treated by se-fortified wheat. Chin. J. Ctrl. Endem. Dis. 1990, 5, 269–272. [Google Scholar]
- Li, J.; Chen, D.; Ren, S.; Liu, W.; Wang, X.; Hou, J.; Fan, L.; Jia, Z.; Xu, L.; Zhang, X.; et al. Effect of applying selenium fertilizer to improve soil and increase selenium level of food on prevention and treatment of kashin-beck disease. Chin. J. Endemiol. 1991, 10, 69–75. [Google Scholar]
- Guo, X.; Zhang, S.; Mo, D. Observation of the effect of 5-year selenium supplement on children with kashin-beck disease. Natl. Med. J. China 1991, 71, 700–701. [Google Scholar]
- Guo, X. Progression and prospect of etiology and pathogenesis of kashin-beck disease. Chin. J. Xi’an Jiaotong Univ. (Med. Sci.) 2008, 29, 481–488. [Google Scholar]
- Letsiou, S.; Nomikos, T.; Panagiotakos, D.B.; Pergantis, S.A.; Fragopoulou, E.; Pitsavos, C.; Stefanadis, C.; Antonopoulou, S. Gender-specific distribution of selenium to serum selenoproteins: Associations with total selenium levels, age, smoking, body mass index, and physical activity. Biofactors 2014, 40, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.A.; Longnecker, M.P.; Veillon, C.; Howe, M.; Levander, O.A.; Taylor, P.R.; Mcadam, P.A.; Brown, C.C.; Stampfer, M.J.; Willett, W.C. Selenium intake, age, gender, and smoking in relation to indexes of selenium status of adults residing in a seleniferous area. Am. J. Clin. Nutr. 1990, 52, 858–862. [Google Scholar] [PubMed]
- Ducros, V.; Ferry, M.; Faure, P.; Belin, N.; Renversez, J.C.; Ruffieux, D.; Favier, A. Distribution of selenium in plasma of french women: Relation to age and selenium status. Clin. Chem. 2000, 46, 731–733. [Google Scholar] [PubMed]
- Ellis, D.R.; Salt, D.E. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- Aguilar, F.; Charrondiere, U.R.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Grilli, S.; Guertler, R.; Kass, G.E.N.; Koenig, J.; et al. Scientific opinion of the panel on food additives and nutrient sources added to food on l-selenomethionine as a source of selenium added for nutritional purposes to food supplements. Eur. Food Saf. Auth. 2009, 1082, 1–39. [Google Scholar]
- Campbell, D.; Bunker, V.W.; Thomas, A.J.; Clayton, B.E. Selenium and vitamin e status of healthy and institutionalized elderly subjects: Analysis of plasma, erythrocytes and platelets. Br. J. Nutr. 1989, 62, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Bunker, V.W.; Lawson, M.S.; Stansfield, M.F.; Clayton, B.E. Selenium balance studies in apparently healthy and housebound elderly people eating self-selected diets. Br. J. Nutr. 1988, 59, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.P.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lv, X.; Liang, S. An investigation on the nutritional condition of inhabitants in Kashin-Beck disease affected areas. Endemic Dis. Bull. 1990, 5, 85–90. [Google Scholar]
- Chen, C.; Liang, S. An analysis of on the nutritional condition of inhabitants in Kashin-Beck disease affected areas in yongshou county. Chin. J. Endemiol. 1992, 11, 119–121. [Google Scholar]
- Hu, Y.; Yu, Z.; Chen, X.; Wang, T.; Ding, R.; Hong, G.; Yang, F. An epidemiological survey of Kashin-Beck disease in qinling mountainous area. Chin. J. Endemiol. 1991, 10, 144–147. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Y.; Wang, X.; Wang, S.; Zhang, F.; Zhang, L.; Lei, Y.; Guo, X. Is It the Appropriate Time to Stop Applying Selenium Enriched Salt in Kashin-Beck Disease Areas in China? Nutrients 2015, 7, 6195-6212. https://doi.org/10.3390/nu7085276
Ning Y, Wang X, Wang S, Zhang F, Zhang L, Lei Y, Guo X. Is It the Appropriate Time to Stop Applying Selenium Enriched Salt in Kashin-Beck Disease Areas in China? Nutrients. 2015; 7(8):6195-6212. https://doi.org/10.3390/nu7085276
Chicago/Turabian StyleNing, Yujie, Xi Wang, Sen Wang, Feng Zhang, Lianhe Zhang, Yanxia Lei, and Xiong Guo. 2015. "Is It the Appropriate Time to Stop Applying Selenium Enriched Salt in Kashin-Beck Disease Areas in China?" Nutrients 7, no. 8: 6195-6212. https://doi.org/10.3390/nu7085276




