Gastroprotective Efficacy and Safety Evaluation of Scoparone Derivatives on Experimentally Induced Gastric Lesions in Rodents
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
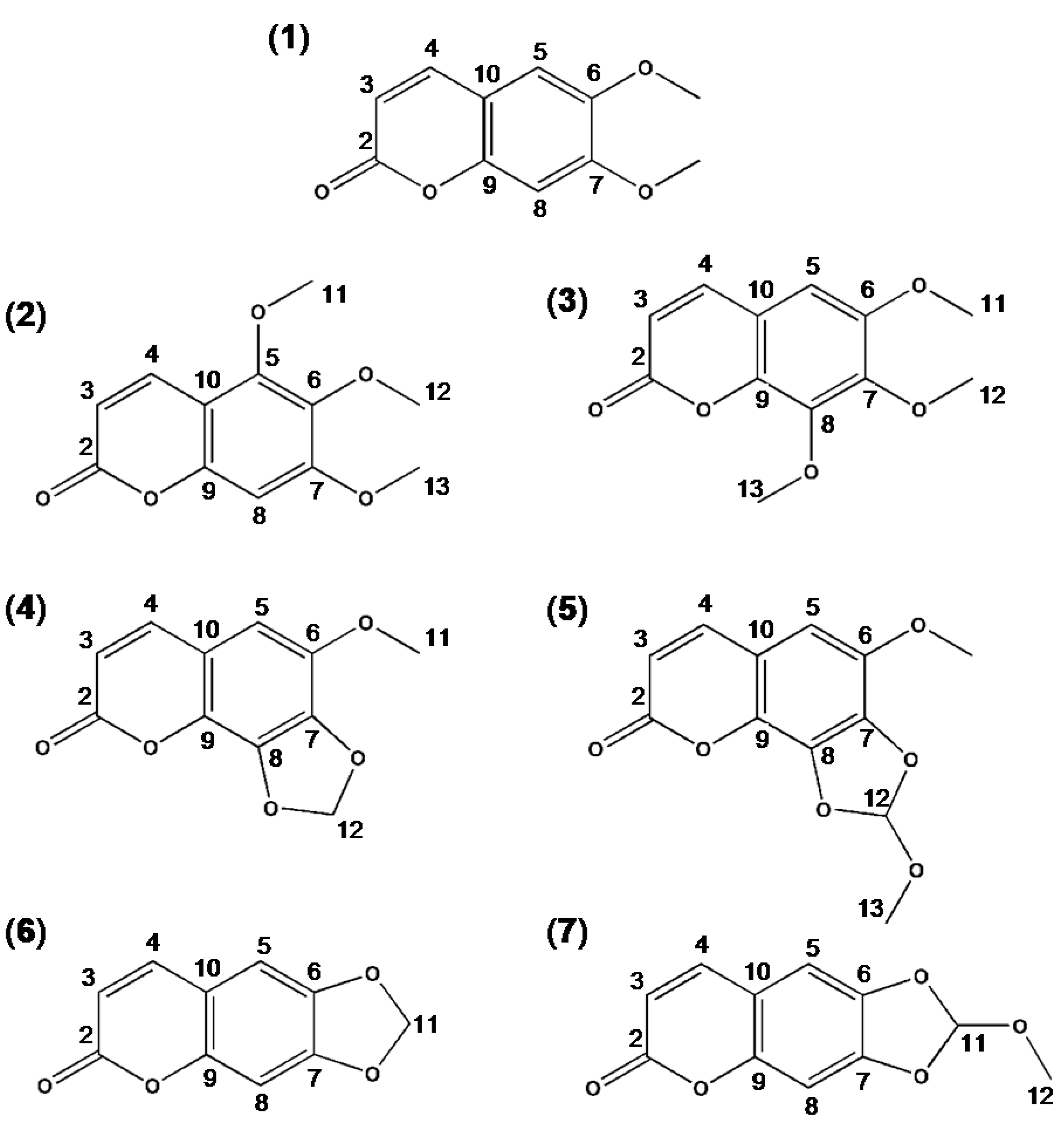
2.2. Synthesis and Spectral Data of Scoparone Derivatives
2.2.1. 5,6,7-Trimehoxycoumarin
2.2.2. 6,7,8-Trimethoxycoumarin
2.2.3. 6-Methoxy-7,8-methylenedioxycoumarin
| C* | Compound | |||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | |
| C-2 | 160.15 | 160.52 | 159.81 | 159.74 | 161.26 | 161.35 |
| C-3 | 112.08 | 115.70 | 114.44 | 114.44 | 113.97 | 114.28 |
| C-4 | 137.68 | 144.73 | 143.63 | 138.24 | 140.78 | 143.30 |
| C-5 | 148.73 | 105.48 | 105.05 | 104.76 | 112.68 | 112.43 |
| C-6 | 139.06 | 151.15 | 141.11 | 143.72 | 143.51 | 144.54 |
| C-7 | 156.93 | 143.72 | 133.97 | 133.31 | 144.91 | 152.02 |
| C-8 | 95.94 | 146.76 | 135.05 | 140.48 | 105.03 | 103.59 |
| C-9 | 150.90 | 141.83 | 139.97 | 133.48 | 151.24 | 150.55 |
| C-10 | 106.43 | 115.50 | 114.33 | 114.23 | 113.39 | 113.34 |
| C-11 | 56.42 | 56.60 | 56.82 | 56.83 | 102.36 | 121.53 |
| C-12 | 60.71 | 61.92 | 103.60 | 121.55 | - | 50.81 |
| C-13 | 61.81 | 61.54 | - | 50.64 | - | - |
| C* | Compound | |||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | |
| C-2 | - | - | - | - | - | - |
| C-3 | 6.23, 6.25(d, 1H) | 6.30, 6.33(d, 1H) | 6.26, 6.29(d, 1H) | 6.28, 6.31(d, 1H) | 6.25, 6.29(d, 1H) | 6.15, 6.30(d, 1H) |
| C-4 | 7.95, 7.97(d, 1H) | 7.87, 7.89(d, 1H) | 7.57, 7.59(d, 1H) | 7.59, 7.61(d, 1H) | 7.57, 7.60(d, 1H) | 7.79, 7.93(d, 1H) |
| C-5 | - | 7.03(s, 1H) | 6.59(s, 1H) | 6.61(s, 1H) | 6.83(s, 1H) | 6.59(s, 1H) |
| C-6 | - | - | - | - | - | - |
| C-7 | - | - | - | - | - | - |
| C-8 | 6.86(s, 1H) | - | - | - | 6.96(s, 1H) | 6.80(s, 1H) |
| C-9 | - | - | - | - | - | - |
| C-10 | - | - | - | - | - | - |
| C-11 | 3.75(s, 3H) | 3.89(s, 3H) | - | 3.95(s, 3H) | 6.09(s, 2H) | 7.06(s, 1H) |
| C-12 | 3.88(s, 3H) | 3.93(s, 3H) | 5.17(s, 2H) | 7.04(s, 1H) | - | 3.45(s, 3H) |
| C-13 | 3.93(s, 3H) | 3.97(s, 3H) | - | 3.49(s, 3H) | - | - |
2.2.4. 6-Methoxy-7,8-(1-methoxy) Methylenedioxycomarin
2.2.5. 6,7-Methylenedioxycomarin
2.2.6. 6,7-(1-Methoxy)methylenedioxycomarin
2.3. Animals and Ethics Statement
2.4. Gastroprotective Efficacy Assessments
2.4.1. HCl/Ethanol-Induced Gastritis in Rats
2.4.2. Indomethacin-Induced Gastritis in Rats
2.4.3. Measurement of Adherent Gastric Mucus
2.5. Toxicological Safety Assessments
2.5.1. Acute Toxicity Studies
2.5.2. Cytochrome P450 Enzyme Activity Assay in vitro
2.6. Statistical Analysis
3. Results
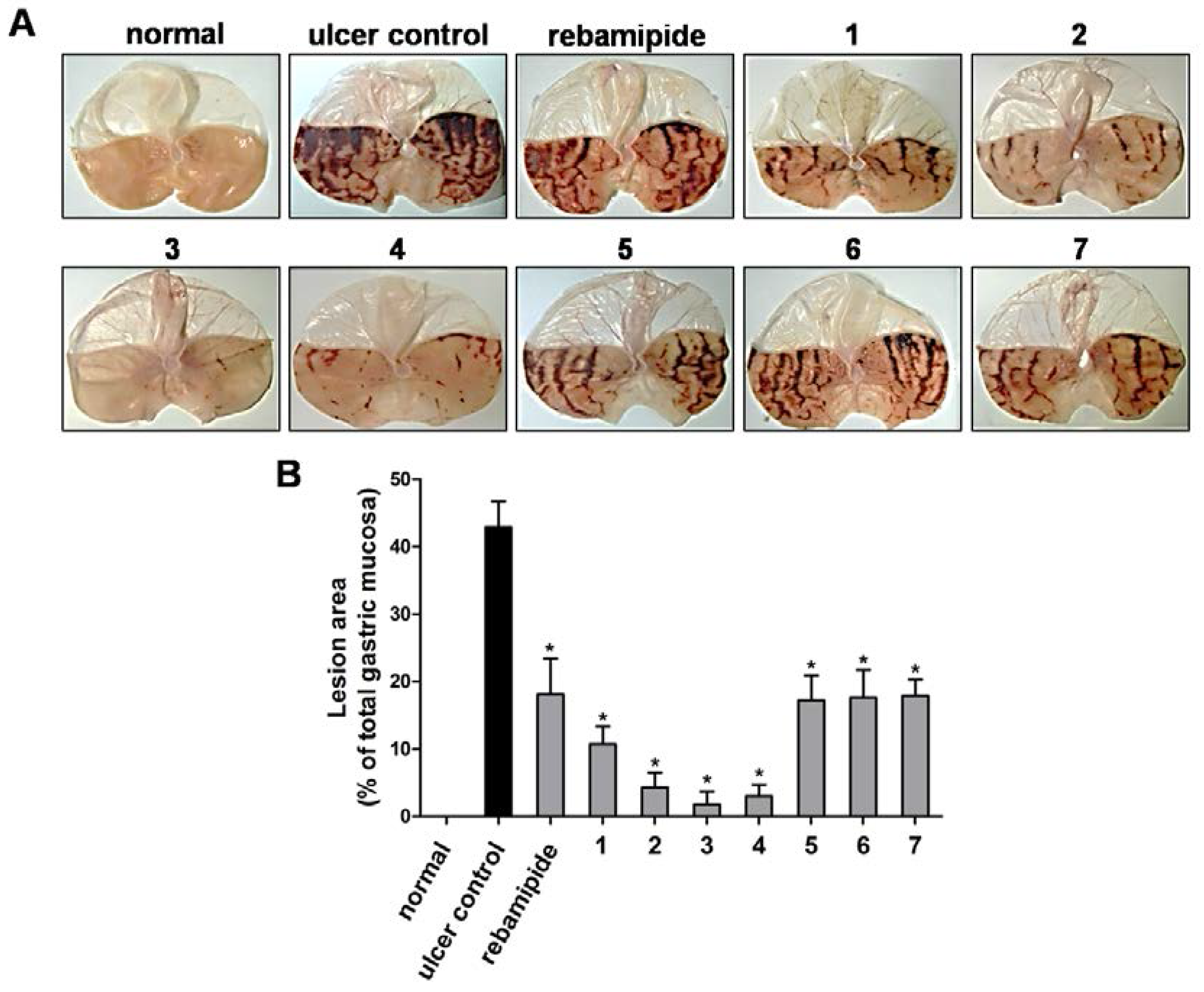
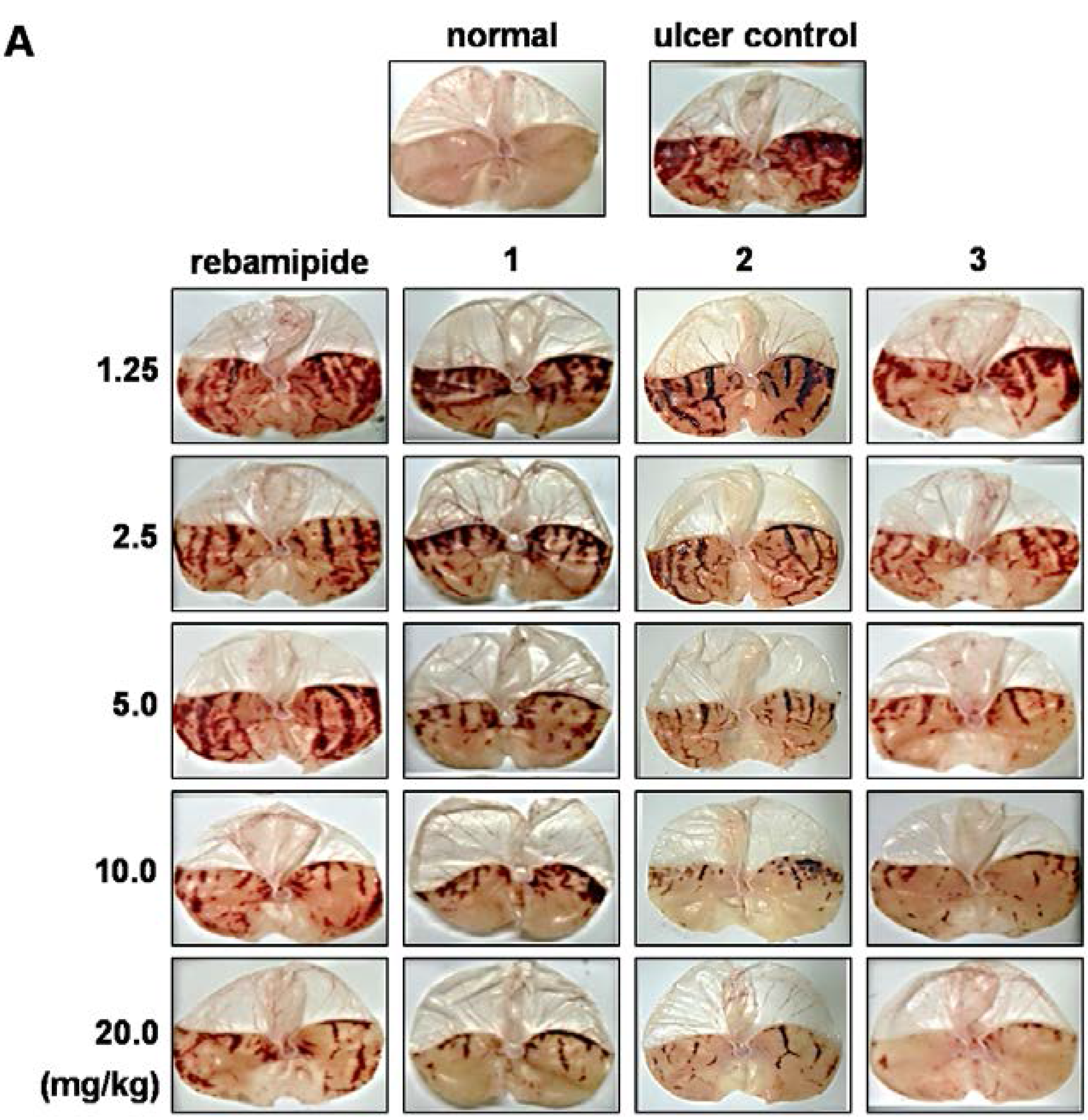
3.1. Effect of Scoparone Derivatives on Gastric Ulcer
3.2. Effect of Scoparone Derivatives on Adherent Gastric Mucus

| Treatment | Dose (mg/kg body weight) | Adherent Mucus (μg alcian blue/g tissue) |
|---|---|---|
| Normal control | - | 1618 ± 64* |
| Ulcer control | - | 456 ± 74* |
| Rebamipide | 20 | 1584 ± 80 * |
| 5,6,7-trimethoxycoumarin (2) | 20 | 1424 ± 83 * |
| 6,7,8-trimethoxycoumarin (3) | 20 | 1876 ± 51 * |
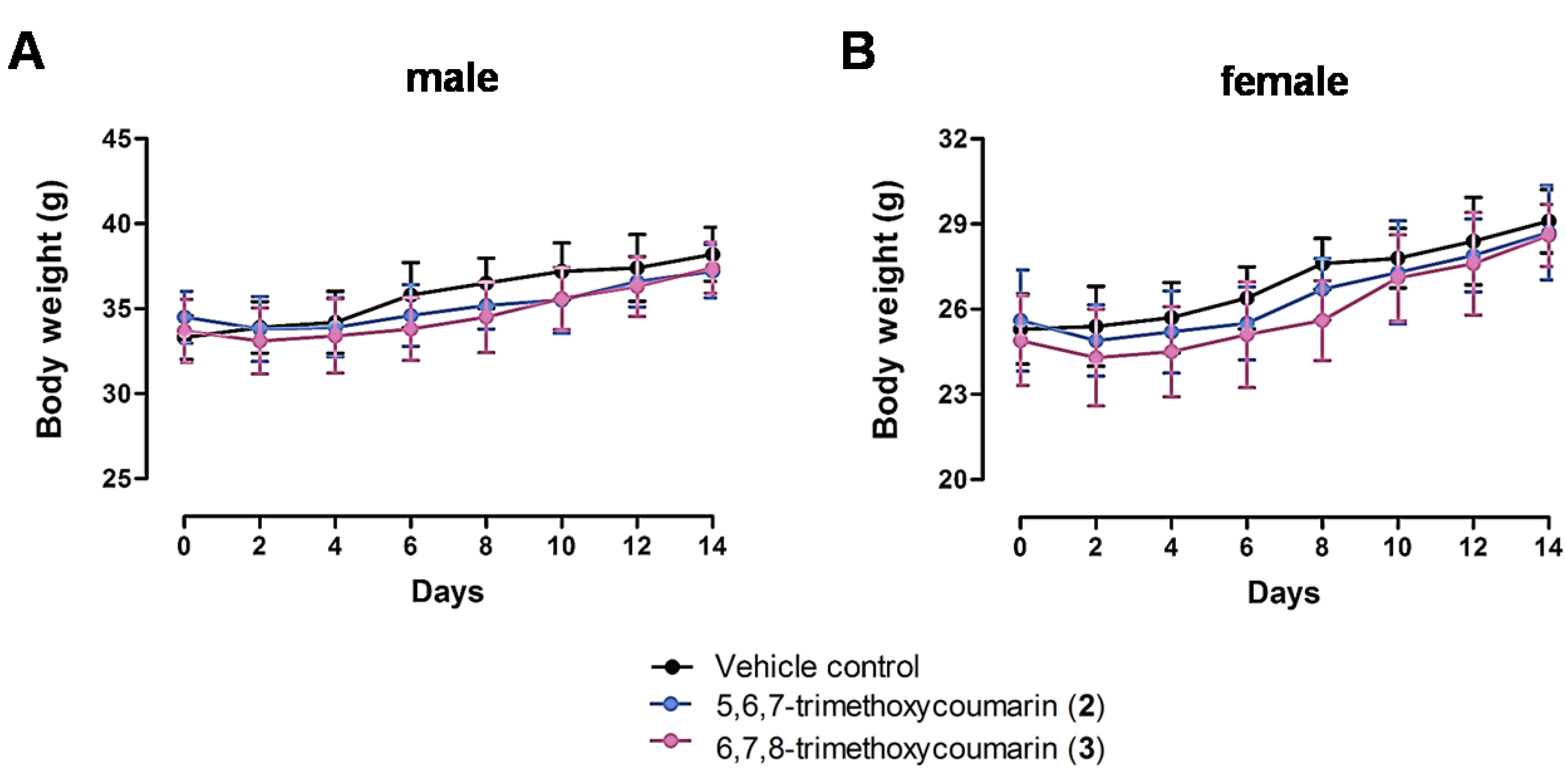
3.3. Acute Toxicity Assay Results
| Treatment | LD50 (mg/kg body weight) | |
|---|---|---|
| Male | Female | |
| 5,6,7-trimethoxycoumarin (2) | 3812 | 3857 |
| 6,7,8-trimethoxycoumarin (3) | 2111 | 3500 |
3.4. Effect of Scoparone Derivatives on Human Recombinant CYP Enzyme Activity
| Treatment | Concentration (μM) | Inhibition (%) | |
|---|---|---|---|
| CYP3A4 | CYP2C9 | ||
| Ketoconazole | 20 | 97.5 | 0 |
| Sulfaphenazole | 20 | 0 | 93.4 |
| Scoparone (1) | 10 | 32.7 | 0 |
| 5,6,7-trimethoxycoumarin (2) | 10 | 19.1 | 0 |
| 6,7,8-trimethoxycoumarin (3) | 10 | 15.8 | 0 |
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rogers, K. The Digestive System, 1st ed.; Britannica Educational Pub. in Association with Rosen Educational Services: New York, NY, USA, 2011. [Google Scholar]
- El-Serag, H.B. Time trends of gastroesophageal reflux disease: A systematic review. Clin. Gastroenterol. Hepatol. 2007, 5, 17–26. [Google Scholar]
- Schubert, M.L.; Peura, D.A. Control of gastric acid secretion in health and disease. Gastroenterology 2008, 134, 1842–1860. [Google Scholar]
- Saxena, B.; Krishnamurthy, S.; Singh, S. Gastroprotective potential of risperidone, an atypical antipsychotic, against stress and pyloric ligation induced gastric lesions. Chem. Biol. Interact. 2011, 190, 155–164. [Google Scholar]
- Sostres, C.; Gargallo, C.J.; Lanas, A. Interaction between infection, nonsteroidal anti-inflammatory drugs and/or low-dose aspirin use: Old question new insights. World J. Gastroenterol. 2014, 20, 9439–9450. [Google Scholar]
- Verhoef, T.I.; Redekop, W.K.; Daly, A.K.; van Schie, R.M.; de Boer, A.; Maitland-van der Zee, A.H. Pharmacogenetic-guided dosing of coumarin anticoagulants: Algorithms for warfarin, acenocoumarol and phenprocoumon. Br. J. Clin. Pharmacol. 2014, 77, 626–641. [Google Scholar]
- Jain, M.; Surin, W.R.; Misra, A.; Prakash, P.; Singh, V.; Khanna, V.; Kumar, S.; Siddiqui, H.H.; Raj, K.; Barthwal, M.K.; et al. Antithrombotic activity of a newly synthesized coumarin derivative 3-(5-hydroxy-2,2-dimethyl-chroman-6-yl)-n-{2-[3-(5-hydroxy-2,2-dimethyl-chroman-6 -yl)-propionylamino]-ethyl}-propionamide. Chem. Biol. Drug Des. 2013, 81, 499–508. [Google Scholar]
- Peng, X.M.; Damu, G.L.; Zhou, C. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930. [Google Scholar]
- Asadipour, A.; Alipour, M.; Jafari, M.; Khoobi, M.; Emami, S.; Nadri, H.; Sakhteman, A.; Moradi, A.; Sheibani, V.; Homayouni Moghadam, F.; et al. Novel coumarin-3-carboxamides bearing N-benzylpiperidine moiety as potent acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2013, 70, 623–630. [Google Scholar]
- Huang, L.; Su, T.; Li, X. Natural products as sources of new lead compounds for the treatment of Alzheimer’s disease. Curr. Top. Med. Chem. 2013, 13, 1864–1878. [Google Scholar]
- Patil, P.O.; Bari, S.B.; Firke, S.D.; Deshmukh, P.K.; Donda, S.T.; Patil, D.A. A comprehensive review on synthesis and designing aspects of coumarin derivatives as monoamine oxidase inhibitors for depression and Alzheimer’s disease. Bioorg. Med. Chem. 2013, 21, 2434–2450. [Google Scholar]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida, L.D., Jr.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar]
- Guinez, R.F.; Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Olea-Azar, C.; Maya, J.D. Synthesis and evaluation of antioxidant and trypanocidal properties of a selected series of coumarin derivatives. Future Med. Chem. 2013, 5, 1911–1922. [Google Scholar]
- Bubols, G.B.; Vianna Dda, R.; Medina-Remon, A.; von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Garcia, S.C. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar]
- Kang, Y.F.; Liu, C.M.; Kao, C.L.; Chen, C.Y. Antioxidant and anticancer constituents from the leaves of liriodendron tulipifera. Molecules 2014, 19, 4234–4245. [Google Scholar]
- Lin, M.H.; Cheng, C.H.; Chen, K.C.; Lee, W.T.; Wang, Y.F.; Xiao, C.Q.; Lin, C.W. Induction of ros-independent jnk-activation-mediated apoptosis by a novel coumarin-derivative, DMAC, in human colon cancer cells. Chem. Biol. Interact. 2014, 218, 42–49. [Google Scholar]
- Yadagiri, B.; Holagunda, U.D.; Bantu, R.; Nagarapu, L.; Kumar, C.G.; Pombala, S.; Sridhar, B. Synthesis of novel building blocks of benzosuberone bearing coumarin moieties and their evaluation as potential anticancer agents. Eur. J. Med. Chem. 2014, 79, 260–265. [Google Scholar]
- Nasr, T.; Bondock, S.; Youns, M. Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives. Eur. J. Med. Chem. 2014, 76, 539–548. [Google Scholar]
- Zou, Q.; Fang, Y.; Zhao, Y.; Zhao, H.; Wang, Y.; Gu, Y.; Wu, F. Synthesis and in vitro photocytotoxicity of coumarin derivatives for one- and two-photon excited photodynamic therapy. J. Med. Chem. 2013, 56, 5288–5294. [Google Scholar]
- Barraja, P.; Spano, V.; Patrizia, D.; Carbone, A.; Cirrincione, G.; Vedaldi, D.; Salvador, A.; Viola, G.; Dall’acqua, F. Pyrano[2,3-e]isoindol-2-ones, new angelicin heteroanalogues. Bioorg. Med. Chem. Lett. 2009, 19, 1711–1714. [Google Scholar]
- Lee, J.H.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and the virulence of escherichia coli o157:H7. Phytomedicine 2014, 21, 1037–1042. [Google Scholar]
- Rea, A.; Tempone, A.G.; Pinto, E.G.; Mesquita, J.T.; Rodrigues, E.; Silva, L.G.; Sartorelli, P.; Lago, J.H. Soulamarin isolated from Calophyllum brasiliense (Clusiaceae) induces plasma membrane permeabilization of Trypanosoma cruzi and mytochondrial dysfunction. PLoS Negl. Trop. Dis. 2013, 7, e2556. [Google Scholar] [CrossRef]
- Rehman, S.; Ikram, M.; Baker, R.J.; Zubair, M.; Azad, E.; Min, S.; Riaz, K.; Mok, K.; Rehman, S.U. Synthesis, characterization, in vitro antimicrobial, and U2OS tumoricidal activities of different coumarin derivatives. Chem. Cent. J. 2013, 7, 68. [Google Scholar] [CrossRef]
- Salas-Sarduy, E.; Cabrera-Munoz, A.; Cauerhff, A.; Gonzalez-Gonzalez, Y.; Trejo, S.A.; Chidichimo, A.; Chavez-Planes Mde, L.; Cazzulo, J.J. Antiparasitic effect of a fraction enriched in tight-binding protease inhibitors isolated from the caribbean coral plexaura homomalla. Exp. Parasitol. 2013, 135, 611–622. [Google Scholar]
- Vila-Nova, N.S.; de Morais, S.M.; Falcao, M.J.; Alcantara, T.T.; Ferreira, P.A.; Cavalcanti, E.S.; Vieira, I.G.; Campello, C.C.; Wilson, M. Different susceptibilities of Leishmania spp. Promastigotes to the Annona muricata acetogenins annonacinone and corossolone, and the Platymiscium floribundum coumarin scoparone. Exp. Parasitol. 2013, 133, 334–338. [Google Scholar]
- Sashidhara, K.V.; Kumar, A.; Dodda, R.P.; Krishna, N.N.; Agarwal, P.; Srivastava, K.; Puri, S.K. Coumarin-trioxane hybrids: Synthesis and evaluation as a new class of antimalarial scaffolds. Bioorg. Med. Chem. Lett. 2012, 22, 3926–3930. [Google Scholar]
- Hemshekhar, M.; Sunitha, K.; Thushara, R.M.; Sebastin Santhosh, M.; Shanmuga Sundaram, M.; Kemparaju, K.; Girish, K.S. Antiarthritic and antiinflammatory propensity of 4-methylesculetin, a coumarin derivative. Biochimie 2013, 95, 1326–1335. [Google Scholar]
- Stefani, H.A.; Gueogjan, K.; Manarin, F.; Farsky, S.H.; Zukerman-Schpector, J.; Caracelli, I.; Pizano Rodrigues, S.R.; Muscara, M.N.; Teixeira, S.A.; Santin, J.R.; et al. Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: Effect on inducible nitric oxide synthase. Eur. J. Med. Chem. 2012, 58, 117–127. [Google Scholar]
- Jang, H.L.; El-Gamal, M.I.; Choi, H.E.; Choi, H.Y.; Lee, K.T.; Oh, C.H. Synthesis of tricyclic fused coumarin sulfonates and their inhibitory effects on LPS-induced nitric oxide and PGE2 productions in raw 264.7 macrophages. Bioorg. Med. Chem. Lett. 2014, 24, 571–575. [Google Scholar]
- Okuyama, S.; Minami, S.; Shimada, N.; Makihata, N.; Nakajima, M.; Furukawa, Y. Anti-inflammatory and neuroprotective effects of auraptene, a citrus coumarin, following cerebral global ischemia in mice. Eur. J. Pharmacol. 2013, 699, 118–123. [Google Scholar]
- Sekiguchi, H.; Takabayashi, F.; Irie, K.; Murakami, A. Auraptene attenuates gastritis via reduction of helicobacter pylori colonization and pro-inflammatory mediator production in c57bl/6 mice. J. Med. Food 2012, 15, 658–663. [Google Scholar]
- Sekiguchi, H.; Irie, K.; Murakami, A. Suppression of CD74 expression and helicobacter pylori adhesion by auraptene targeting serum starvation-activated Erk1/2 in NCI-n87 gastric carcinoma cells. Biosci. Biotechnol. Biochem. 2010, 74, 1018–1024. [Google Scholar]
- Bighetti, A.E.; Antonio, M.A.; Kohn, L.K.; Rehder, V.L.; Foglio, M.A.; Possenti, A.; Vilela, L.; Carvalho, J.E. Antiulcerogenic activity of a crude hydroalcoholic extract and coumarin isolated from Mikania laevigata Schultz BIP. Phytomedicine 2005, 12, 72–77. [Google Scholar]
- Goel, R.K.; Maiti, R.N.; Manickam, M.; Ray, A.B. Antiulcer activity of naturally occurring pyrano-coumarin and isocoumarins and their effect on prostanoid synthesis using human colonic mucosa. Indian J. Exp. Biol. 1997, 35, 1080–1083. [Google Scholar]
- Choi, W.S.; Jang, D.Y.; Nam, S.W.; Park, B.S.; Lee, H.S.; Lee, S.E. Antiulcerogenic activity of scoparone on Hcl/ethanol-induced gastritis in rats. J. Korean Soc. Appl. Biol. 2012, 55, 159–163. [Google Scholar]
- Choi, W.S.; Kim, Y.S.; Yang, J.A.; Lee, Y.H.; Park, B.S.; Lee, S.E. Curative effects of extracts of hericium erinaceum hypha cultivated with Artemisia capillaris (HEAC) and their primary active compounds on rat liver disease. J. Korean Soc. Appl. Biol. 2011, 54, 531–537. [Google Scholar]
- Kitagawa, H.; Takeda, F.; Kohei, H. A simple method for estimation of gastric mucus and effects of antiulcerogenic agents on the decrease in mucus during water-immersion stress in rats. Arzneim. Forsch. 1986, 36, 1240–1244. [Google Scholar]
- Kushima, W.; Hiruma-Lima, C.A.; Santos, M.A.; Viana, E.; Coelho-Ferreira, M.; Brito, A.R.M.S. Gastroprotective activity of pradosia huberi on experimentally induced gastric lesions in rodents: Role of endogenous sulphydryls and nitric oxide. J. Ethnopharmacol. 2005, 101, 61–67. [Google Scholar]
- Zhang, A.; Sun, H.; Yuan, Y.; Sun, W.; Jiao, G.; Wang, X. An in vivo analysis of the therapeutic and synergistic properties of chinese medicinal formula yin-chen-hao-tang based on its active constituents. Fitoterapia 2011, 82, 1160–1168. [Google Scholar]
- Zhang, A.; Sun, H.; Wang, X. Urinary metabolic profiling of rat models revealed protective function of scoparone against alcohol induced hepatotoxicity. Sci. Rep. 2014, 4, 6768. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, D.W.; Choi, J.S.; Kim, Y.S.; Lee, S.M. Scoparone attenuates d-galactosamine/lipopolysaccharide-induced fulminant hepatic failure through inhibition of toll-like receptor 4 signaling in mice. Food Chem. Toxicol. 2013, 57, 132–139. [Google Scholar]
- Atmaca, M.; Bilgin, H.M.; Obay, B.D.; Diken, H.; Kelle, M.; Kale, E. The hepatoprotective effect of coumarin and coumarin derivates on carbon tetrachloride-induced hepatic injury by antioxidative activities in rats. J. Physiol. Biochem. 2011, 67, 569–576. [Google Scholar]
- Niu, N.; Li, B.; Hu, Y.; Li, X.; Li, J.; Zhang, H. Protective effects of scoparone against lipopolysaccharide-induced acute lung injury. Int. Immunopharmacol. 2014, 23, 127–133. [Google Scholar]
- Choi, Y.H.; Yan, G.H. Anti-allergic effects of scoparone on mast cell-mediated allergy model. Phytomedicine 2009, 16, 1089–1094. [Google Scholar]
- Kim, J.K.; Kim, J.Y.; Kim, H.J.; Park, K.G.; Harris, R.A.; Cho, W.J.; Lee, J.T.; Lee, I.K. Scoparone exerts anti-tumor activity against DU145 prostate cancer cells via inhibition of stat3 activity. PLoS One 2013, 8, e80391. [Google Scholar] [CrossRef]
- Kielbus, M.; Skalicka-Wozniak, K.; Grabarska, A.; Jeleniewicz, W.; Dmoszynska-Graniczka, M.; Marston, A.; Polberg, K.; Gawda, P.; Klatka, J.; Stepulak, A. 7-Substituted coumarins inhibit proliferation and migration of laryngeal cancer cells in vitro. Anticancer Res. 2013, 33, 4347–4356. [Google Scholar]
- Mogana, R.; Adhikari, A.; Debnath, S.; Hazra, S.; Hazra, B.; Teng-Jin, K.; Wiart, C. The antiacetylcholinesterase and antileishmanial activities of canarium patentinervium MIQ. BioMed Res. Int. 2014, 2014, 903529. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lee, H.J.; Huang, H.S.; Lee, B.K.; Choi, H.S.; Lim, S.C.; Lee, C.K.; Lee, M.K. Effects of scoparone on dopamine biosynthesis and l-dopa-induced cytotoxicity in PC12 cells. J. Neurosci. Res. 2009, 87, 1929–1937. [Google Scholar]
- Lee, S.H.; Jang, H.D. Scoparone attenuates RANKL-induced osteoclastic differentiation through controlling reactive oxygen species production and scavenging. Exp. Cell Res. 2015, 331, 267–277. [Google Scholar]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar]
- Allen, A.; Flemstrom, G. Gastroduodenal mucus bicarbonate barrier: Protection against acid and pepsin. Am. J. Physiol. 2005, 288, C1–C19. [Google Scholar]
- Yang, H.; Xu, L.N.; Sui, Y.J.; Liu, X.; He, C.Y.; Fang, R.Y.; Liu, J.; Hao, F.; Ma, T.H. Stimulation of airway and intestinal mucosal secretion by natural coumarin CFTR activators. Front. Pharmacol. 2011, 2, 52. [Google Scholar]
- Greaves, P. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation, 3rd ed.; Elsevier/AP: Amsterdam, The Netherlands; Boston, MA, USA, 2007; p. 288. [Google Scholar]
- Schmidt, L.E.; Dalhoff, K. Food-drug interactions. Drugs 2002, 62, 1481–1502. [Google Scholar]
- Bushra, R.; Aslam, N.; Khan, A.Y. Food-drug interactions. Oman Med. J. 2011, 26, 77–83. [Google Scholar]
- Obach, R.S.; Zhang, Q.Y.; Dunbar, D.; Kaminsky, L.S. Metabolic characterization of the major human small intestinal cytochrome p450s. Drug Metab. Dispos. 2001, 29, 347–352. [Google Scholar]
- Kanazu, T.; Yamaguchi, Y.; Okamura, N.; Baba, T.; Koike, M. Model for the drug-drug interaction responsible for Cyp3a enzyme inhibition. II: Establishment and evaluation of dexamethasone-pretreated female rats. Xenobiot. Fate Foreign Compd. Biol. Syst. 2004, 34, 403–413. [Google Scholar]
- Rettie, A.E.; Jones, J.P. Clinical and toxicological relevance of Cyp2c9: Drug-drug interactions and pharmacogenetics. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 477–494. [Google Scholar]
- Kirchheiner, J.; Brockmoller, J. Clinical consequences of cytochrome p450 2c9 polymorphisms. Clin. Pharmacol. Ther. 2005, 77, 1–16. [Google Scholar]
- Gurley, B.J.; Gardner, S.F.; Hubbard, M.A.; Williams, D.K.; Gentry, W.B.; Cui, Y.; Ang, C.Y. Cytochrome p450 phenotypic ratios for predicting herb-drug interactions in humans. Clin. Pharmacol. Ther. 2002, 72, 276–287. [Google Scholar]
- Zhou, S.; Lim, L.Y.; Chowbay, B. Herbal modulation of p-glycoprotein. Drug Metab. Rev. 2004, 36, 57–104. [Google Scholar]
- Wittkowsky, A.K. Drug interactions update: Drugs, herbs, and oral anticoagulation. J. Thromb. Thromb. 2001, 12, 67–71. [Google Scholar]
- Kayser, O.; Kolodziej, H. Antibacterial activity of extracts and constituents of pelargonium sidoides and pelargonium reniforme. Planta Med. 1997, 63, 508–510. [Google Scholar]
- Satyanarayana, P.; Kumar, K.A.; Singh, S.K.; Rao, G.N. A new phorbol diester from aleurites moluccana. Fitoterapia 2001, 72, 304–306. [Google Scholar]
- Lee, S.; Kim, K.S.; Shim, S.H.; Park, Y.M.; Kim, B.K. Constituents from the non-polar fraction of Artemisia apiacea. Arch. Pharm. Res. 2003, 26, 902–905. [Google Scholar]
- Uddin, S.J.; Shilpi, J.A.; Middleton, M.; Byres, M.; Shoeb, M.; Nahar, L.; Sarker, S.D. Swarnalin and cis-swarnalin, two new tetrahydrofuran derivatives with free radical scavenging activity, from the aerial parts of Cuscuta reflexa. Nat. Prod. Res. 2007, 21, 663–668. [Google Scholar]
- Saeed, M.A.; Sabir, A.W. Irritant and cytotoxic coumarins from Angelica glauca edgew roots. J. Asian Nat. Prod. Res. 2008, 10, 49–58. [Google Scholar]
- Lee, S.J.; Kim, H.M.; Lee, J.M.; Park, H.S.; Lee, S. Artemisterol, a new steryl ester from the whole plant of Artemisia apiacea. J. Asian Nat. Prod. Res. 2008, 10, 281–283. [Google Scholar]
- Traore, M.; Jaroszewski, J.W.; Olsen, C.E.; Ouedraogo, J.B.; Pierre, G.I.; Nacoulma, O.G.; Guiguemde, T.R.; Christensen, S.B. A new oxygenated ursane derivative from Canthium multiflorum. Planta Med. 2008, 74, 560–562. [Google Scholar]
- Li, Q.J.; Wang, M.L.; Yang, X.S.; Ma, L.; Hao, X.J. Two new coumarin glycosides from Chimonanthus nitens. J. Asian Nat. Prod. Res. 2013, 15, 270–275. [Google Scholar]
- Kolodziej, H. Fascinating metabolic pools of pelargonium sidoides and pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine umckaloabo. Phytomedicine 2007, 14 (Suppl. 6), 9–17. [Google Scholar]
- Estevez-Braun, A.; Estevez-Reyes, R.; Moujir, L.M.; Ravelo, A.G.; Gonzalez, A.G. Antibiotic activity and absolute configuation of 8s-heptadeca-2(z),9(z)-diene-4,6-diyne-1,8-diol from bupleurum salicifolium. J. Nat. Prod. 1994, 57, 1178–1182. [Google Scholar]
- Mbwambo, Z.H.; Lee, S.K.; Mshiu, E.N.; Pezzuto, J.M.; Kinghorn, A.D. Constituents from the stem wood of euphorbia quinquecostata with phorbol dibutyrate receptor-binding inhibitory activity. J. Nat. Prod. 1996, 59, 1051–1055. [Google Scholar]
- Rollinger, J.M.; Schuster, D.; Danzl, B.; Schwaiger, S.; Markt, P.; Schmidtke, M.; Gertsch, J.; Raduner, S.; Wolber, G.; Langer, T.; et al. In silico target fishing for rationalized ligand discovery exemplified on constituents of Ruta graveolens. Planta Med. 2009, 75, 195–204. [Google Scholar]
- Gao, W.; Li, Q.; Chen, J.; Wang, Z.; Hua, C. Total synthesis of six 3,4-unsubstituted coumarins. Molecules 2013, 18, 15613–15623. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, D.J.; Lee, G.R.; Oh, S.; Lee, S.E.; Choi, W.S. Gastroprotective Efficacy and Safety Evaluation of Scoparone Derivatives on Experimentally Induced Gastric Lesions in Rodents. Nutrients 2015, 7, 1945-1964. https://doi.org/10.3390/nu7031945
Son DJ, Lee GR, Oh S, Lee SE, Choi WS. Gastroprotective Efficacy and Safety Evaluation of Scoparone Derivatives on Experimentally Induced Gastric Lesions in Rodents. Nutrients. 2015; 7(3):1945-1964. https://doi.org/10.3390/nu7031945
Chicago/Turabian StyleSon, Dong Ju, Gyung Rak Lee, Sungil Oh, Sung Eun Lee, and Won Sik Choi. 2015. "Gastroprotective Efficacy and Safety Evaluation of Scoparone Derivatives on Experimentally Induced Gastric Lesions in Rodents" Nutrients 7, no. 3: 1945-1964. https://doi.org/10.3390/nu7031945




