Increased Intake of Selected Vegetables, Herbs and Fruit may Reduce Bone Turnover in Post-Menopausal Women
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Participants

2.3. Study Diets
2.4. Dietary Analysis
| Group A fruit/vegetable/herb dietary recommendations | ||||||
| Herbs 1 | Fruit 1 | Vegetables 1 Green leafy | At least 5 servings/day of any vegetable (non Group B); examples of commonly-consumed vegetables | |||
| Basil, mint, oregano | Apple | Banana | Other fruit (not citrus or prunes) | Spinach, silver beet, white/green cabbage | Carrot, pumpkin, peas, cauliflower, courgettes, | |
| No. of servings/day | 1 culinary serving | 1 | 1 | 1 | 1 | |
| Group B fruit/vegetable/herb dietary recommendations | ||||||
| Herbs | Fruit | Vegetables Green leafy | Other vegetables (≥2–3 servings from this category) and ≤2 servings self-selected | |||
| No. of servings | Parsley sage, rosemary, thyme, garlic | Prunes | Oranges/other citrus | Other fruit (not banana or apple) | Chinese cabbage, e.g., bok choy, red cabbage, lettuce, rocket | Onions, broccoli, tomatoes, mushrooms, cucumber, leeks, green beans |
| 1 culinary serving | 1 | 1 | 1 | 1 | ||
| Group C dietary recommendations—Continue with usual diet (no change) | ||||||
2.5. Specimen Collection and Analysis
2.6. Bone Densitometry
2.7. Statistical Analyses
3. Results
3.1. Compliance
| Urinary Mineral Excretion mmol/24 h | n | Baseline | n | End | Change | % Change | p Change 1 < |
|---|---|---|---|---|---|---|---|
| Calcium A | 29 | 3.9 ± 2.02 | 29 | 2.7 ± 1.5 2 | −1.2 (−1.8–−0.5) 3 | −26 | 0.002 |
| Calcium B | 36 | 4.8 ± 2.1 | 36 | 3.5 ± 1.8 | −1.3 (−2.0–−0.6) | −24 | 0.001 |
| Calcium C | 22 | 4.2 ± 1.6 | 22 | 3.6 ± 1.6 | −0.61 (−1.6–−0.2) | 2 | 0.14 |
| p change between groups | N/S | <0.05 | |||||
| Potassium A | 29 | 135 ± 91 | 29 | 221 ± 199 | 86 (−5–167) | 93 | 0.04 |
| Potassium B | 36 | 174 ± 189 | 36 | 260 ± 217 | 85 (−9–178) | 141 | 0.07 |
| Potassium C | 22 | 187 ± 150 | 22 | 291 ± 265 | 104 (−27–235) | 93 | 0.10 |
| p change between groups | NS | NS | |||||
| Sodium A | 29 | 256 ± 119 | 29 | 90 ± 50 | −164 (−117–−212) | −48 | 0.001 |
| Sodium B | 36 | 274 ± 114 | 36 | 110 ± 64 | −166 (131–−202) | −50 | 0.001 |
| Sodium C | 22 | 260 ± 108 | 22 | 103 ± 54 | −166 (−120–−213) | −49 | 0.02 |
| p change between groups | NS | NS | |||||
| Magnesium A | 29 | 4.0 ± 1.9 | 29 | 3.9 ± 1.4 | −0.04 (−0.8–0.8) | −28 | 0.9 |
| Magnesium B | 36 | 4.7 ± 1.6 | 36 | 4.4 ± 2.3 | −0.08 (−0.9–0.7) | −5 | 0.9 |
| Magnesium C | 22 | 4.1 ± 1.5 | 22 | 4.3 ± 1.5 | 0.1 (−0.6–0.8) | 10 | 0.8 |
| p change between groups | NS | NS | |||||
| Creatinine A | 29 | 8929 ± 3191 | 29 | 9881 ± 2999 | 952 (−659–2563) | N/A | 0.2 |
| Creatinine B | 36 | 9017 ± 2591 | 36 | 8344 ± 3279 | −481 (−1992–1029) | N/A | 0.5 |
| Creatinine C | 22 | 9169 ± 2602 | 22 | 10,069 ± 2768 | 899 (−717–2516) | N/A | 0.3 |
| p change between groups | NS |
3.2. Anthropometric and Bone Mineral Density Measurements
| Characteristics 1 | Group A n = 49 | Group B n = 50 | Group C n = 43 |
|---|---|---|---|
| Age (years) 2 | 60 ± 4 (53–68,60) 2 | 60 ± 4 (51–71,61) | 61 ± 5 (51–71,61) |
| YSM 3 | 11 ± 5 (9–12) | 11 ± 4 (9–12) | 11 ± 5 (9–12) |
| Weight (kg) (baseline) | 72 ± 13 (68–76) | 70 ± 13 (66–73) | 66 ± 11 (63–70) |
| Weight (kg) (end) | 72 ± 12.5 (68–74) | 70 ± 13 (66–73) | 66 ± 11 (62–70) |
| Height (metres) | 1.6 ± .5 (1.61–1.64) | 1.65 ± 7 (1.62–1.167) | 1.63 ± 7 (1.61–1.66) |
| BMI 4 (kg/m2) | 27 ± 5 (26–29) | 25 ± 5 (24–27) | 24 ± 5 (23–26) |
| Body fat % | 40 ± 6.6 (38–42) | 38 ± 7 (36–40) | 37 ± 6 (35–39) |
| BP 5 systolic (mmHg) base | 131 ± 16 (126–135) | 132 ± 18 (127–137) | 129 ± 18 (124–135) |
| BP systolic (mmHg) end | 131 ± 15 (126–135) | 129 ± 17 (125–134) | 127 ± 18 (122–133) |
| BP diastolic(mmHg) base | 80 ± 10 (77–83) | 79 ± 10 (76–81) | 78 ± 9 (75–81) |
| BP diastolic (mmHg) end | 79 ± 10 (76–82) | 79 ± 10 (76–82) | 78 ± 9 (75–80) |
| BMD 6 (n = 142) | |||
| Normal BMD (%) | 20 (40) | 16 (32) | 15 (36) |
| Osteopenia (%) | 26 (52) | 26 (52) | 22 (54) |
| Osteoporotic (%) | 4 (8) | 8(16) | 4 (10) |
| Spine BMC (g/cm) | 53 ± 13 (49–57) | 55 ± 10 (52–58) | 55 ± 13 (50–59) |
| Spine BMD (g/cm2) | 0.95 ± 15 (0.90–0.99) | 0.94 ± 14 (0.90–0.98) | 0.97 ± 0.20 (0.90–1.00) |
| Spine t-score | −0.8 ± 1.2 (−1.1–−0.5) | −0.9 ± 1.3 (−1.3–−0.6) | −0.6 ± 1.6 (−1.1–−0.1) |
| Hip BMC (g/cm) | 30 ± 5 (29–32) | 29 ± 5 (28–31) | 30 ± 5 (29–32) |
| Hip BMD (g/cm2) | 0.88 ±11 (0.85–0.91) | 0.86 ± 0.14 (0.82–0.89) | 0.88 ± 0.01 (0.84–0.92) |
| Hip t-score | −0.5 ± 0.9 (−0.8–−0.3) | −0.69 ± 1.1 (−1.0–−0.3) | −0.48 ± 1.0 (−0.8–−0.2) |
| Femoral neck hip BMC (g/cm) | 3.7 ±0.7 (3.5–3.9) | 3.5 ± 0.8 (3.3–3.8) | 3.8 ± 0.6 (3.6–4.0) |
| Femoral neck hip BMD (g/cm2) | 0.76 ± 0.12 (0.72–0.79) | 0.72 ± 0.11 (0.69–0.75) | 0.75 ± 0.12 (0.72–0.79) |
| Femoral neck hip t-score | −0.8 ± 1.0 (−1.2–−0.5) | −1.2 ± 1.0 (−1.5–−0.9) | −0.9 ± 1.1 (−1.2–−0.5) |
3.3. Changes in Food Group Servings
| Servings 1 /Day | Group A n = 47 | Group B n = 50 | Group C (Control) n = 41 | p 2 |
|---|---|---|---|---|
| Fruit (baseline) 3 | 2.0 ± 0.9 (0–3.8) | 1.8 ± 0 .9 (0.3–3.8) | 2.2 ± 1.2 (0–4.7) | 0.15 |
| Fruit (final) | 2.9 ± 1.4 (0.50–7.0) | 3.0 ± 1.0 (1.3–5.3) | 2.2 ± 1.4 (0–6.3) | 0.003 |
| Change 4 | 0.9 ± 1.5 (−2.3–4.8) | 1.3 ± 1.1 (−0.8–4.0) | −0.06 ± 1.0 (−2.0–2.0) | 0.001 |
| P < 0.001 | p < 0.001 | p = 0.51 | ||
| Vegetables (baseline) | 3.4 ± 1.2 (1.3–6.0) | 3.3 ± 1.3 (1.0–7.8) | 3.7 ± 1.3 (1.2–7.0) | 0.21 |
| Vegetables(end) | 5. 5 ± 1.9 (1.7–10) | 6.1 ± 1.9 (2.7–12.2) | 4.0 ± 1.2 (0.7–6.3) | 0.001 |
| Change 4 | 2.0 ± 2.2 (−3.7–7.3) | 2.8 ± 2.1 (−2.5–8.2) | 0.2 ± 1.5 (−3.7–2.8) | 0.001 |
| p < 0.001 | p < 0.001 | p = 0.49 | ||
| Bread/cereals (baseline) | 4.5 ± 1.60 (1.0–8.3) | 4.4 ± 1.4 (0.3–7.5) | 4.2 ± 1.4 (1.7–7.5) | 0.54 |
| Bread/cereals (end) | 3.4 ± 1.52 (0.83–7.8) | 3.3 ± 1.5 (0–7.0) | 4.1 ± 0.7 (0.5–8.0) | 0.03 |
| Change | −1.4 ± 1.9 (−5.7–2.3) | −1.2 ± 1.5 (−5.7–1.5) | −0.22 ± 1.5 (−4.7–2.2) | 0.03 |
| p < 0.001 | p < 0.001 | p = 0.48 | ||
| Dairy (baseline) | 1.5 ± 0.74 (0.3–2.8) | 1.5 ± 0.9 (0.2–4.2) | 1.8 ± 1 (0.3–5.3) | 0.28 |
| Dairy (end) | 1.3 ± 1.0 (0.0–5.0) | 1.5 ± 0.8 (0.2–3.5) | 1.4 ± 0.0 (0.0–4.5) | 0.77 |
| Change | −0.2 ± 1.0 (−2.0–3.3) | −0.08 ± 0.8 (−2.3–1.7) | −0.4 ± 0.8 (−2.0–1.5) | 0.24 |
| p < 0.03 | p = 0.5 | p < 0.003 | ||
| Meat and protein (baseline) | 1.9 ± 0.9 (0.3–4.3) | 1.8 ± 0.8 (0.3–5.0) | 2.0 ± 0.8 (0.5–4.3) | 0.43 |
| Meat and protein (end) | 1.8 ± 1.0 (0.2–4.8) | 1.7 ± 0.8 (0.3–4.0) | 2.0 ± 1.0 (0.2–6.3) | 0.34 |
| Change | −0.1 ± 1.0 (−2.7–2.5) | −0.1 ± 0.9 (−2.0–2.0) | −0.1 ± 1.0 (−2.2–3.8) | 0.99 |
| p = 0.46 | p = 0.40 | p = 0.69 | ||
| PRAL (baseline) mEq/day 5 | −0.1 ± 16 (−36–31) | −1.2 ± 15 (−34–26) | −1.8 ± 13 (−43–27) | 0.76 |
| PRAL (end) mEq/day | −17 ± 17 (−45–27) | −23 ± 16 (−65–10) | −3 ± 16 (−36–40) | 0.001 |
| Change 5 | −17± 17 (−55–15) | −22 ± 25 (−65–10) | −1.6 ± 18 (−30–60) | 0.001 |
| p < 0.001 | p < 0.001 | p = 0.57 |
3.4. Changes in Dietary Nutrient Intake
| Nutrientse | Group A Intervention n = 47 | Group B Intervention n = 50 | Group C Control n = 41 | EAR 1/AI 2 RDI 3/SDT 4 | p 5 |
|---|---|---|---|---|---|
| Baseline | |||||
| Protein (g) 6 | 86 ± 17 (49–128) | 82 ± 18 (46–128) | 83.5 ± 13.6 (56–117) | 37 1/46 3 | |
| Change | 2.2 ± 19 (−67–35) | 2.0 ± 17 (−34–45) | 0.10 ± 16 (−53–35) | 0.8 | |
| Fat (g) | 72 ± 16 (35–98) | 75 ± 14 (47–102) | 74 ± 17 (42–112) | N/A | |
| Change | 2.30 ± 15 (−36–24) | 2 ± 19 (−49–57) | 4 ± 20 (−69–41) | 0.9 | |
| CHO (g) | 220 ± 43 (150–350) | 216 ± 32 (107–275) | 220 ± 41 (132–304) | N/A | |
| Change | 19 ± 72 (−115–195) | 5.2 ± 52 (−148–133) | 6 ± 65 (−155–124) | 0.5 | |
| Fibre (g) | 27 ± 8 (14–50) | 27 ± 8 (12–56) | 28 ± 8 (13–43) | 25 2/28 4 | |
| Change | 5.7 ± 6.6 (−22–7) | 6.4 ± 10.3 (−28–18) | −0.5 ± 0.0 (−24–24) | 0.01 | |
| Folate (μg) | 368 ± 153 (102–812) | 380 ± 225 (151–1646) | 381 ± 134 (205–687) | 400 3/600 4 | |
| Change | 109 ± 213 (−865–447) | 121 ± 250 (−561–1177) | −9 ± 16 (−237–548) | 0.01 | |
| Sodium (mg) | 2345 ± 618 (1261–4046) | 2766 ± 900 (1242–4593) | 2551 ± 979 (947–6405) | 1600 4 | |
| Change | −63 ± 887 (−2466–1566) | −376 ± 943 (−1561–2461) | 24.5 ± 1256 (−2147–3230) | 0.2 | |
| Potassium (mg) | 3695 ± 766 (2400–6261) | 3643 ± 1000 (2013–6902) | 3781 ± 790 (2410–6027) | 2800 2/4700 4 | |
| Change | 935 ± 953 (−3246–775) | 1393 ± 1375 (−5401–2483) | 64 ± 933 (−1758–2414) | 0.001 | |
| Magnesium (mg) | 365 ± 930 (232–694) | 366 ± 105 (208–667) | 385 ± 112 (231–941) | 265 1/320 3 | |
| Change | 73 ± 134 (−548–298) | 54 ± 106 (−285–229) | −20 ± 113 (−143–532) | 0.001 | |
| Calcium (mg) | 850 ± 257 (375–1532) | 872 ± 347 (404–2,166) | 905 ± 270 (396–1632) | 1100 1/1300 3 | |
| Change | 181 ± 353 (−935–635) | 164 ± 41 (−974–1514) | 5 ± 293 (−621–679) | 0.03 |
3.5. Urinary pH and Vegetable/Herb/Fruit Intake
3.6. Urinary Mineral Excretion
3.7. Bone Turnover Markers
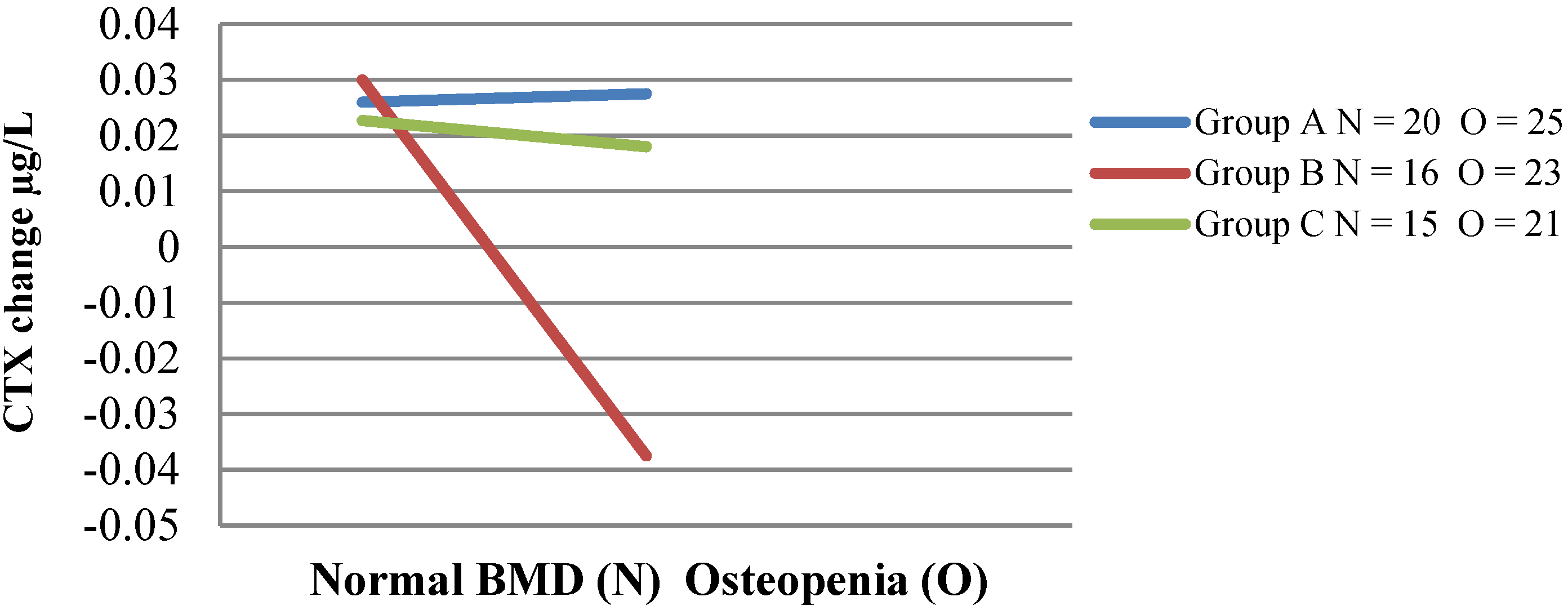
| Bone Markers | n | Intervention Group A | n | Intervention Group B | n | Non Intervention Group C | p between Groups 1 |
|---|---|---|---|---|---|---|---|
| CTX baseline μg/L 2 | 48 | 0.37 (0.34–0.41) | 50 | 0.42 (0.38–0.45) | 43 | 0.39 (0.33–0.45) | 0.4 |
| CTX mid μg/L | 41 | 0.39 (0.35–0.43) | 47 | 0.43 (0.39–0.47) | 0.2 | ||
| CTX final μg/L | 47 | 0.40 (0.36–0.44) | 47 | 0.41 (0.38–0.45) | 39 | 0.40 (0.35–0.46) | 0.8 |
| Change in CTX | 0.03 (0.01–0.03) | −0.01 (−0.03–0.03) | 0.01 (−0.01–0.03) | 0.4 | |||
| p-change | p < 0.03 | p = 0.9 | p = 0.3 | ||||
| P1NP μg/L | 48 | 44.2 (39.8–48.7) | 50 | 49.7 (45.1–54.2) | 43 | 45.0 (39.0–50.8) | 0.2 |
| P1NP mid μg/L | 41 | 43.8 (38.9–48.6) | 47 | 46.7 (42.5–50.9) | 0.4 | ||
| P1NP end μg/L | 47 | 43.3 (39.3–47.3) | 47 | 45.9 (42.4–49.5) | 39 | 46.6 (38.5–54.6) | 0.6 |
| Change in P1NP | −0.9 (−2.4–4.3) | −3.2 (−5.9–0.4) | 1.8 (−3.1–6.6) | 0.2 | |||
| p-change | p = 0.6 | p < 0.01 | p = 0.5 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dawson-Hughes, B.; Harris, S.; Palermo, N.; Castaneda-Sceppa, C.; Rasmussen, H.; Dallal, G. Treatment with potassium bicarbonate lowers calcium excretion and bone resorption in older men and women. J. Clin. Endocrinol. Metab. 2009, 94, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; National Osteoporosis Foundation Committee. A revised clinician’s guide to the prevention and treatment of osteoporosis. J. Clin. Endocrinol. Metab. 2008, 93, 2463–2465. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; McNeill, R.; Leung, W.; Radwan, E.; Willingdale, J. Current and future economic burden of osteoporosis in New Zealand. Appl. Health Economic Health Policy 2011, 9, 111–123. [Google Scholar] [CrossRef]
- Ashwell, M.; Stone, E.; Mathers, J.; Barnes, S.; Compston, J.; Francis, R.M.; Key, T.; Cashman, K.D.; Cooper, C.; Khaw, K.T.; et al. Nutrition and bone health projects funded by the UK Food Standards Agency: Have they helped to inform public health policy? Br. J. Nutr. 2008, 99, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Devine, A.; Prince, R. The effects of high potassium consumption on bone mineral density in a prospective cohort study of elderly postmenopausal women. Osteoporosis Int. 2009, 20, 335–340. [Google Scholar] [CrossRef]
- Weaver, C.; Alekel, D.; Ward, W.; Ronis, M. Flavonoid intake and bone health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; McDonald, H. Interaction of dietary patterns, systemic inflammation, and bone health. In Nutritional Influences on Bone Health; Burckhardt, P., Dawson-Hughes, B., Weaver, C., Eds.; Springer: London, UK, 2013; pp. 19–30. [Google Scholar]
- Nordin, B.E.C.; Need, A.G.; Morris, H.A.; Horowitz, M. The nature and significance of the relationship between urinary sodium and urinary calcium in women. J. Nutr. 1993, 123, 1615–1622. [Google Scholar] [PubMed]
- New, S.; MacDonald, H.; Campbell, M.; Martin, J.; Garton, M.; Robins, S.; Reid, D. Lower estimates of net endogenous non-carbonic acid production are positively associated with indexes of bone health in premenopausal and perimenopausal women. Am. J. Clin. Nutr. 2004, 79, 131–138. [Google Scholar] [PubMed]
- Shi, L.; Libuda, L.; Schonau, E.; Frassetto, L.; Remer, T. Long term higher urinary calcium excretion within the normal physiologic range predicts impaired bone status of the proximal radius in healthy children with higher potential renal acid load. Bone 2012, 50, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Investig. 2011, 41, 1361–1366. [Google Scholar] [CrossRef]
- Wachman, A.; Bernstein, D. Diet and osteoporosis. Lancet 1968, 291, 958–959. [Google Scholar] [CrossRef]
- New, S.; Bolton-Smith, C.; Grubb, D.; Reid, D. Nutritional influences on bone mineral density: A cross-sectional study in premenopausal women. Am. J. Clin. Nutr. 1997, 65, 1831–1839. [Google Scholar] [PubMed]
- Prynne, C.; Mishra, G.; O’Connell, M.; Muniz, G.; Laskey, M.; Yan, L.; Prentice, A.; Ginty, F. Fruit and vegetable intakes and bone mineral status: A cross sectional study in 5 age and sex cohorts. Am. J. Clin. Nutr. 2006, 83, 1420–1428. [Google Scholar] [PubMed]
- Welch, A.; Bingham, S.; Reeve, J.; Khaw, K.T. More acidic dietary acid-base load is associated with reduced calcaneal broadband ultrasound attenuation in women but not in men: Results from the Epic-Norfolk Cohort Study. Am. J. Clin. Nutr. 2007, 85, 1134–1141. [Google Scholar] [PubMed]
- New, S. Intake of fruit and vegetables: Implications for bone health. Proc. Nutr. Soc. 2003, 62, 889–899. [Google Scholar] [PubMed]
- Moseley, K.; Weaver, C.; Appel, L.; Sebastian, A.; Sellmeyer, D. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J. Bone Miner. Res. 2013, 28, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Sebastian, A. Long-term persistence of the urine calcium-lowering effect of potassium bicarbonate in post-menopausal women. J. Clin. Endocrinol. Metab. 2005, 90, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; Black, A.J.; Aucott, L.; Duthie, G.; Duthie, S.; Sandison, R.; Hardcastle, A.C.; Lanham New, S.A.; Fraser, W.D.; Reid, D.M. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: A randomized controlled trial. Am. J. Clin. Nutr. 2008, 88, 465–474. [Google Scholar] [PubMed]
- Lin, P.; Ginty, F.; Appel, L.; Aickin, M.; Bohannon, A.; Garnero, P.; Barclay, D.; Svetkey, L.P. The Dash diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J. Nutr. Educ. Behav. 2003, 133, 3130–3136. [Google Scholar]
- Nowson, C.; Patchett, A.; Wattanapenpaiboon, N. The effects of a low-sodium base-producing diet including red meat compared with a high-carbohydrate, low-fat diet on bone turnover markers in women aged 45–75 years. Br. J. Nutr. 2009, 102, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K.; Maalouf, N.M.; Abrams, S.A.; Pak, C.Y. Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J. Clin. Endocrinol. Metab. 2005, 90, 3528–3533. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Muhlbauer, R.; Tang, C.; Chen, H.; Chang, G. Onion decreases the ovariectomy-induced osteopenia in young adult rats. Bone 2008, 42, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Mühlbauer, R.; Lozano, A.; Reinli, A. Onion and a mixture of vegetables, salads, and herbs affect bone resorption in the rat by a mechanism independent of their base excess. J. Bone Miner. Res. 2002, 17, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Mühlbauer, R. Are vegetables, salads, herbs, mushrooms, fruits and red wine residue that inhibit bone resorption in the rat a promise of osteoporosis prevention? Curr. Nutr. Food Sci. 2006, 2, 69–78. [Google Scholar] [CrossRef]
- Mühlbauer, R.; Li, F. Effect of vegetables on bone metabolism. Nature 1999, 401, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.; Bu, S.Y.; Lerner, M.R.; Lancaster, E.A.; Bellmer, D.; Marlow, D.; Lightfoot, S.A.; Arjmandi, B.H.; Brackett, D.J.; Lucas, E.A.; et al. Dried plum prevents bone loss in a male osteoporosis model via IgF-I and the RANK pathway. Bone 2006, 39, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Arjmandi, B.H. Viewpoint: Dried plum, an emerging functional food that may effectively improve bone health. Ageing Res. Rev. 2009, 8, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Horcajada-Molteni, M.-N.L.; Crespy, V.; Coxam, V.R.; Davicco, M.-J.; Remesy, C.; Barlet, J.-P. Rutin inhibits ovariectomy-induced osteopenia in rats. J. Bone Miner. Res. 2000, 15, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Matheson, E.M.; Mainous, A.G.I.; Carnemolla, M.A. The association between onion consumption and bone density in perimenopausal and postmenopausal non-hispanic white women 50 years and older. Menopause 2009, 16, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.; Clifford, M. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Product Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Phytochemicals suppress nuclear factor-kappa B signaling: Impact on health span and the aging process. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Horcajada, M.N. Phenolic phytochemicals and bone. Phytochem. Rev. 2008, 7, 313–344. [Google Scholar] [CrossRef]
- Trzeciakiewicz, A.; Habauzit, V.; Horcajada, M.-N. When nutrition interacts with osteoblast function: Molecular mechanisms of polyphenols. Nutr. Res. Rev. 2009, 22, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, L.; Ferrandiz, M.; Brines, R.; Guede, D.; Cuadrado, A.; Alcaraz, M.J. Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxidative Med. Cell. Longev. 2014, 2014, 9. [Google Scholar] [CrossRef]
- Gunn, C.; Weber, J.; Kruger, M. Midlife women, bone health, vegetables, herbs and fruit study. The scarborough fair study protocol. BMC Public Health 2013, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Muhlbauer, R.; Lozano, A.M.; Reinil, A.; Wetli, H. Various selected vegetables, fruit, mushrooms and red wine residue inhibit bone resorption in rats. J. Nutr. 2003, 133, 3592–3597. [Google Scholar] [PubMed]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S4–S24. [Google Scholar] [PubMed]
- Frassetto, L.; Lanham-New, S.; Macdonald, H.; Remer, T.; Sebastian, A.; Tucker, K.; Tylavsky, F. Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J. Nutr. 2007, 137, 1491–1492. [Google Scholar] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [PubMed]
- World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis; WHO: Geneva, Switzerland, 1994. [Google Scholar]
- World Health Organization. Assessment of Osteoporosis at Primary Health Care Level; World Health Organisation: Brussels, Belgium, 2004. [Google Scholar]
- Kanis, J.A.; Adachi, J.D.; Cooper, C.; Clark, P.; Cummings, S.R.; Diaz-Curiel, M.; Harvey, N.; Hiligsmann, M.; Papaioannou, A.; Pierroz, D.D. Standardising the descriptive epidemiology of osteoporosis: Recommendations from the epidemiology and quality of life working group of IOF. Osteoporosis Int. 2013, 24, 2763–2764. [Google Scholar] [CrossRef]
- Frassetto, L.; Morris, R.; Sebastian, A. A practical approach to the balance between acid production and renal acid excretion in humans. J. Nephrol. 2006, 19 (Suppl. 9), S33–S40. [Google Scholar] [PubMed]
- Mühlbauer, R.; Lozano, A.; Palacio, S.; Reinli, A.; Felix, R. Common herbs, essential oils, and monoterpenes potently modulate bone metabolism. Bone 2003, 32, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, G.N. Pragmatic trials: How to adjust for the “Hawthorne effect”? Thorax 2012, 67, 562. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Riesen, W.; Muser, J.; Hulter, H.; Krapf, R. Neutralization of western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am. J. Physiol. Renal Physiol. 2003, 284, F32–F40. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J.; Frassetto, L.; Katzinger, J. Diet induced acidosis: Is it real and clinically relevant? Br. J. Nutr. 2010, 103, 1185–1194. [Google Scholar] [PubMed]
- Rafferty, K.; Heaney, R.; Davies, K. Potassium intake and the calcium economy. J. Am. College Nutr. 2005, 24, 99–106. [Google Scholar] [CrossRef]
- Heaney, R. Role of dietary sodium in osteoporosis. J. Am. College Nutr. 2006, 25, 271S–276S. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Fisheries. New Zealand Total Diet Study; Ministry of Agriculture and Fisheries: Wellington, New Zealand, 2009.
- Ministry of Health (NZ). Nutrient reference values of Australia and New Zealand; Ministry of Health (NZ): Wellington, New Zealand, 2006.
- USDA; USDHHS. Dietary guidelines for Americans; Government Printing Office: Washington, DC, USA, 2010.
- Drewnowski, A.; Maillot, M.; Rehm, C. Reducing the sodium-potassium ratio in the US diet: A challenge for public health. Am. J. Clin. Nutr. 2012, 96, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.; Hohman, E. Comparison of natural products for effects on bone balance. In Nutritional Influences on Bone Health; Burckhardt, P., Dawson-Hughes, B., Weaver, C., Eds.; Springer: London, UK, 2013; pp. 147–156. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunn, C.A.; Weber, J.L.; McGill, A.-T.; Kruger, M.C. Increased Intake of Selected Vegetables, Herbs and Fruit may Reduce Bone Turnover in Post-Menopausal Women. Nutrients 2015, 7, 2499-2517. https://doi.org/10.3390/nu7042499
Gunn CA, Weber JL, McGill A-T, Kruger MC. Increased Intake of Selected Vegetables, Herbs and Fruit may Reduce Bone Turnover in Post-Menopausal Women. Nutrients. 2015; 7(4):2499-2517. https://doi.org/10.3390/nu7042499
Chicago/Turabian StyleGunn, Caroline Ann, Janet Louise Weber, Anne-Thea McGill, and Marlena Cathorina Kruger. 2015. "Increased Intake of Selected Vegetables, Herbs and Fruit may Reduce Bone Turnover in Post-Menopausal Women" Nutrients 7, no. 4: 2499-2517. https://doi.org/10.3390/nu7042499





