Vitamin D Status and Acute Respiratory Infection: Cross Sectional Results from the United States National Health and Nutrition Examination Survey, 2001–2006
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source Data
2.2. Data Collection
2.3. Data Abstraction
2.4. Statistical Analysis
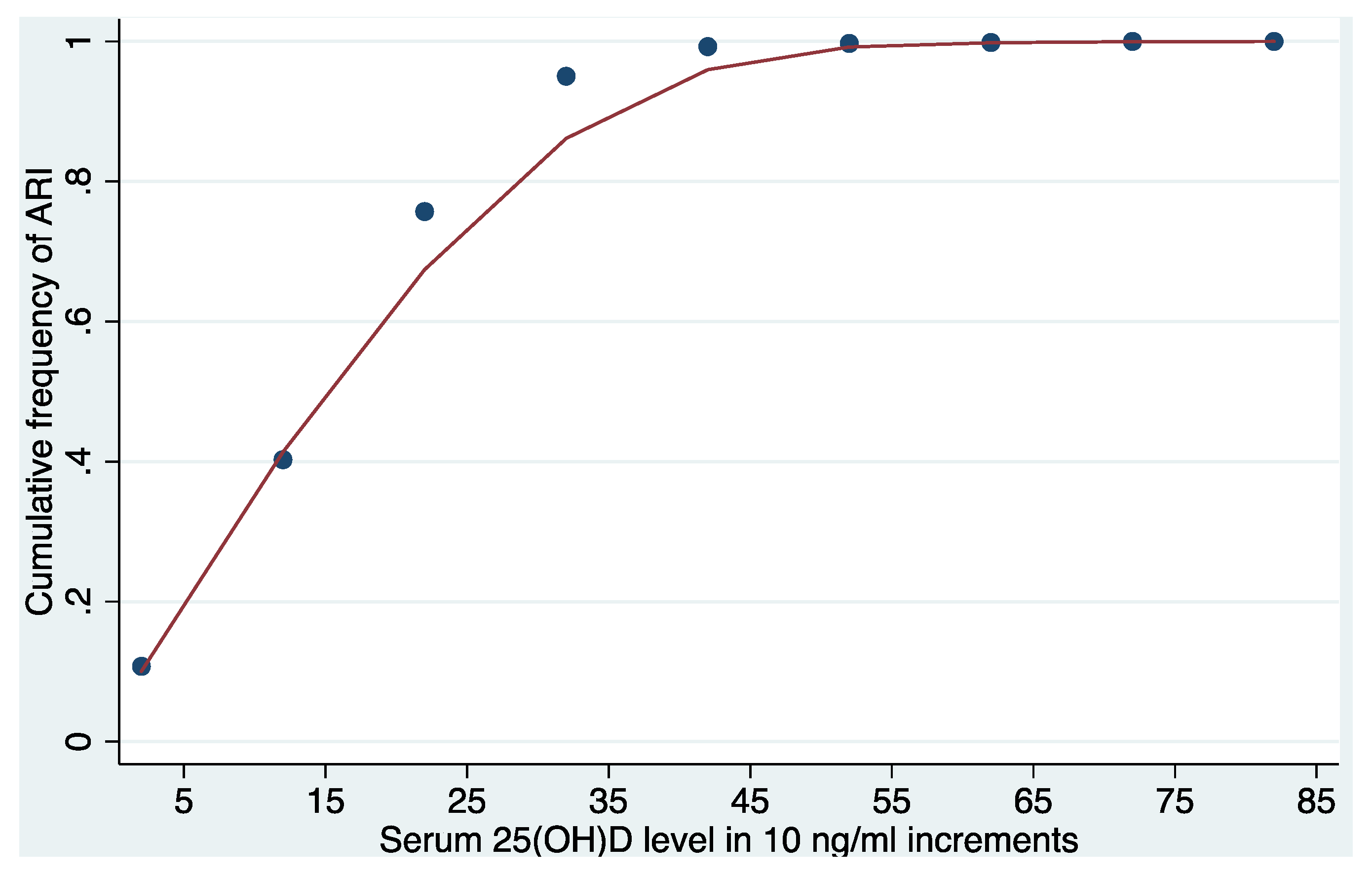
3. Results
| Covariate | Total Number of Observations | Number Reporting Acute Respiratory Infection (Row %) | p-value |
|---|---|---|---|
| 25-hydroxyvitamin D | |||
| <10 ng/mL | 1168 | 90 (7.7%) | <0.001 |
| 10–19.9 ng/mL | 4958 | 262 (5.3%) | |
| 20–29.9 ng/mL | 5416 | 245 (4.5%) | |
| ≥30 ng/mL | 2566 | 82 (3.2%) | |
| Season | |||
| High ambient ultraviolet B radiation | 7468 | 236 (3.2%) | <0.001 |
| Low ambient ultraviolet B radiation | 6640 | 443 (6.7%) | |
| Age | |||
| 17–39 years | 5934 | 285 (4.8%) | 0.06 |
| 40–59 years | 3836 | 208 (5.4%) | |
| ≥60 years | 4338 | 186 (4.3%) | |
| Sex | |||
| Female | 7252 | 386 (5.3%) | 0.004 |
| Male | 6856 | 293 (4.3%) | |
| Race | |||
| Non-white | 7178 | 303 (4.2%) | 0.001 |
| White | 6930 | 376 (5.4%) | |
| Poverty-to-income ratio | |||
| ≤Federal poverty limit | 2408 | 148 (6.2%) | <0.001 |
| >Federal poverty limit | 10,361 | 454 (4.4%) | |
| Body mass index | |||
| <20 kg/m2 | 744 | 34 (4.6%) | 0.06 |
| 20–24.9 kg/m2 | 3787 | 160 (4.2%) | |
| 25–29.9 kg/m2 | 4668 | 220 (4.7%) | |
| ≥30 kg/m2 | 4390 | 241 (5.5%) | |
| Active smoker | |||
| Yes | 2801 | 173 (6.2%) | <0.001 |
| No | 3416 | 127 (3.7%) | |
| Second-hand smoke | |||
| Yes | 2767 | 176 (6.4%) | <0.001 |
| No | 11,227 | 494 (4.4%) | |
| Alcohol consumption | |||
| ≤30 drinks/month | 952 | 37 (3.9%) | 0.53 |
| >30 drinks/month | 7167 | 310 (4.3%) | |
| Pneumococcal vaccination | |||
| Yes | 867 | 35 (4.0%) | 0.33 |
| No | 3278 | 158 (4.8%) | |
| Asthma | |||
| Yes | 1716 | 129 (7.5%) | <0.001 |
| No | 12,374 | 550 (4.4%) | |
| Chronic obstructive pulmonary disease | |||
| Yes | 929 | 87 (9.4%) | <0.001 |
| No | 11,694 | 504 (4.3%) | |
| Congestive heart failure | |||
| Yes | 421 | 36 (8.6%) | <0.001 |
| No | 12,916 | 556 (4.6%) | |
| Diabetes mellitus | |||
| Yes | 1290 | 72 (5.6%) | 0.15 |
| No | 12,612 | 591 (4.7%) | |
| Chronic kidney disease | |||
| eGFR < 60 mL/min/1.73m2 | 1697 | 82 (4.8%) | 0.97 |
| eGFR ≥ 60 mL/min/1.73m2 | 12,108 | 588 (4.9%) | |
| Stroke | |||
| Yes | 459 | 37 (8.1%) | <0.001 |
| No | 12,191 | 556 (4.6%) | |
| Neutropenia | |||
| WBC ≥ 3.5 × 109/L | 13,958 | 672 (4.8%) | 0.84 |
| WBC < 3.5 × 109/L | 135 | 6 (4.4%) |
| Risk Factor | Odds Ratio (95% Confidence Interval), p-value |
|---|---|
| 25OHD (<30 ng/mL versus ≥30 ng/mL) | 1.58 (1.07–2.33), p = 0.023 |
| Low ambient UVB radiation (1 November–30 April) | 2.19 (1.69–2.85), p < 0.001 |
| Poverty-to-income ratio (≤FPL versus >FPL) | 1.47 (1.10–1.96), p = 0.009 |
| Active smoking | 1.39 (1.01–1.91), p = 0.046 |
| Chronic obstructive pulmonary disease | 1.94 (1.39–2.72), p < 0.001 |
| Congestive heart failure | 1.85 (1.15–2.97), p = 0.012 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cherry, D.K.; Hing, E.; Woodwell, D.A.; Rechtsteiner, E.A. National ambulatory medical care survey: 2006 summary. Natl. Health Stat. Rep. 2008, 3, 1–39. [Google Scholar]
- Allan, G.M.; Arroll, B. Prevention and treatment of the common cold: Making sense of the evidence. CMAJ 2014, 186, 190–199. [Google Scholar]
- Lozano, R. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2014, 380, 2095–2128. [Google Scholar]
- Fendrick, A.M.; Monto, A.S.; Nightengale, B.; Sarnes, M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003, 163, 487–494. [Google Scholar]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar]
- Bariffi, F.; Sanduzzi, A.; Ponticiello, A. Epidemiology of lower respiratory tract infections. J. Chemother. 1995, 7, 263–276. [Google Scholar]
- File, T.M., Jr.; Marrie, T.J. Burden of community-acquired pneumonia in North American adults. Postgrad. Med. 2010, 122, 130–141. [Google Scholar]
- Watkins, R.R.; Lemonovich, T.L. Diagnosis and management of community-acquired pneumonia in adults. Am. Fam. Physician 2011, 83, 1299–1306. [Google Scholar]
- Centers for Disease Control and Prevention. Fast Stats: Pneumonia Deaths and Mortality. Available online: http://www.cdc.gov/nchs/fastats/pneumonia.htm (accessed on 15 August 2014).
- Feikin, D.R.; Schuchat, A.; Kolczak, M.; Barrett, N.L.; Harrison, L.H.; Lefkowitz, L.; McGeer, A.; Farley, M.M.; Vugia, D.J.; Lexau, C.; et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am. J. Public Health 2000, 90, 223–229. [Google Scholar]
- File, T.M. The epidemiology of respiratory tract infections. Semin. Respir. Infect. 2000, 15, 184–194. [Google Scholar]
- National Institute of Allergy and Infectious Diseases. Common Cold: Protect Yourself and Others. Available online: http://www.niaid.nih.gov/topics/commoncold/Pages/default.aspx (accessed on 15 August 2014).
- World Lung Foundation: The Acute Respiratory Infections Overcrowding. Available online: http://www.ariatlas.org/drivers_of_aris/overcrowding (accessed on 15 August 2014).
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar]
- Wong, V.W.; Cowling, B.J.; Aiello, A.E. Hand hygiene and risk of influenza virus infections in the community: A systematic review and meta-analysis. Epidemiol. Infect. 2014, 142, 922–932. [Google Scholar]
- Passioti, M.; Maggina, P.; Megremis, S.; Papadopoulos, N.G. The common cold: Potential for future prevention or cure. Curr. Allergy Asthma Rep. 2014, 14, 413. [Google Scholar] [CrossRef]
- Barrelet, C.; Bourrier, M.; Burton-Jeangros, C.; Schinder, M. Unresolved issues in risk communication research: The case of the H1N1 pandemic (2009–2011). Influenza Other Respir. Vir. 2013, 7 (Suppl. S2), 114–119. [Google Scholar]
- World Health Organization. Limiting Spread: Limiting the Spread of Pandemic, Zoonotic, and Seasonal Epidemic Influenza. Available online: http://www.who.int/influenza/resources/research/research_agenda_influenza_stream_2_limiting_spread.pdf (accessed on 15 August 2014).
- Evans, S.E.; Tuvim, M.J.; Fox, C.J.; Sachdev, N.; Gibiansky, L.; Dickey, B.F. Inhaled innate immune ligands to prevent pneumonia. Br. J. Pharmacol. 2011, 163, 195–206. [Google Scholar]
- Hui, D.S.; Lee, N. Adjunctive therapies and immunomodulating agents for severe influenza. Influenza Other Respir. Vir. 2013, 7 (Suppl. S3), 52–59. [Google Scholar]
- Darwish, I.; Mubareka, S.; Liles, W.C. Immunomodulatory therapy for severe influenza. Expert Rev. Anti Infect. Ther. 2011, 9, 807–822. [Google Scholar]
- Sallenave, J.M. Secretory leukocyte protease inhibitor and elafin/trappin-2: Versatile mucosal antimicrobials and regulators of immunity. Am. J. Respir. Cell Mol. Biol. 2010, 42, 635–643. [Google Scholar]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar]
- Taylor, C.E.; Camargo, C.A., Jr. Impact of micronutrients on respiratory infections. Nutr. Rev. 2010, 69, 259–269. [Google Scholar]
- Quraishi, S.A.; Camargo, C.A., Jr. Vitamin D in acute stress and critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 625–634. [Google Scholar]
- Quraishi, S.A.; Bittner, E.A.; Christopher, K.B.; Camargo, C.A., Jr. Vitamin D status and community-acquired pneumonia: Results from the third National Health and Nutrition Examination Survey. PLoS One 2013, 8, e81120. [Google Scholar] [CrossRef]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar]
- Hirani, V. Associations between vitamin D and self-reported respiratory disease in older people from a nationally representative population survey. J. Am. Geriatr. Soc. 2013, 61, 969–973. [Google Scholar]
- Zhao, G.; Ford, E.S.; Tsai, J.; Li, C.; Croft, J.B. Low concentrations of serum 25-hydroxyvitamin D associated with increased risk for chronic bronchitis among US adults. Br. J. Nutr. 2013, 107, 1386–1392. [Google Scholar]
- Berry, D.J.; Hesketh, K.; Power, C.; Hyppönen, E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011, 106, 1433–1440. [Google Scholar]
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National Health and Nutrition Examination Survey: Plan and operations, 1999–2010. Vital Health Stat. 2013, 56, 1–37. [Google Scholar]
- Centers for Disease Control and Prevention. Revised Analytical Note for NHANES 2001–2006 and NHANES III (1988–1994) 25-hydroxyvitamin D analysis. Available online: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/VID_D.htm#Analytic_Notes (accessed on 15 August 2014).
- Cleveland, W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar]
- Cleveland, W.S.; Devlin, S.J. Locally weighted regression: An approach to regression analysis by local fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar]
- Adams, J.S.; Hewison, M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 80–90. [Google Scholar]
- Kankova, M.; Luini, W.; Pedrazzoni, M.; Riganti, F.; Sironi, M.; Bottazzi, B.; Mantovani, A.; Vecchi, A. Impairment of cytokine production in mice fed a vitamin D3-deficient diet. Immunology 1991, 73, 466–471. [Google Scholar]
- Fabri, M.; Stenger, S.; Shin, D.M.; Yuk, J.M.; Liu, P.T.; Realegeno, S.; Lee, H.M.; Krutzik, S.R.; Schenk, M.; Sieling, P.A.; et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 31, 1770–1773. [Google Scholar]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar]
- Bhalla, A.K.; Amento, E.P.; Krane, S.M. Differential effects of 1,25-dihydroxyvitamin D3 on human lymphocytes and monocyte/macrophages: Inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell Immunol. 1986, 98, 311–322. [Google Scholar]
- Pinheiro da Silva, F.; Machado, M.C. Antimicrobial peptides: Clinical relevance and therapeutic implications. Peptides 2012, 36, 308–314. [Google Scholar]
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar]
- Tollin, M.; Bergman, P.; Svenberg, T.; Jörnvall, H.; Gudmundsson, G.H.; Agerberth, B. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides 2003, 24, 523–530. [Google Scholar]
- Mao, S.; Huang, S. Vitamin D supplementation and risk of respiratory tract infections: A meta-analysis of randomized controlled trials. Scand. J. Infect. Dis. 2013, 45, 696–702. [Google Scholar]
- Charan, J.; Goyal, J.P.; Saxena, D.; Yadav, P. Vitamin D for prevention of respiratory tract infections: A systematic review and meta-analysis. J. Pharmacol. Pharmacother. 2012, 3, 300–303. [Google Scholar]
- Bergman, P.; Lindh, A.U.; Björkhem-Bergman, L.; Lindh, J.D. Vitamin D and respiratory tract infections: A systematic review and meta-analysis of randomized controlled trials. PLoS One 2013, 8, e65835. [Google Scholar] [CrossRef]
- Platz, E.A.; Leitzmann, M.F.; Hollis, B.W.; Willett, W.C.; Giovannucci, E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control 2004, 15, 255–265. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monlezun, D.J.; Bittner, E.A.; Christopher, K.B.; Camargo, C.A., Jr.; Quraishi, S.A. Vitamin D Status and Acute Respiratory Infection: Cross Sectional Results from the United States National Health and Nutrition Examination Survey, 2001–2006. Nutrients 2015, 7, 1933-1944. https://doi.org/10.3390/nu7031933
Monlezun DJ, Bittner EA, Christopher KB, Camargo CA Jr., Quraishi SA. Vitamin D Status and Acute Respiratory Infection: Cross Sectional Results from the United States National Health and Nutrition Examination Survey, 2001–2006. Nutrients. 2015; 7(3):1933-1944. https://doi.org/10.3390/nu7031933
Chicago/Turabian StyleMonlezun, Dominique J., Edward A. Bittner, Kenneth B. Christopher, Carlos A. Camargo, Jr., and Sadeq A. Quraishi. 2015. "Vitamin D Status and Acute Respiratory Infection: Cross Sectional Results from the United States National Health and Nutrition Examination Survey, 2001–2006" Nutrients 7, no. 3: 1933-1944. https://doi.org/10.3390/nu7031933




