The Fate of Fat: Pre-Exposure Fat Losses during Nasogastric Tube Feeding in Preterm Newborns
Abstract
:1. Introduction
2. Development of Oral Feeding Skills
3. The Fate of Fat: Pre-Exposure Fat Losses during Nasogastric Tube Feeding
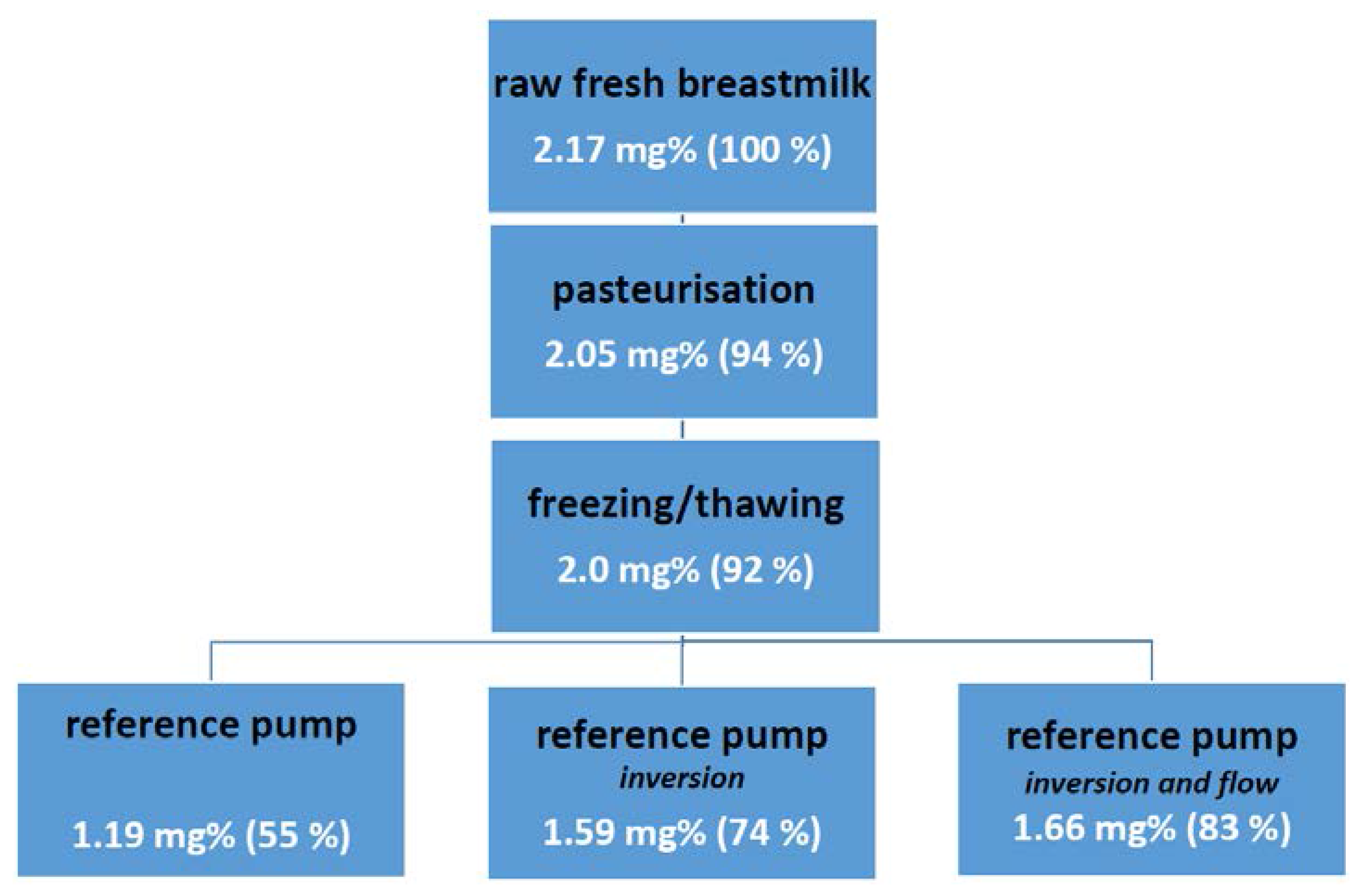
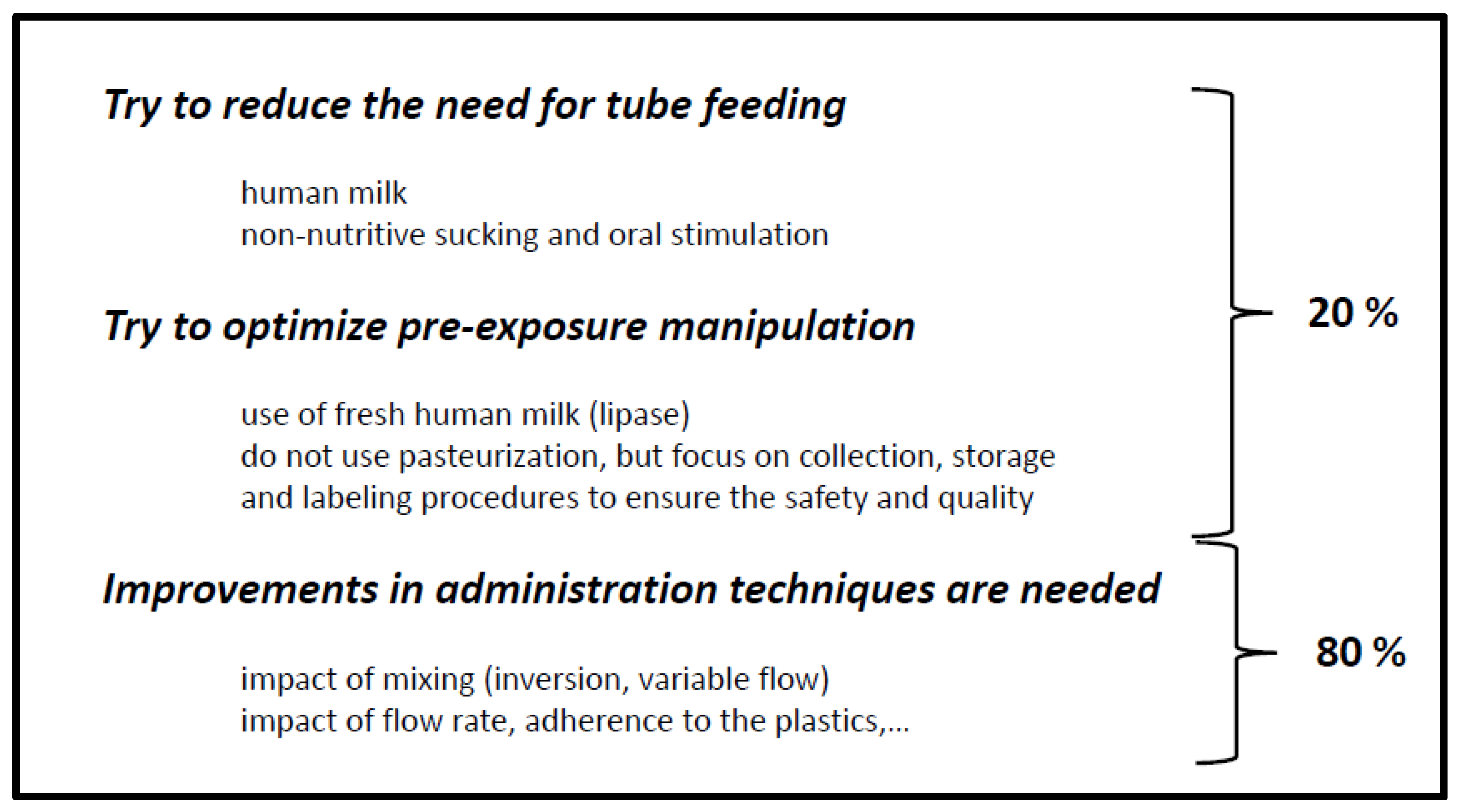
4. Similarities between Developmental Drug and Developmental Nutritional Product Development
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ehrenkranz, R.A. Early nutritional support and outcomes in elbw infants. Early Hum. Dev. 2010, 86 (Suppl. 1), 21–25. [Google Scholar] [CrossRef] [PubMed]
- Loys, C.M.; Maucort-Boulch, D.; Guy, B.; Putet, G.; Picaud, J.C.; Hays, S. Extremely low birthweight infants: How neonatal intensive care unit teams can reduce postnatal malnutrition and prevent growth retardation. Acta Paediatr. 2013, 102, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.A.; Carvalho, M.; Costa, A.C.; Moreira, M.E. Variables associated with extra uterine growth restriction in very low birth weight infants. J. Pediatr. 2014, 90, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A. Extrauterine growth restriction: Is it preventable? J. Pediatr. 2014, 90, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Singh, B.; Chessell, L.; Wilson, J.; Janes, M.; McDonald, K.; Shahid, S.; Gardner, V.A.; Hjartarson, A.; Purcha, M.; et al. Guidelines for feeding very low birth weight infants. Nutrients 2015, 7, 423–442. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, J.; Martin, C.R.; Allred, E.; Chen, M.L.; Ehrenkranz, R.A.; Dammann, O.; Leviton, A. Risk factors and correlates of neonatal growth velocity in extremely low gestational age newborns: The elgan study. Neonatology 2013, 104, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, W.E.; Kouwenhoven, S.M.; van Goudoever, J.B. Optimal growth of preterm infants. World Rev. Nutr. Diet. 2013, 106, 149–155. [Google Scholar] [PubMed]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid quality in infant nutrition: Current knowledge and future opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, A. Enteral and parenteral lipid requirements of preterm infants. World Rev. Nutr. Diet. 2014, 110, 82–98. [Google Scholar] [PubMed]
- Vlaardingerbroek, H.; van Goudoever, J.B. Intravenous lipids in preterm infants: Impact on laboratory and clinical outcomes and long-term consequences. World Rev. Nutr. Diet. 2015, 112, 71–80. [Google Scholar] [PubMed]
- Rayyan, M.; Devlieger, H.; Jochum, F.; Allegaert, K. Short-term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium-chain triglycerides, and fish oil: A randomized double-blind study in preterm infants. J. Parenter. Enter. Nutr. 2012, 36, 81S–94S. [Google Scholar] [CrossRef] [PubMed]
- Picaud, J.C.; Buffin, R.; Guy, B.; Putet, G. How to optimize nutritonal value of human milk for very preterm infant? In Progrès en Néonatologie; Corlet, A.N., Ed.; Assoiation de Néontaologie de Port-Royal: Paris, France, 2014; Volume 34, pp. 443–457. [Google Scholar]
- Corpeleijn, W.E.; Kouwenhoven, S.M.; Paap, M.C.; van Vliet, I.; Scheerder, I.; Muizer, Y.; Helder, O.K.; van Goudoever, J.B.; Vermeulen, M.J. Intake of own mother’s milk during the first days of life is associated with decreased morbidity and mortality in very low birth weight infants during the first 60 days of life. Neonatology 2012, 102, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Rochow, N.; Fusch, G.; Choi, A.; Chessell, L.; Elliott, L.; McDonald, K.; Kuiper, E.; Purcha, M.; Turner, S.; Chan, E.; et al. Target fortification of breast milk with fat, protein, and carbohydrates for preterm infants. J. Pediatr. 2013, 163, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- De Halleux, V.; Close, A.; Stalport, S.; Studzinski, F.; Habibi, F.; Rigo, J. Advantages of individualized fortification of human milk for preterm infants. Arch. Pédiatr. 2007, 14 (Suppl. 1), S5–S10. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Ziegler, E.E. Adjustable fortification of human milk fed to preterm infants: Does it make a difference? J. Perinatol. 2006, 26, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Rochow, N.; Fusch, G.; Zapanta, B.; Ali, A.; Barui, S.; Fusch, C. Target fortification of breast milk: How often should milk analysis be done? Nutrients 2015, 7, 2297–2310. [Google Scholar] [CrossRef] [PubMed]
- Bakewell-Sachs, S.; Medoff-Cooper, B.; Escobar, G.J.; Silber, J.H.; Lorch, S.A. Infant functional status: The timing of physiologic maturation of premature infants. Pediatrics 2009, 123, e878–e886. [Google Scholar] [CrossRef] [PubMed]
- Jadcherla, S.R.; Wang, M.; Vijayapal, A.S.; Leuthner, S.R. Impact of prematurity and co-morbidities on feeding milestones in neonates: A retrospective study. J. Perinatol. 2010, 30, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Samara, M.; Johnson, S.; Lamberts, K.; Marlow, N.; Wolke, D. Eating problems at age 6 years in a whole population sample of extremely preterm children. Dev. Med. Child Neurol. 2010, 52, e16–e22. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Steinwender, S. Timing introduction and transition to oral feeding in preterm infants: Current trends and practice. Newborn Infant Nurs. Rev. 2008, 8, 153–189. [Google Scholar] [CrossRef]
- Klingenberg, C.; Embleton, N.D.; Jacobs, S.E.; O’Connell, L.A.; Kuschel, C.A. Enteral feeding practices in very preterm infants: An international survey. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F56–F61. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, A.I. Breastfeeding and the use of human milk: An analysis of the american academy of pediatrics 2012 breastfeeding policy statement. Breastfeeding Med. 2012, 7, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Knafl, G.; Thoyre, S.; Brandon, D. Factors associated with feeding progression in extremely preterm infants. Nurs. Res. 2015, 64, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Dodrill, P.; Donovan, T.; Cleghorn, G.; McMahon, S.; Davies, P.S. Attainment of early feeding milestones in preterm neonates. J. Perinatol. 2008, 28, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lyu, T.; Hu, X.; Shi, P.; Cao, Y.; Latour, J.M. Effect of nonnutritive sucking and oral stimulation on feeding performance in preterm infants: A randomized controlled trial. Pediatr. Crit. Care Med. 2014, 15, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Shaker, C.S. Cue-based feeding in the nicu: Using the infant’s communication as a guide. Neonatal Netw. 2013, 32, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Stocks, R.J.; Davies, D.P.; Allen, F.; Sewell, D. Loss of breast milk nutrients during tube feeding. Arch. Dis. Child. 1985, 60, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Jarjour, J.; Juarez, A.M.; Kocak, D.K.; Liu, N.J.; Tabata, M.M.; Hawthorne, K.M.; Ramos, R.F.; Abrams, S.A. A novel approach to improving fat delivery in neonatal enteral feeding. Nutrients 2015, 7, 5051–5064. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.P.; Hicks, P.D.; Hamzo, M.; Veit, L.E.; Abrams, S.A. Continuous feedings of fortified human milk lead to nutrient losses of fat, calcium and phosphorous. Nutrients 2010, 2, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Brennan-Behm, M.; Carlson, G.E.; Meier, P.; Engstrom, J. Caloric loss from expressed mother’s milk during continuous gavage infusion. Neonatal Netw. 1994, 13, 27–32. [Google Scholar] [PubMed]
- Andersson, Y.; Savman, K.; Blackberg, L.; Hernell, O. Pasteurization of mother’s own milk reduces fat absorption and growth in preterm infants. Acta Paediatr. 2007, 96, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Montjaux-Regis, N.; Cristini, C.; Arnaud, C.; Glorieux, I.; Vanpee, M.; Casper, C. Improved growth of preterm infants receiving mother’s own raw milk compared with pasteurized donor milk. Acta Paediatr. 2011, 100, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.A.; Soares, F.V.; Pimenta, H.P.; Abranches, A.D.; Moreira, M.E. Analysis of the influence of pasteurization, freezing/thawing, and offer processes on human milk’s macronutrient concentrations. Early Hum. Dev. 2011, 87, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Giribaldi, M.; Baro, C.; Giancotti, V.; Pazzi, M.; Peila, C.; Tonetto, P.; Arslanoglu, S.; Moro, G.E.; Cavallarin, L.; et al. Effect of prolonged refrigeration on the lipid profile, lipase activity, and oxidative status of human milk. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.R.; Hamosh, M.; Bitman, J.; Wood, D.L. Adherence of medium-chain fatty acids to feeding tubes during gavage feeding of human milk fortified with medium-chain triglycerides. J. Pediatr. 1988, 112, 474–476. [Google Scholar] [CrossRef]
- Cossey, V.; Vanhole, C.; Eerdekens, A.; Rayyan, M.; Fieuws, S.; Schuermans, A. Pasteurization of mother’s own milk for preterm infants does not reduce the incidence of late-onset sepsis. Neonatology 2013, 103, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Casper, C.; Carnielli, V.P.; Hascoet, J.M.; Lapillonne, A.; Maggio, L.; Timdahl, K.; Olsson, B.; Vagero, M.; Hernell, O. Rhbssl improves growth and lcpufa absorption in preterm infants fed formula or pasteurized breast milk. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Swedish Orphan Biovitrum AB. Available online: http://hugin.info/134557/R/1771859/603492.pdf (accessed on 27 July 2015).
- Premji, S.S.; Chessell, L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Tabata, M.; Abdelrahman, K.; Hair, A.B.; Hawthorne, K.M.; Chen, Z.; Abrams, S.A. Fortifier and cream improve fat delivery in continuous enteral infant feeding of breast milk. Nutrients 2015, 7, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Aynsley-Green, A.; Adrian, T.E.; Bloom, S.R. Feeding and the development of enteroinsular hormone secretion in the preterm infant: Effects of continuous gastric infusions of human milk compared with intermittent boluses. Acta Paediatr. Scand. 1982, 71, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Fiorotto, M.L.; Suryawan, A. Bolus vs. Continuous feeding to optimize anabolism in neonates. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Verbesselt, R.; Naulaers, G.; van den Anker, J.N.; Rayyan, M.; Debeer, A.; de Hoon, J. Developmental pharmacology: Neonates are not just small adults. Acta Clin. Belg. 2008, 63, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, C.M.; Medlicott, N.J.; Reith, D.M.; Broadbent, R.S. Intravenous drug delivery in neonates: Lessons learnt. Arch. Dis. Child. 2014, 99, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.; Kulo, A.; de Hoon, J.N.; Allegaert, K. Pharmacokinetics of drugs in neonates: Pattern recognition beyond compound specific observations. Curr. Pharm. Des. 2012, 18, 3119–3146. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003754.pdf (accessed on 27 July 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayyan, M.; Rommel, N.; Allegaert, K. The Fate of Fat: Pre-Exposure Fat Losses during Nasogastric Tube Feeding in Preterm Newborns. Nutrients 2015, 7, 6213-6223. https://doi.org/10.3390/nu7085279
Rayyan M, Rommel N, Allegaert K. The Fate of Fat: Pre-Exposure Fat Losses during Nasogastric Tube Feeding in Preterm Newborns. Nutrients. 2015; 7(8):6213-6223. https://doi.org/10.3390/nu7085279
Chicago/Turabian StyleRayyan, Maissa, Nathalie Rommel, and Karel Allegaert. 2015. "The Fate of Fat: Pre-Exposure Fat Losses during Nasogastric Tube Feeding in Preterm Newborns" Nutrients 7, no. 8: 6213-6223. https://doi.org/10.3390/nu7085279





