Impact of Weight Loss on Plasma Leptin and Adiponectin in Overweight-to-Obese Post Menopausal Breast Cancer Survivors
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Participants
2.3. Adverse Events
2.4. Non Intervention Control
2.5. Intervention
2.6. Laboratory Analyses
2.7. Statistical Methods
3. Results
3.1. Anthropometric Determinates
| Variable | Group | Baseline | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 |
|---|---|---|---|---|---|---|---|---|
| Body Weight (kg) | CTRL | 53, 79.7 ± 9.3 (11.7) | 53, 79.4 ± 10.1 (12.7) | |||||
| LC | 65, 79.8 ± 8.7 (10.9) | 65, 76.0 ± 8.4 (11.1) | 65, 74.0 ± 8.4 (11.4) | 64, 72.3 ± 8.4 (11.7) | 62, 71.2 ± 8.7 (12.2) | 61, 70.2 ± 8.9 (12.6) | 65, 69.4 ± 9.0 (13.0) | |
| LF | 73, 77.6 ± 7.7 (9.9) | 73, 74.2 ± 7.4 (10.0) | 71, 72.3 ± 7.5 (10.3) | 69, 71.1 ± 7.5 (10.5) | 69, 70.0 ± 7.4 (10.6) | 67, 69.0 ± 7.6 (11.0) | 73, 68.3 ± 7.5 (11.0) | |
| Fat Mass (kg) | CTRL | 53, 34.9 ± 7.3 (20.9) | 53, 34.9 ± 8.2 (23.4) | |||||
| LC | 65, 35.0 ± 6.1 (17.4) | 65, 31.9 ± 5.7 (18.0) | 65, 30.2 ± 5.6 (18.7) | 64, 28.5 ± 5.8 (20.5) | 62, 27.4 ± 6.2 (22.5) | 61, 26.1 ± 6.4 (24.5) | 65, 25.4 ± 6.6 (26.1) | |
| LF | 73, 33.0 ± 5.8 (17.6) | 73, 29.8 ± 5.7 (19.2) | 71, 28.1 ± 5.6 (19.8) | 69, 26.7 ± 5.8 (21.6) | 69, 25.7 ± 5.6 (21.8) | 67, 24.8 ± 5.9 (23.9) | 73, 24.1 ± 5.7 (23.7) | |
| Waist (cm) | CTRL | 53, 94.9 ± 8.3 (8.7) | 53, 94.8 ± 8.8 (9.3) | |||||
| LC | 65, 94.3 ± 6.9 (7.4) | 65, 91.6 ± 7.5 (8.2) | 65, 89.8 ± 7.6 (8.4) | 64, 87.8 ± 7.6 (8.6) | 62, 87.1 ± 7.7 (8.9) | 61, 86.0 ± 7.7 (8.9) | 65, 85.0 ± 7.5 (8.8) | |
| LF | 73, 91.6 ± 7.2 (7.9) | 73, 89.0 ± 6.8 (7.6) | 71, 86.7 ± 7.0 (8.1) | 69, 85.6 ± 7.8 (9.1) | 69, 84.4 ± 7.0 (8.3) | 67, 83.5 ± 7.3 (8.7) | 73, 83.1 ± 7.4 (8.9) | |
| Hip (cm) | CTRL | 53, 110.5 ± 7.4 (6.7) | 53, 111.0 ± 9.2 (8.3) | |||||
| LC | 65, 112.0 ± 7.2 (6.4) | 65, 109.3 ± 6.9 (6.3) | 65, 107.4 ± 6.9 (6.5) | 64, 106.1 ± 7.3 (6.9) | 62, 104.7 ± 7.3 (7.0) | 61, 103.9 ± 8.2 (7.9) | 65, 103.2 ± 7.3 (7.1) | |
| LF | 73, 110.7 ± 5.8 (5.2) | 73, 107.6 ± 5.8 (5.3) | 71, 105.9 ± 6.2 (5.8) | 69, 105.1 ± 5.4 (5.1) | 69, 103.7 ± 5.3 (5.1) | 67, 102.8 ± 6.1 (6.0) | 73, 102.2 ± 5.6 (5.5) | |
| WHR | CTRL | 53, 0.9 ± 0.1 (8.2) | 53, 0.9 ± 0.1 (9.1) | |||||
| LC | 65, 0.8 ± 0.1 (7.9) | 65, 0.8 ± 0.1 (7.9) | 65, 0.8 ± 0.1 (9.0) | 64, 0.8 ± 0.1 (8.1) | 62, 0.8 ± 0.1 (7.6) | 61, 0.8 ± 0.1 (8.5) | 65, 0.8 ± 0.1 (7.5) | |
| LF | 73, 0.8 ± 0.1 (6.7) | 73, 0.8 ± 0.1 (6.4) | 71, 0.8 ± 0.1 (6.7) | 69, 0.8 ± 0.1 (7.5) | 69, 0.8 ± 0.1 (6.7) | 67, 0.8 ± 0.1 (6.7) | 73, 0.8 ± 0.1 (6.8) |
3.2. Fat Mass and Plasma Concentrations of Leptin and Adiponectin
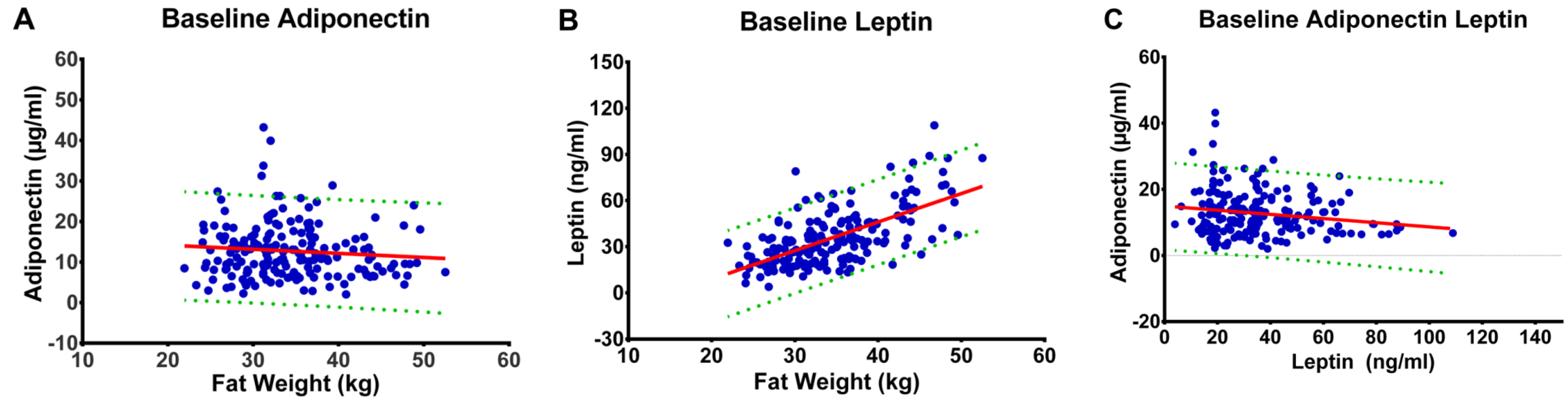
3.3. Pattern of Change
| Variable | Group | Baseline | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adiponectin (μg/mL) | CTRL | 53, 12.1 ± 7.2 (59.8) | 52, 12.5 ± 6.8 (54.0) | |||||||||||||
| LC | 65, 11.9 ± 7.3 (61.3) | 65, 11.4 ± 6.8 (59.6) | 65, 11.6 ± 6.1 (52.6) | 63, 12.1 ± 6.3 (52.3) | 62, 12.3 ± 6.2 (50.7) | 61, 13.0 ± 6.4 (49.4) | 65, 12.7 ± 6.7 (52.9) | |||||||||
| LF | 72, 14.0 ± 5.7 (41.0) | 73, 12.5 ± 5.4 (43.1) | 71, 12.8 ± 5.4 (42.4) | 69, 13.6 ± 5.6 (40.8) | 69, 13.7 ± 5.4 (39.2) | 66, 13.9 ± 5.9 (42.2) | 72, 15.0 ± 6.3 (42.1) | |||||||||
| Adiponectin (%) ∆ from baseline | CTRL | 52, 10.6 ± 38.7 (363) | ||||||||||||||
| LC | 65, −3.4 ± 20.7 (−615) | 65, 2.5 ± 23.2 (925) | 63, 6.8 ± 25.7 (381) | 62, 10.5 ± 32.3 (309) | 61, 14.1 ± 32.7 (231) | 65, 13.0 ± 25.7 (198) | ||||||||||
| LF | 72, −7.8 ± 21.8(−279) | 71, −6.0 ± 25.1 (−420) | 69, 1.1 ± 23.3 (2093) | 69, 2.9 ± 27.7 (961) | 66, 2.7 ± 24.5 (918) | 71, 12.1 ± 27.0 (223) | ||||||||||
| Leptin (ng/mL) | CTRL | 53, 34.6 ± 17.7 (51.2) | 52, 35.1 ± 19.4 (55.3) | |||||||||||||
| LC | 65, 36.5 ± 16.2 (44.5) | 65, 20.0 ± 9.7 (48.5) | 65, 18.4 ± 10.5 (57.2) | 63, 17.3 ± 10.4 (60.0) | 62, 16.5 ± 10.6 (64.4) | 61, 16.4 ± 11.1 (68.0) | 65, 16.4 ± 11.8 (71.8) | |||||||||
| LF | 72, 35.1 ± 20.7 (59.1) | 73, 18.9 ± 11.2 (59.0) | 71, 17.7 ± 11.7 (66.4) | 69, 17.6 ± 11.3 (63.9) | 69, 15.8 ± 9.0 (57.2) | 66, 15.0 ± 10.5 (69.7) | 72, 15.3 ± 9.8 (64.0) | |||||||||
| Leptin (%) ∆ from baseline | CTRL | 52, 6.5 ± 41.7 (643) | ||||||||||||||
| LC | 65, −43 ± 20.1 (−47) | 65, −46 ± 25.9 (−56) | 63, −50 ± 23.5 (−47) | 62, −53 ± 24.5 (−46) | 61, −53 ± 26.2 (−49) | 65, −52 ± 30.6 (−59) | ||||||||||
| LF | 72, −44 ± 17.6 (−40) | 71, −48 ± 20.5 (−42) | 69, −48 ± 24.0 (−50) | 69, −53 ± 18.6 (−35) | 66, −54 ± 24.2 (−45) | 71, −51 ± 31.9 (−62) | ||||||||||
| Ratio Adiponectin/eptin | CTRL | 53, 0.5 ± 0.4 (87.0) | 52, 0.5 ± 0.4 (78.0) | |||||||||||||
| LC | 65, 0.4 ± 0.5 (119) | 65, 0.8 ± 1.0 (123) | 65, 0.9 ± 1.0 (112) | 63, 1.1 ± 1.3 (121) | 62, 1.2 ± 1.3 (114) | 61, 1.4 ± 2.5 (175) | 65, 1.4 ± 2.1 (144) | |||||||||
| LF | 72, 0.6 ± 0.4 (75.1) | 73, 0.9 ± 0.6 (68.5) | 71, 1.1 ± 0.9 (83.5) | 69, 1.2 ± 1.0 (87.0) | 69, 1.3 ± 1.1 (85.4) | 66, 1.4 ± 1.4 (96.7) | 72, 1.6 ± 1.7 (108) | |||||||||

3.4. Impact of Weight Loss Magnitude
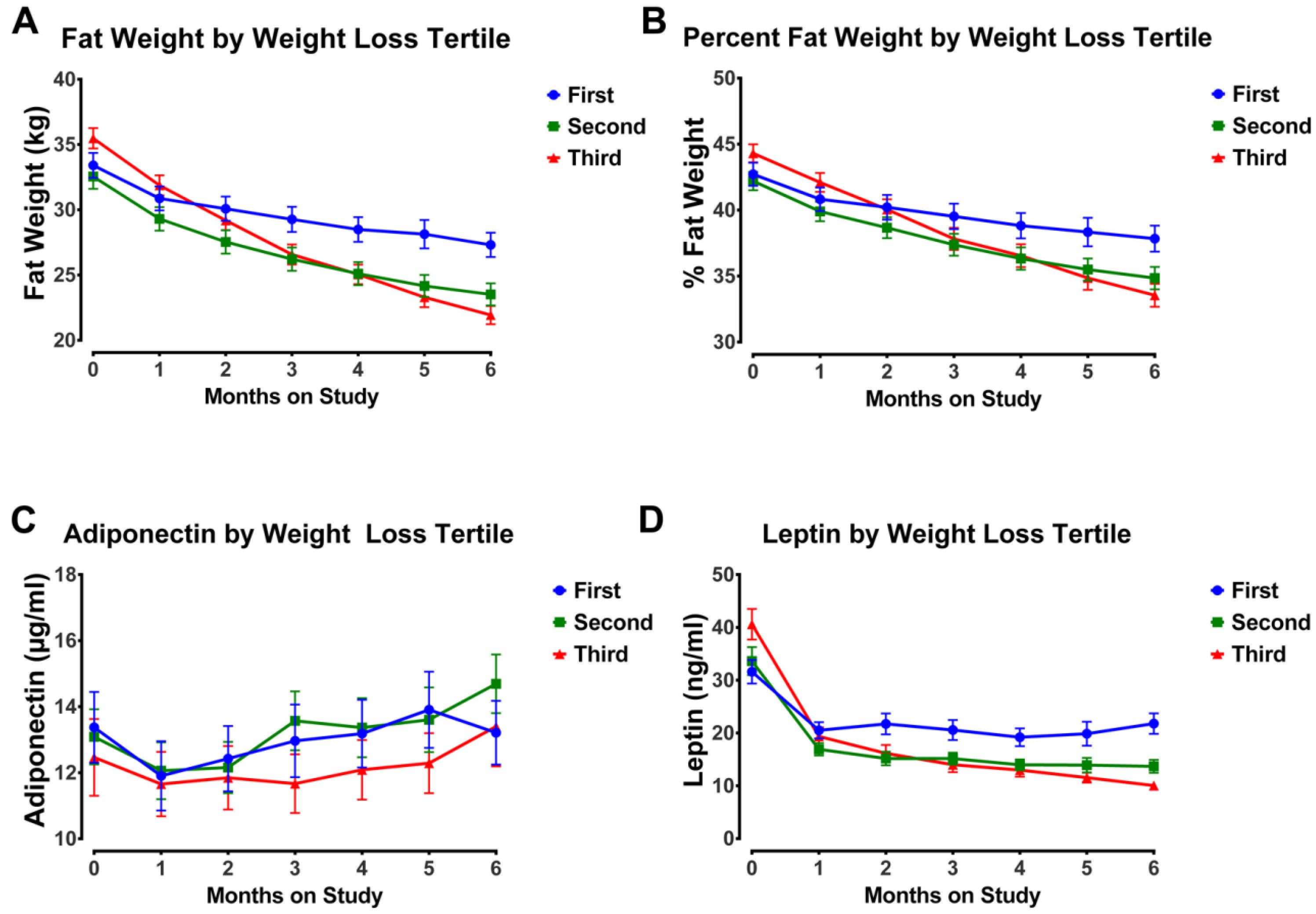
3.5. Clinical Relevance of Changes in Plasma Adipokines
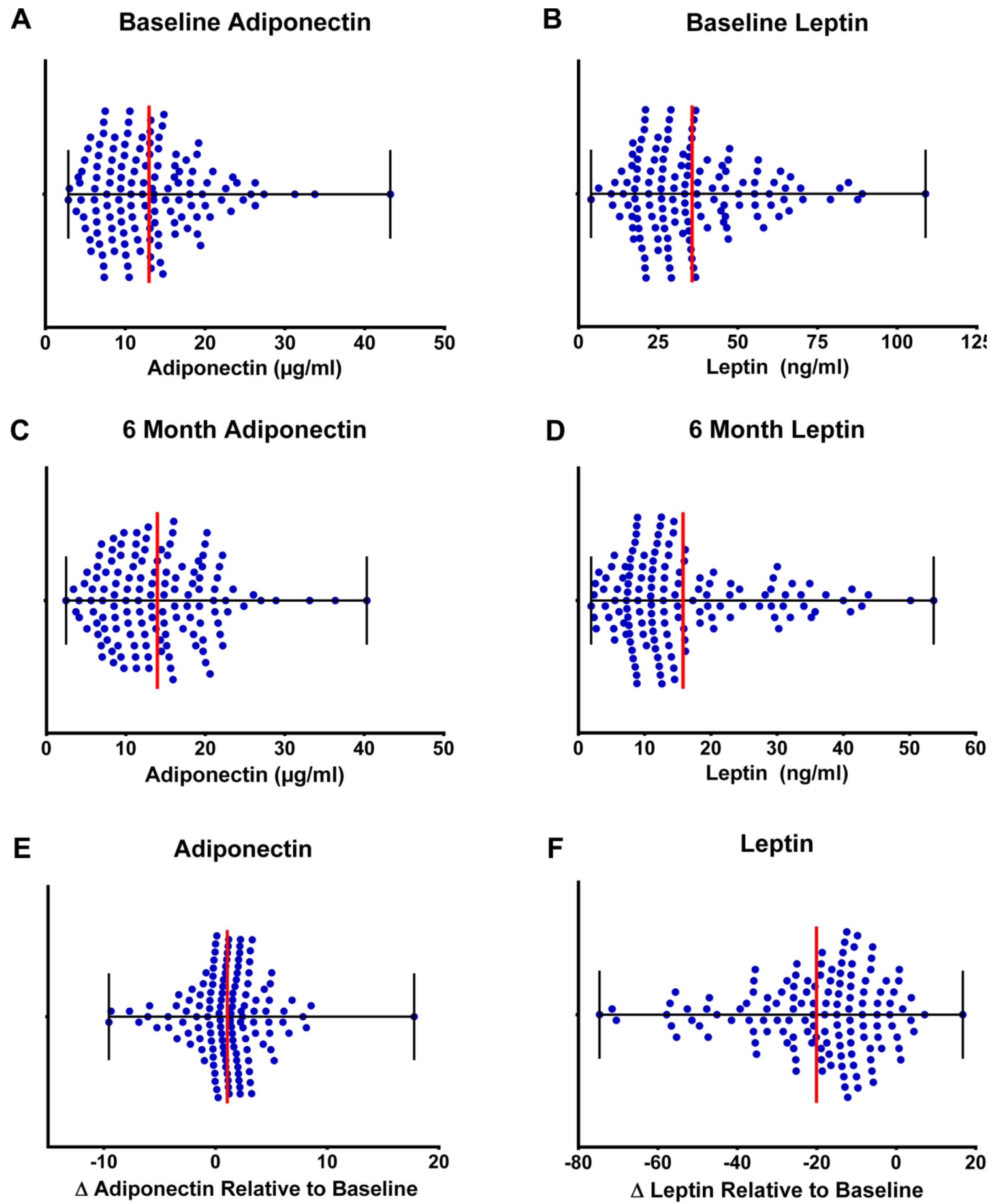
4. Discussion
4.1. Leptin
4.2. Adiponectin
4.3. Adiponectin to Leptin Ratio and Causal Mechanisms
4.4. Strengths and Limitations
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ewertz, M.; Jensen, M.B.; Gunnarsdottir, K.A.; Hojris, I.; Jakobsen, E.H.; Nielsen, D.; Stenbygaard, L.E.; Tange, U.B.; Cold, S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011, 29, 25–31. [Google Scholar]
- Demark-Wahnefried, W.; Platz, E.A.; Ligibel, J.A.; Blair, C.K.; Courneya, K.S.; Meyerhardt, J.A.; Ganz, P.A.; Rock, C.L.; Schmitz, K.H.; Wadden, T.; et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol. Biomarkers Prev. 2012, 21, 1244–1259. [Google Scholar]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar]
- Al-Delaimy, W.K.; Flatt, S.W.; Natarajan, L.; Laughlin, G.A.; Rock, C.L.; Gold, E.B.; Caan, B.J.; Parker, B.A.; Pierce, J.P. IGF1 and risk of additional breast cancer in the WHEL study. Endocr. Relat. Cancer 2011, 18, 235–244. [Google Scholar]
- Oh, S.W.; Park, C.Y.; Lee, E.S.; Yoon, Y.S.; Lee, E.S.; Park, S.S.; Kim, Y.; Sung, N.J.; Yun, Y.H.; Lee, K.S.; et al. Adipokines, insulin resistance, metabolic syndrome, and breast cancer recurrence: A cohort study. Breast Cancer Res. 2011, 13, R34. [Google Scholar]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; Trudeau, M.E.; Koo, J.; Taylor, S.K.; Hood, N. Insulin- and obesity-related variables in early-stage breast cancer: Correlations and time course of prognostic associations. J. Clin. Oncol. 2012, 30, 164–171. [Google Scholar]
- Macis, D.; Gandini, S.; Guerrieri-Gonzaga, A.; Johansson, H.; Magni, P.; Ruscica, M.; Lazzeroni, M.; Serrano, D.; Cazzaniga, M.; Mora, S.; et al. Prognostic effect of circulating adiponectin in a randomized 2 × 2 trial of low-dose tamoxifen and fenretinide in premenopausal women at risk for breast cancer. J. Clin. Oncol. 2012, 30, 151–157. [Google Scholar]
- Cho, Y.A.; Sung, M.K.; Yeon, J.Y.; Ro, J.; Kim, J. Prognostic role of interleukin-6, interleukin-8, and leptin levels according to breast cancer subtype. Cancer Res. Treat. 2013, 45, 210–219. [Google Scholar]
- Wadden, T.A.; Butryn, M.L.; Wilson, C. Lifestyle modification for the management of obesity. Gastroenterology 2007, 132, 2226–2238. [Google Scholar]
- Foster-Schubert, K.E.; Alfano, C.M.; Duggan, C.R.; Xiao, L.; Campbell, K.L.; Kong, A.; Bain, C.E.; Wang, C.Y.; Blackburn, G.L.; McTiernan, A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity 2012, 20, 1628–1638. [Google Scholar]
- Thomson, C.A.; Thompson, P.A. Dietary patterns, risk and prognosis of breast cancer. Future Oncol. 2009, 5, 1257–1269. [Google Scholar]
- Gold, E.B.; Pierce, J.P.; Natarajan, L.; Stefanick, M.L.; Laughlin, G.A.; Caan, B.J.; Flatt, S.W.; Emond, J.A.; Saquib, N.; Madlensky, L.; et al. Dietary pattern influences breast cancer prognosis in women without hot flashes: The women’s healthy eating and living trial. J. Clin. Oncol. 2009, 27, 352–359. [Google Scholar]
- Prentice, R.L.; Anderson, G.L. The women’s health initiative: Lessons learned. Annu. Rev. Public Health 2008, 29, 131–150. [Google Scholar]
- Prentice, R.L. Observational studies, clinical trials, and the women’s health initiative. Lifetime Data Anal. 2007, 13, 449–462. [Google Scholar]
- Pierce, J.P.; Faerber, S.; Wright, F.A.; Rock, C.L.; Newman, V.; Flatt, S.W.; Kealey, S.; Jones, V.E.; Caan, B.J.; Gold, E.B.; et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: The Women’s Healthy Eating and Living (WHEL) Study. Control Clin. Trials 2002, 23, 728–756. [Google Scholar]
- Ho, V.W.; Hamilton, M.J.; Dang, N.H.; Hsu, B.E.; Adomat, H.H.; Guns, E.S.; Weljie, A.; Samudio, I.; Bennewith, K.L.; Krystal, G. A low carbohydrate, high protein diet combined with celecoxib markedly reduces metastasis. Carcinogenesis 2014, 35, 2291–2299. [Google Scholar]
- Liebman, M. When and why carbohydrate restriction can be a viable option. Nutrition 2014, 30, 748–754. [Google Scholar]
- Huebner, J.; Marienfeld, S.; Abbenhardt, C.; Ulrich, C.; Muenstedt, K.; Micke, O.; Muecke, R.; Loeser, C. Counseling patients on cancer diets: A review of the literature and recommendations for clinical practice. Anticancer Res. 2014, 34, 39–48. [Google Scholar]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar]
- Nilsson, L.M.; Winkvist, A.; Johansson, I.; Lindahl, B.; Hallmans, G.; Lenner, P.; Van, G.B. Low-carbohydrate, high-protein diet score and risk of incident cancer; a prospective cohort study. Nutr. J. 2013, 12, 58. [Google Scholar]
- Busetto, L.; Marangon, M.; De, S.F. High-protein low-carbohydrate diets: What is the rationale? Diabetes Metab. Res. Rev. 2011, 27, 230–232. [Google Scholar]
- Thomson, C.A.; Stopeck, A.T.; Bea, J.W.; Cussler, E.; Nardi, E.; Frey, G.; Thompson, P.A. Changes in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets. Nutr. Cancer 2010, 62, 1142–1152. [Google Scholar]
- Boeke, C.E.; Eliassen, A.H.; Chen, W.Y.; Cho, E.; Holmes, M.D.; Rosner, B.; Willett, W.C.; Tamimi, R.M. Dietary fat intake in relation to lethal breast cancer in two large prospective cohort studies. Breast Cancer Res. Treat. 2014, 146, 383–392. [Google Scholar]
- Michels, K.B.; Willett, W.C. The women’s health initiative randomized controlled dietary modification trial: A post-mortem. Breast Cancer Res. Treat. 2009, 114, 1–6. [Google Scholar]
- Bilsborough, S.A.; Crowe, T.C. Low-carbohydrate diets: What are the potential short- and long-term health implications? Asia Pac. J. Clin. Nutr. 2003, 12, 396–404. [Google Scholar]
- McTiernan, A. Obesity and cancer: The risks, science, and potential management strategies. Oncology 2005, 19, 871–881. [Google Scholar]
- Chen, X.; Wang, Y. Adiponectin and breast cancer. Med. Oncol. 2011, 28, 1288–1295. [Google Scholar]
- Garofalo, C.; Koda, M.; Cascio, S.; Sulkowska, M.; Kanczuga-Koda, L.; Golaszewska, J.; Russo, A.; Sulkowski, S.; Surmacz, E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: Possible role of obesity-related stimuli. Clin. Cancer Res. 2006, 12, 1447–1453. [Google Scholar]
- Gaudet, M.M.; Falk, R.T.; Gierach, G.L.; Lacey, J.V., Jr.; Graubard, B.I.; Dorgan, J.F.; Brinton, L.A. Do adipokines underlie the association between known risk factors and breast cancer among a cohort of United States women? Cancer Epidemiol. 2010, 34, 580–586. [Google Scholar]
- Grossmann, M.E.; Ray, A.; Nkhata, K.J.; Malakhov, D.A.; Rogozina, O.P.; Dogan, S.; Cleary, M.P. Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010, 29, 641–653. [Google Scholar]
- Grossmann, M.E.; Cleary, M.P. The balance between leptin and adiponectin in the control of carcinogenesis—Focus on mammary tumorigenesis. Biochimie 2012, 94, 2164–2171. [Google Scholar]
- Havel, P.J. Control of energy homeostasis and insulin action by adipocyte hormones: Leptin, acylation stimulating protein, and adiponectin. Curr. Opin. Lipidol. 2002, 13, 51–59. [Google Scholar]
- Rose, D.P.; Komninou, D.; Stephenson, G.D. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes. Rev. 2004, 5, 153–165. [Google Scholar]
- Forsblom, C.; Thomas, M.C.; Moran, J.; Saraheimo, M.; Thorn, L.; Waden, J.; Gordin, D.; Frystyk, J.; Flyvbjerg, A.; Groop, P.H. Serum adiponectin concentration is a positive predictor of all-cause and cardiovascular mortality in type 1 diabetes. J. Intern. Med. 2011, 270, 346–355. [Google Scholar]
- Trujillo, M.E.; Scherer, P.E. Adiponectin—Journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J. Intern. Med. 2005, 257, 167–175. [Google Scholar]
- Gautron, L.; Elmquist, J.K. Sixteen years and counting: An update on leptin in energy balance. J. Clin. Investig. 2011, 121, 2087–2093. [Google Scholar]
- Harwood, H.J., Jr. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 2012, 63, 57–75. [Google Scholar]
- Sedlacek, S.M.; Playdon, M.C.; Wolfe, P.; McGinley, J.N.; Wisthoff, M.R.; Daeninck, E.A.; Jiang, W.; Zhu, Z.; Thompson, H.J. Effect of a low fat versus a low carbohydrate weight loss dietary intervention on biomarkers of long term survival in breast cancer patients (“CHOICE”): Study protocol. BMC Cancer 2011, 11, 287. [Google Scholar]
- Abbenhardt, C.; McTiernan, A.; Alfano, C.M.; Wener, M.H.; Campbell, K.L.; Duggan, C.; Foster-Schubert, K.E.; Kong, A.; Toriola, A.T.; Potter, J.D.; et al. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J. Intern. Med. 2013, 274, 163–175. [Google Scholar]
- Fabian, C.J.; Kimler, B.F.; Donnelly, J.E.; Sullivan, D.K.; Klemp, J.R.; Petroff, B.K.; Phillips, T.A.; Metheny, T.; Aversman, S.; Yeh, H.W.; et al. Favorable modulation of benign breast tissue and serum risk biomarkers is associated with >10% weight loss in postmenopausal women. Breast Cancer Res. Treat. 2013, 142, 119–132. [Google Scholar]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar]
- Swarbrick, M.M.; Havel, P.J. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab. Syndr. Relat. Disord. 2008, 6, 87–102. [Google Scholar]
- Harris, H.R.; Tworoger, S.S.; Hankinson, S.E.; Rosner, B.A.; Michels, K.B. Plasma leptin levels and risk of breast cancer in premenopausal women. Cancer Prev. Res. 2011, 4, 1449–1456. [Google Scholar]
- Tian, Y.F.; Chu, C.H.; Wu, M.H.; Chang, C.L.; Yang, T.; Chou, Y.C.; Hsu, G.C.; Yu, C.P.; Yu, J.C.; Sun, C.A. Anthropometric measures, plasma adiponectin, and breast cancer risk. Endocr. Relat. Cancer 2007, 14, 669–677. [Google Scholar]
- Tworoger, S.S.; Eliassen, A.H.; Kelesidis, T.; Colditz, G.A.; Willett, W.C.; Mantzoros, C.S.; Hankinson, S.E. Plasma adiponectin concentrations and risk of incident breast cancer. J. Clin. Endocrinol. Metab. 2007, 92, 1510–1516. [Google Scholar]
- Woo, H.Y.; Park, H.; Ki, C.S.; Park, Y.L.; Bae, W.G. Relationships among serum leptin, leptin receptor gene polymorphisms, and breast cancer in Korea. Cancer Lett. 2006, 237, 137–142. [Google Scholar]
- Wu, M.H.; Chou, Y.C.; Chou, W.Y.; Hsu, G.C.; Chu, C.H.; Yu, C.P.; Yu, J.C.; Sun, C.A. Circulating levels of leptin, adiposity and breast cancer risk. Br. J. Cancer 2009, 100, 578–582. [Google Scholar]
- Ye, J.; Jia, J.; Dong, S.; Zhang, C.; Yu, S.; Li, L.; Mao, C.; Wang, D.; Chen, J.; Yuan, G. Circulating adiponectin levels and the risk of breast cancer: A meta-analysis. Eur. J. Cancer Prev. 2014, 23, 158–165. [Google Scholar]
- Llanos, A.A.; Krok, J.L.; Peng, J.; Pennell, M.L.; Olivo-Marston, S.; Vitolins, M.Z.; Degraffinreid, C.R.; Paskett, E.D. Favorable effects of low-fat and low-carbohydrate dietary patterns on serum leptin, but not adiponectin, among overweight and obese premenopausal women: A randomized trial. Springerplus 2014, 3, 175. [Google Scholar]
- Rock, C.L.; Pande, C.; Flatt, S.W.; Ying, C.; Pakiz, B.; Parker, B.A.; Williams, K.; Bardwell, W.A.; Heath, D.D.; Nichols, J.F. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin. Breast Cancer 2013, 13, 188–195. [Google Scholar]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar]
- Viguerie, N.; Vidal, H.; Arner, P.; Holst, C.; Verdich, C.; Avizou, S.; Astrup, A.; Saris, W.H.; Macdonald, I.A.; Klimcakova, E.; et al. Adipose tissue gene expression in obese subjects during low-fat and high-fat hypocaloric diets. Diabetologia 2005, 48, 123–131. [Google Scholar]
- Beasley, J.M.; Ange, B.A.; Anderson, C.A.; Miller, E.R., III; Erlinger, T.P.; Holbrook, J.T.; Sacks, F.M.; Appel, L.J. Associations between macronutrient intake and self-reported appetite and fasting levels of appetite hormones: Results from the Optimal Macronutrient Intake Trial to Prevent Heart Disease. Am. J. Epidemiol. 2009, 169, 893–900. [Google Scholar]
- Bluher, M.; Rudich, A.; Kloting, N.; Golan, R.; Henkin, Y.; Rubin, E.; Schwarzfuchs, D.; Gepner, Y.; Stampfer, M.J.; Fiedler, M.; et al. Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care 2012, 35, 342–349. [Google Scholar]
- Ebbeling, C.B.; Swain, J.F.; Feldman, H.A.; Wong, W.W.; Hachey, D.L.; Garcia-Lago, E.; Ludwig, D.S. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 2012, 307, 2627–2634. [Google Scholar]
- Esposito, K.; Di, P.C.; Maiorino, M.I.; Petrizzo, M.; Bellastella, G.; Siniscalchi, I.; Giugliano, D. Long-term effect of mediterranean-style diet and calorie restriction on biomarkers of longevity and oxidative stress in overweight men. Cardiol. Res. Pract. 2011, 2011, 293916. [Google Scholar]
- Ganji, V.; Kafai, M.R.; McCarthy, E. Serum leptin concentrations are not related to dietary patterns but are related to sex, age, body mass index, serum triacylglycerol, serum insulin, and plasma glucose in the US population. Nutr. Metab. 2009, 6, 3. [Google Scholar]
- Hatami, Z.Z.; Salehi, M.; Heydari, S.T.; Babajafari, S. The effects of 6 isocaloric meals on body weight, lipid profiles, leptin, and adiponectin in overweight subjects (BMI > 25). Int. Cardiovasc. Res. J. 2014, 8, 52–56. [Google Scholar]
- Jacobs, D.R., Jr.; Sluik, D.; Rokling-Andersen, M.H.; Anderssen, S.A.; Drevon, C.A. Association of 1-y changes in diet pattern with cardiovascular disease risk factors and adipokines: Results from the 1-y randomized Oslo Diet and Exercise Study. Am. J. Clin. Nutr. 2009, 89, 509–517. [Google Scholar]
- Jafari-Vayghan, H.; Tarighat-Esfanjani, A.; Jafarabadi, M.A.; Ebrahimi-Mameghani, M.; Ghadimi, S.S.; Lalezadeh, Z. Association between dietary patterns and serum leptin-to-adiponectin ratio in apparently healthy adults. J. Am. Coll. Nutr. 2015, 34, 49–55. [Google Scholar]
- Saneei, P.; Hashemipour, M.; Kelishadi, R.; Esmaillzadeh, A. The Dietary Approaches to Stop Hypertension (DASH) diet affects inflammation in childhood metabolic syndrome: A randomized cross-over clinical trial. Ann. Nutr. Metab. 2014, 64, 20–27. [Google Scholar]
- Schoeller, D.A.; Buchholz, A.C. Energetics of obesity and weight control: Does diet composition matter? J. Am. Diet. Assoc. 2005, 105, 24–28. [Google Scholar]
- Sofer, S.; Eliraz, A.; Kaplan, S.; Voet, H.; Fink, G.; Kima, T.; Madar, Z. Changes in daily leptin, ghrelin and adiponectin profiles following a diet with carbohydrates eaten at dinner in obese subjects. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 744–750. [Google Scholar]
- Friedman, J.M.; Mantzoros, C.S. 20 years of leptin: From the discovery of the leptin gene to leptin in our therapeutic armamentarium. Metabolism 2015, 64, 1–4. [Google Scholar]
- Newman, G.; Gonzalez-Perez, R.R. LeptinGÇôcytokine crosstalk in breast cancer. Mol. Cell. Endocrinol. 2014, 382, 570–582. [Google Scholar]
- Khan, S.; Shukla, S.; Sinha, S.; Meeran, S.M. Role of adipokines and cytokines in obesity-associated breast cancer: Therapeutic targets. Cytokine Growth Factor Rev. 2013, 24, 503–513. [Google Scholar]
- Revillion, F.; Charlier, M.; Lhotellier, V.; Hornez, L.; Giard, S.; Baranzelli, M.C.; Djiane, J.; Peyrat, J.P. Messenger RNA expression of leptin and leptin receptors and their prognostic value in 322 human primary breast cancers. Clin. Cancer Res. 2006, 12, 2088–2094. [Google Scholar]
- Fargnoli, J.L.; Fung, T.T.; Olenczuk, D.M.; Chamberland, J.P.; Hu, F.B.; Mantzoros, C.S. Adherence to healthy eating patterns is associated with higher circulating total and high-molecular-weight adiponectin and lower resistin concentrations in women from the Nurses’ Health Study. Am. J. Clin. Nutr. 2008, 88, 1213–1224. [Google Scholar]
- Yannakoulia, M.; Yiannakouris, N.; Melistas, L.; Kontogianni, M.D.; Malagaris, I.; Mantzoros, C.S. A dietary pattern characterized by high consumption of whole-grain cereals and low-fat dairy products and low consumption of refined cereals is positively associated with plasma adiponectin levels in healthy women. Metabolism 2008, 57, 824–830. [Google Scholar]
- Mantzoros, C.S.; Williams, C.J.; Manson, J.E.; Meigs, J.B.; Hu, F.B. Adherence to the Mediterranean dietary pattern is positively associated with plasma adiponectin concentrations in diabetic women. Am. J. Clin. Nutr. 2006, 84, 328–335. [Google Scholar]
- Heidemann, C.; Hoffmann, K.; Spranger, J.; Klipstein-Grobusch, K.; Mohlig, M.; Pfeiffer, A.F.; Boeing, H. A dietary pattern protective against type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)—Potsdam Study cohort. Diabetologia 2005, 48, 1126–1134. [Google Scholar]
- Van Saun, M.N. Molecular pathways: Adiponectin and leptin signaling in cancer. Clin. Cancer Res. 2013, 19, 1926–1932. [Google Scholar]
- Kizer, J.R.; Benkeser, D.; Arnold, A.M.; Mukamal, K.J.; Ix, J.H.; Zieman, S.J.; Siscovick, D.S.; Tracy, R.P.; Mantzoros, C.S.; Defilippi, C.R.; et al. Associations of total and high-molecular-weight adiponectin with all-cause and cardiovascular mortality in older persons: The Cardiovascular Health Study. Circulation 2012, 126, 2951–2961. [Google Scholar]
- Duggan, C.; Irwin, M.L.; Xiao, L.; Henderson, K.D.; Smith, A.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R.; McTiernan, A. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J. Clin. Oncol. 2011, 29, 32–39. [Google Scholar]
- Cleary, M.P.; Ray, A.; Rogozina, O.P.; Dogan, S.; Grossmann, M.E. Targeting the adiponectin:Leptin ratio for postmenopausal breast cancer prevention. Front. Biosci. 2009, 1, 329–357. [Google Scholar]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding nutritional epidemiology and its role in policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, H.J.; Sedlacek, S.M.; Wolfe, P.; Paul, D.; Lakoski, S.G.; Playdon, M.C.; McGinley, J.N.; Matthews, S.B. Impact of Weight Loss on Plasma Leptin and Adiponectin in Overweight-to-Obese Post Menopausal Breast Cancer Survivors. Nutrients 2015, 7, 5156-5176. https://doi.org/10.3390/nu7075156
Thompson HJ, Sedlacek SM, Wolfe P, Paul D, Lakoski SG, Playdon MC, McGinley JN, Matthews SB. Impact of Weight Loss on Plasma Leptin and Adiponectin in Overweight-to-Obese Post Menopausal Breast Cancer Survivors. Nutrients. 2015; 7(7):5156-5176. https://doi.org/10.3390/nu7075156
Chicago/Turabian StyleThompson, Henry J., Scot M. Sedlacek, Pamela Wolfe, Devchand Paul, Susan G. Lakoski, Mary C. Playdon, John N. McGinley, and Shawna B. Matthews. 2015. "Impact of Weight Loss on Plasma Leptin and Adiponectin in Overweight-to-Obese Post Menopausal Breast Cancer Survivors" Nutrients 7, no. 7: 5156-5176. https://doi.org/10.3390/nu7075156





