Effect of Stay-Green Wheat, a Novel Variety of Wheat in China, on Glucose and Lipid Metabolism in High-Fat Diet Induced Type 2 Diabetic Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Determination of the Phytochemical Content and Total Antioxidant Activity in Vitro
2.3. Animal Treatment
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Insulin and Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) Determination
2.6. Blood Biochemical Analysis
2.7. Histopathological Examination
2.8. Statistical Analysis
3. Results
3.1. SGW Phytochemical Content and Total Antioxidant Activity in Vitro
| SGW | Common Wheat | |
|---|---|---|
| Total antioxidant activity (ORAC) (μg TE/g DW) | 30.33 ± 2.66 † | 24.12 ± 1.03 |
| Total phenolic (mg GAE/100 g DW) | 88.82 ± 5.91 | 85.84 ± 3.02 |
| Total flavonoid (mg CE/100 g DW) | 46.14 ± 1.80 † | 20.25 ± 0.78 |
| Total anthocyanin (mg CGE/100 g DW) | 1.73 ± 0.14 | nd |
| Phenolic Composition | ||
| Syringic acid (μg/g) | 7.16 ± 0.63 † | 36.39 ± 1.91 |
| Vanillic acid (μg/g) | 74.54 ± 6.41 † | 22.23 ± 0.93 |
| Caffeic acid (μg/g) | 3.08 ± 0.57 † | 7.87 ± 0.61 |
| Ferulic acid (μg/g) | 3.70 ± 0.89 | 2.51 ± 0.80 |
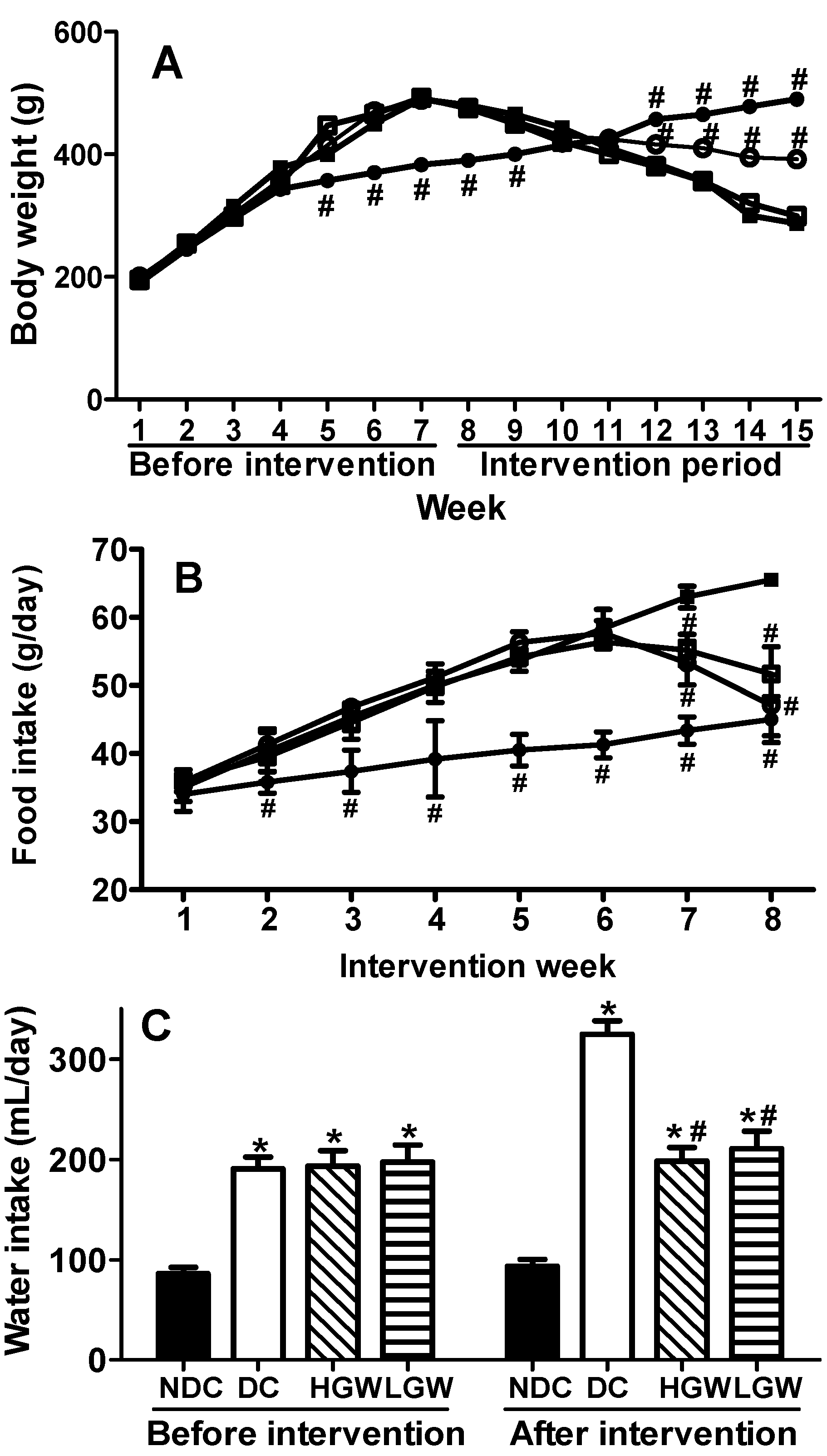
3.2. Rats Body Weight, Food and Water Intakes
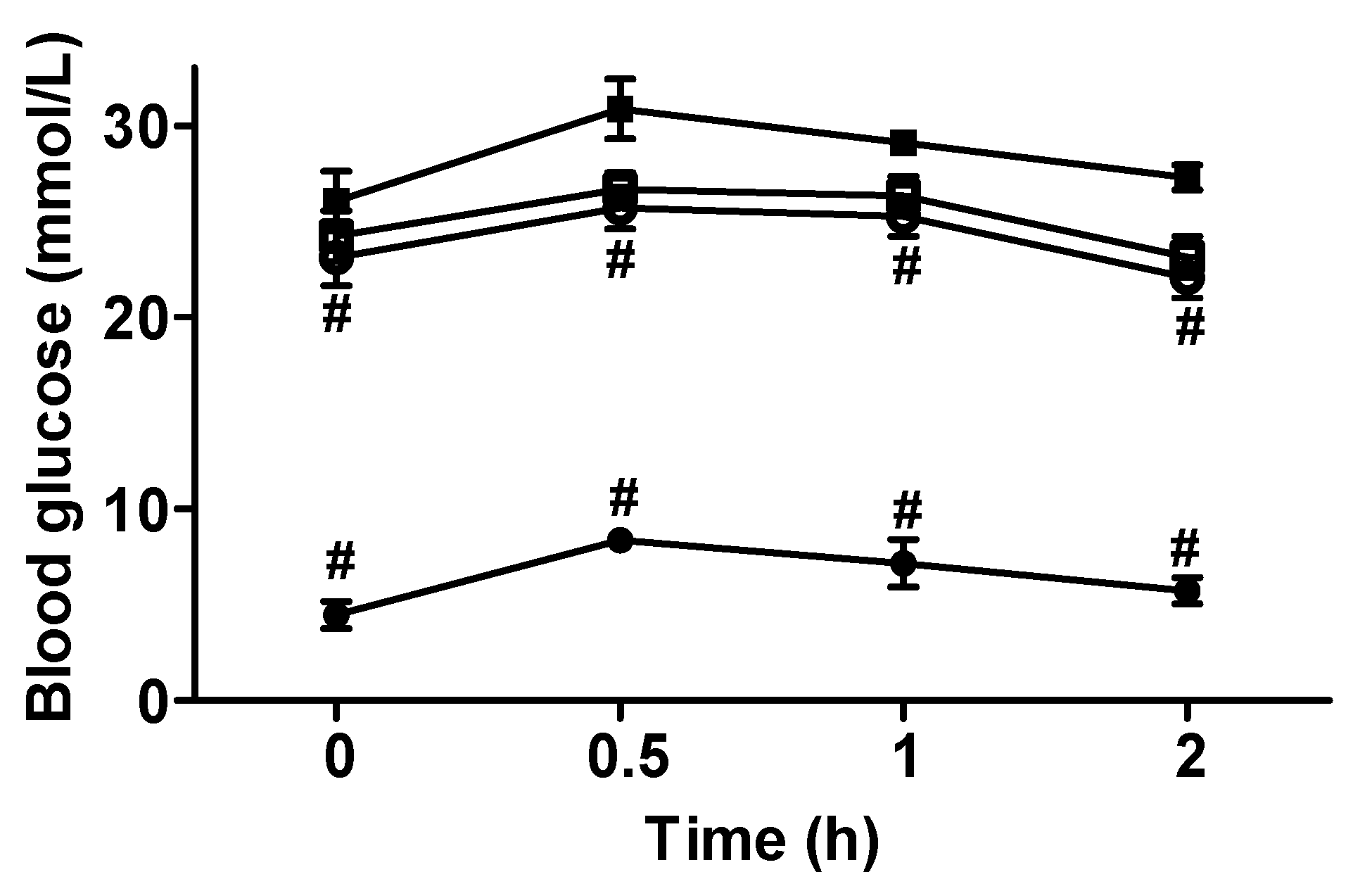
3.3. Blood Glucose and Insulin Level Changes
| Serum Insulin (mIU/L) | HOMA-IR | |
|---|---|---|
| NDC | 11.42 ± 0.23 | 2.78 ± 0.21 |
| DC | 24.56 ± 0.17 * | 23.89 ± 1.48 * |
| HGW | 21.54 ± 0.36 # | 16.83 ± 0.46 # |
| LGW | 22.17 ± 0.22 # | 18.80 ± 0.63 # |
3.4. Blood Lipid Changes
| TC (mg/dL) | TG (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | NEFA (mequiv/L) | |
|---|---|---|---|---|---|
| NDC | 65.27 ± 0.47 | 84.07 ± 3.54 | 26.47 ± 0.50 | 38.62 ± 0.42 | 0.37 ± 0.04 |
| DC | 92.14 ± 0.53 * | 286.73 ± 4.47 * | 19.18 ± 1.15 * | 63.02 ± 0.49 * | 1.03 ± 0.07 * |
| HGW | 83.74 ± 0.63 # | 252.21 ± 7.77 # | 21.12 ± 0.39 | 57.18 ± 0.41 # | 0.70 ± 0.06 # |
| LGW | 85.23 ± 0.32 # | 267.25 ± 7.34 # | 20.34 ± 0.48 | 60.73 ± 0.50 $ | 0.82 ± 0.07 $ |
3.5. Antioxidant Effects
| SOD (U/mL) | GSH-Px (U/mL) | |
|---|---|---|
| NDC | 207.50 ± 12.19 | 537.00 ± 8.35 |
| DC | 148.78 ± 4.53 * | 298.67 ± 11.05 * |
| HGW | 174.62 ± 7.47 $ | 408.87 ± 9.94 # |
| LGW | 171.00 ± 6.24 # | 389.56 ± 8.43 # |
3.6. Histological Changes
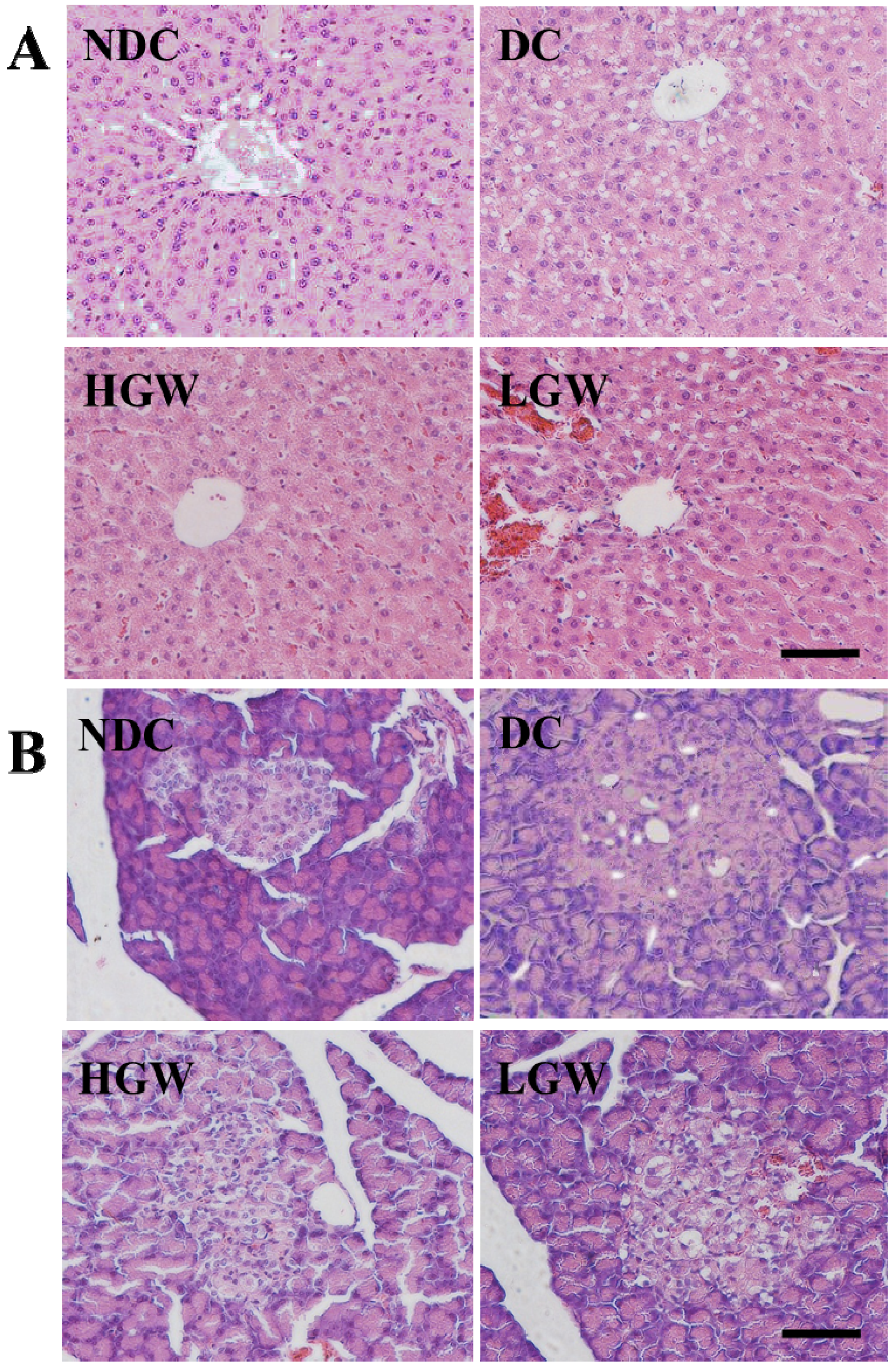
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Zhang, J. Black and green teas equally inhibit diabetic cataracts in a streptozotocin-induced rat model of diabetes. J. Agric. Food Chem. 2005, 53, 3710–3713. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Cho, E.J.; Yokozawa, T. Effects of proanthocyanidin preparations on hyperlipidemia and other biomarkers in mouse model of type 2 diabetes. J. Agric. Food Chem. 2008, 56, 7781–7789. [Google Scholar] [CrossRef] [PubMed]
- Oba, S.; Nanri, A.; Kurotani, K.; Goto, A.; Kato, M.; Mizoue, T.; Noda, M.; Inoue, M.; Tsugane, S.; Japan Public Health Center-based Prospective Study Group. Dietary glycemic index, glycemic load and incidence of type 2 diabetes in Japanese men and women: The Japan Public Health Center-based Prospective Study. Nutr. J. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, M.; Rim, J.S.; Koza, R.A.; Kozak, L.P. Variation in type 2 diabetes—Related traits in mouse strains susceptible to diet-induced obesity. Diabetes 2003, 52, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J. Agric. Food Chem. 2005, 53, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Baublis, A.J.; Lu, C.; Clydesdale, F.M.; Decker, E.A. Potential of wheat-based breakfast cereals as a source of dietary antioxidants. J. Am. Coll. Nutr. 2000, 19, 308S–311S. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Poutanen, K.; Gebruers, K.; Piironen, V.; Lampi, A.M.; Nystrom, L.; Andersson, A.A.; Aman, P.; Boros, D.; Rakszegi, M.; et al. The HEALTHGRAIN cereal diversity screen: concept, results, and prospects. J. Agric. Food Chem. 2008, 56, 9699–9709. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.R.; Vuaran, M.S.; King, R.A.; Noakes, M.; Keogh, J.; Morell, M.K.; Topping, D.L. Wholegrain foods made from a novel high-amylose barley variety (Himalaya 292) improve indices of bowel health in human subjects. Br. J. Nutr. 2008, 99, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, W.; Flis, M.; Antoszkiewicz, Z.; Lipinski, K.; Zdunczyk, Z. Effect of oat by-product antioxidants and vitamin E on the oxidative stability of pork from pigs fed diets supplemented with linseed oil. Arch. Anim. Nutr. 2012, 66, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Okarter, N.; Liu, R.H. Health benefits of whole grain phytochemicals. Crit. Rev. Food Sci. Nutr. 2010, 50, 193–208. [Google Scholar] [CrossRef] [PubMed]
- De Simone, V.; Soccio, M.; Borrelli, G.M.; Pastore, D.; Trono, D. Stay-green trait-antioxidant status interrelationship in durum wheat (Triticum durum) flag leaf during post-flowering. J. Plant. Res. 2014, 127, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Spano, G.; Di Fonzo, N.; Perrotta, C.; Platani, C.; Ronga, G.; Lawlor, D.W.; Napier, J.A.; Shewry, P.R. Physiological characterization of “stay green” mutants in durum wheat. J. Exp. Bot. 2003, 54, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Zhang, H.; Ren, Z.; Luo, P. Effects of paraquat-induced oxidative stress on antioxidants and chlorophyll fluorescence in stay-green wheat (Triticum aestivum L.) flag leaves. Bangladesh J. Bot. 2013, 2, 239–245. [Google Scholar] [CrossRef]
- Okarter, N.; Liu, C.; Sorrells, M.E.; Liu, R.H. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010, 2010, 249–257. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Swapna, M.; Ayesha, S.B.; Zehra, A.; Agawane, S.B.; Madhusudana, K. Identification of proglycemic and antihyperglycemic activity in antioxidant rich fraction of some common food grains. Int. Food Res. J. 2011, 3, 915–923. [Google Scholar]
- Giusti, M.M.; Rodriguez-Saona, L.E.; Wrolstad, R.E. Molar absorptivity and color characteristics of acylated and non-acylated pelargonidin-based anthocyanins. J. Agric. Food Chem. 1999, 47, 4631–4637. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, V.; Nandety, A.; Biesalski, H.K. Antioxidant, free radical scavenging and type II diabetes-related enzyme inhibition properties of traditionally processed Jequirity bean (Abrus precatorius L.). Int. J. Food Sci. Tech. 2011, 2505–2512. [Google Scholar] [CrossRef]
- Sano, T.; Umeda, F.; Hashimoto, T.; Nawata, H.; Utsumi, H. Oxidative stress measurement by in vivo electron spin resonance spectroscopy in rats with streptozotocin-induced diabetes. Diabetologia 1998, 41, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gaziano, J.; Liu, S.; Manson, J.; Buring, J.; Sesso, H. Whole- and refined-grain intakes and the risk of hypertension in women. Am. J. Clin. Nutr. 2007, 86, 1353–1360. [Google Scholar]
- Khalaf, G.; Mohamed, A. Effect of barley (Hordeum vulgare) on the liver of diabetic rats: Histological and biochemical study. Egypt. J. Histol. 2008, 2, 245–255. [Google Scholar]
- Martinez, D.; Ibáñez, G.; Rincón, L. Acido fítico: aspectos nutricionales e implicaciones analíticas. Arch. Latinoam. Nutr. 2002, 52, 219–231. (In Spanish) [Google Scholar]
- Andersson, A.; Tengblad, S.; Karlström, B.; Kamal-Eldin, A.; Landberg, R.; Basu, S.; Aman, P.; Vessby, B. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J. Nutr. 2007, 137, 1401–1407. [Google Scholar] [PubMed]
- Bibak, B.; Khalili, M.; Rajaei, Z.; Soukhtanloo, M.; Hadjzadeh, M.; Hayatdavoudi, P. Effects of melatonin on biochemical factors and food and water consumption in diabetic rats. Adv. Biomed. Res. 2014, 3. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Rahelic, D.; Jenkins, A.; Bozikov, V.; Pavic, E.; Juric, K.; Fairgrieve, C.; Romic, D.; Kokic, S.; Vuksan, V. Glycemic index in diabetes. Coll. Antropol. 2011, 35, 1363–1368. [Google Scholar] [PubMed]
- Yin, J.; Huang, J.; Chen, C.; Gao, N.; Wang, F.; Yu, F.S. Corneal complications in streptozocin-induced type I diabetic rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6589–6596. [Google Scholar] [CrossRef] [PubMed]
- Fridlyand, L.; Philipson, L. Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic beta-cells? Diabetes 2004, 53, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Oxidative stress and beta-cell dysfunction. Eur. J. Physiol. 2010, 460, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Le Bloc’h, J.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. (Berl.) 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Bigagli, E.; Bardini, G.; Rotella, C.M. Lipoperoxidation and antioxidant capacity in patients with poorly controlled type 2 diabetes. Toxicol. Ind. Health 2009, 25, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, T.; Xu, L.; Li, Y.; Zhang, S.; Duan, X. Protective effect of cinnamon polyphenols against STZ-diabetic mice fed high-sugar, high-fat diet and its underlying mechanism. Food Chem. Toxicol. 2013, 51, 419–425. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Zhang, C.; Luo, X.; Wang, L.; Zhang, R.; Wang, Z.; Fan, D.; Yang, H.; Deng, J. Effect of Stay-Green Wheat, a Novel Variety of Wheat in China, on Glucose and Lipid Metabolism in High-Fat Diet Induced Type 2 Diabetic Rats. Nutrients 2015, 7, 5143-5155. https://doi.org/10.3390/nu7075143
Ji J, Zhang C, Luo X, Wang L, Zhang R, Wang Z, Fan D, Yang H, Deng J. Effect of Stay-Green Wheat, a Novel Variety of Wheat in China, on Glucose and Lipid Metabolism in High-Fat Diet Induced Type 2 Diabetic Rats. Nutrients. 2015; 7(7):5143-5155. https://doi.org/10.3390/nu7075143
Chicago/Turabian StyleJi, Jinshan, Chao Zhang, Xiaoqin Luo, Li Wang, Ruijuan Zhang, Zhenlin Wang, Daidi Fan, Haixia Yang, and Jianjun Deng. 2015. "Effect of Stay-Green Wheat, a Novel Variety of Wheat in China, on Glucose and Lipid Metabolism in High-Fat Diet Induced Type 2 Diabetic Rats" Nutrients 7, no. 7: 5143-5155. https://doi.org/10.3390/nu7075143




