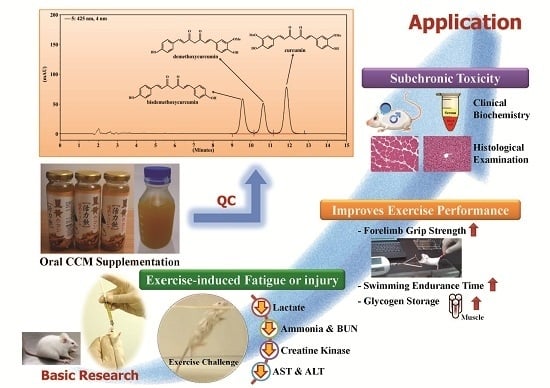
Effect of Curcumin Supplementation on Physiological Fatigue and Physical Performance in Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Animals and Treatment
2.3. Forelimb Grip Strength
2.4. Swimming Exercise Performance Test
2.5. Determination of Blood Biochemical Variables Related to Physical Fatigue and Tissue Injury
2.6. Clinical Biochemical Profile Assay
2.7. Tissue Glycogen Determination
2.8. Histological Staining of Tissues
2.9. Statistical Analysis
3. Results
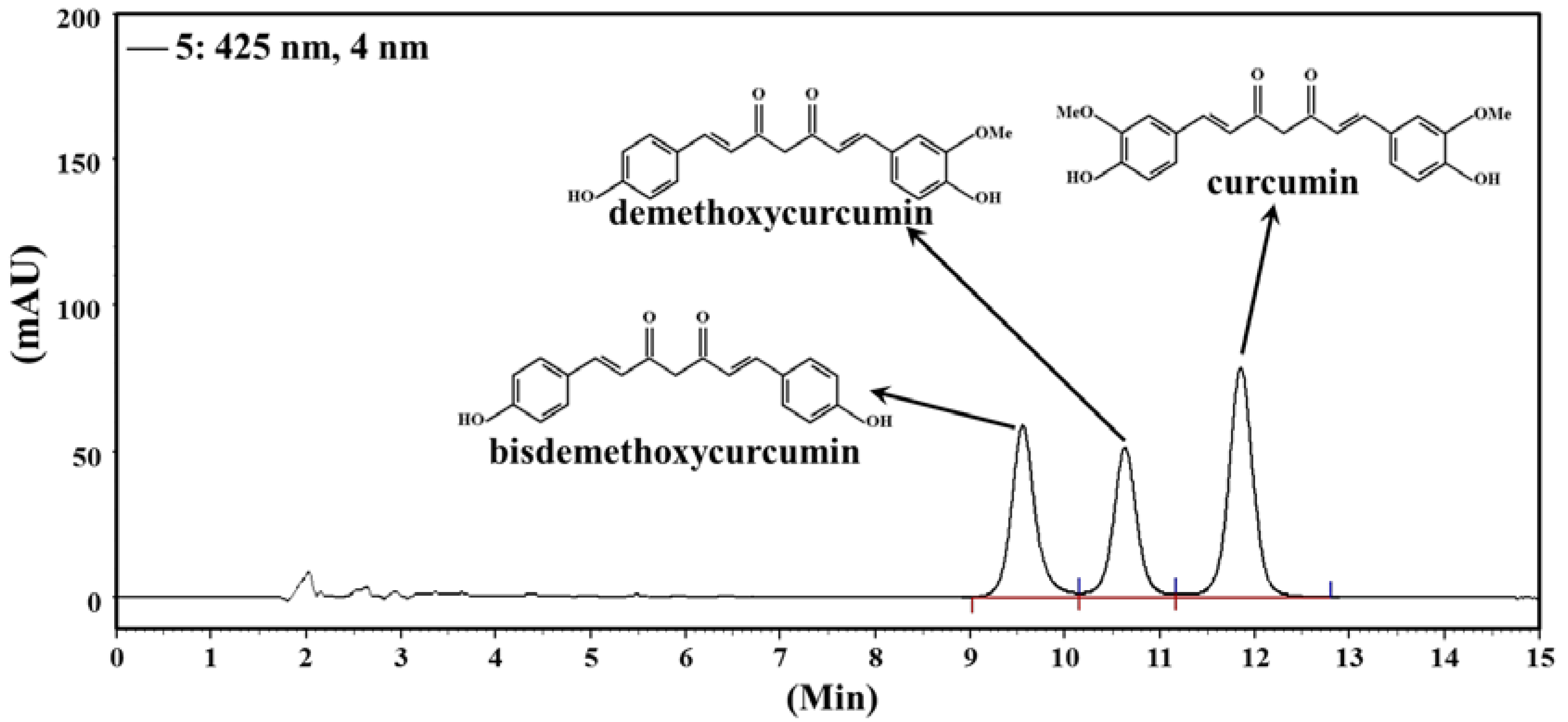
3.1. Content of Curcuminoids in CCM
3.2. Effect of CCM Supplementation on Forelimb Grip Strength

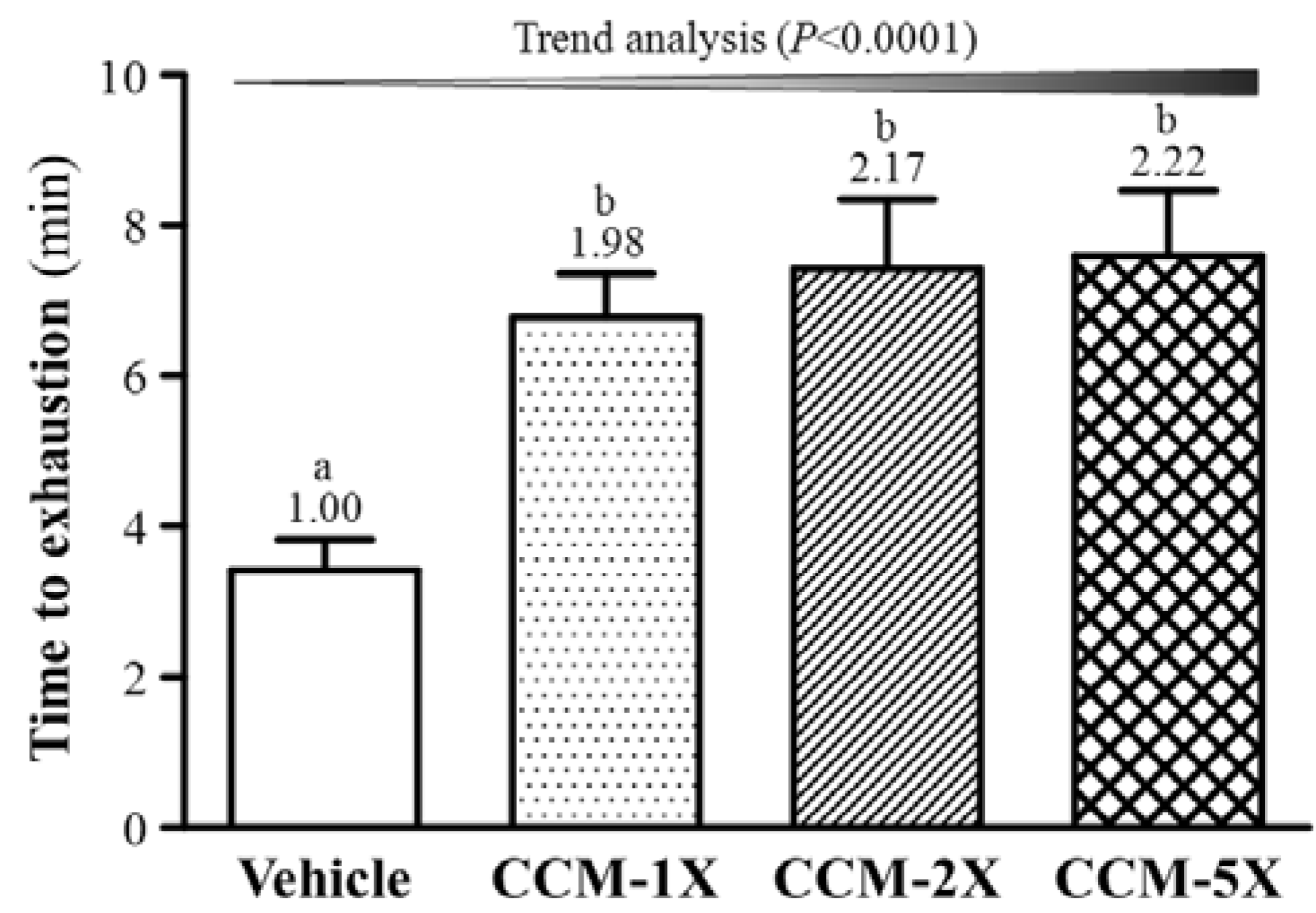
3.3. Effect of CCM Supplementation on Exhaustive Swimming Test
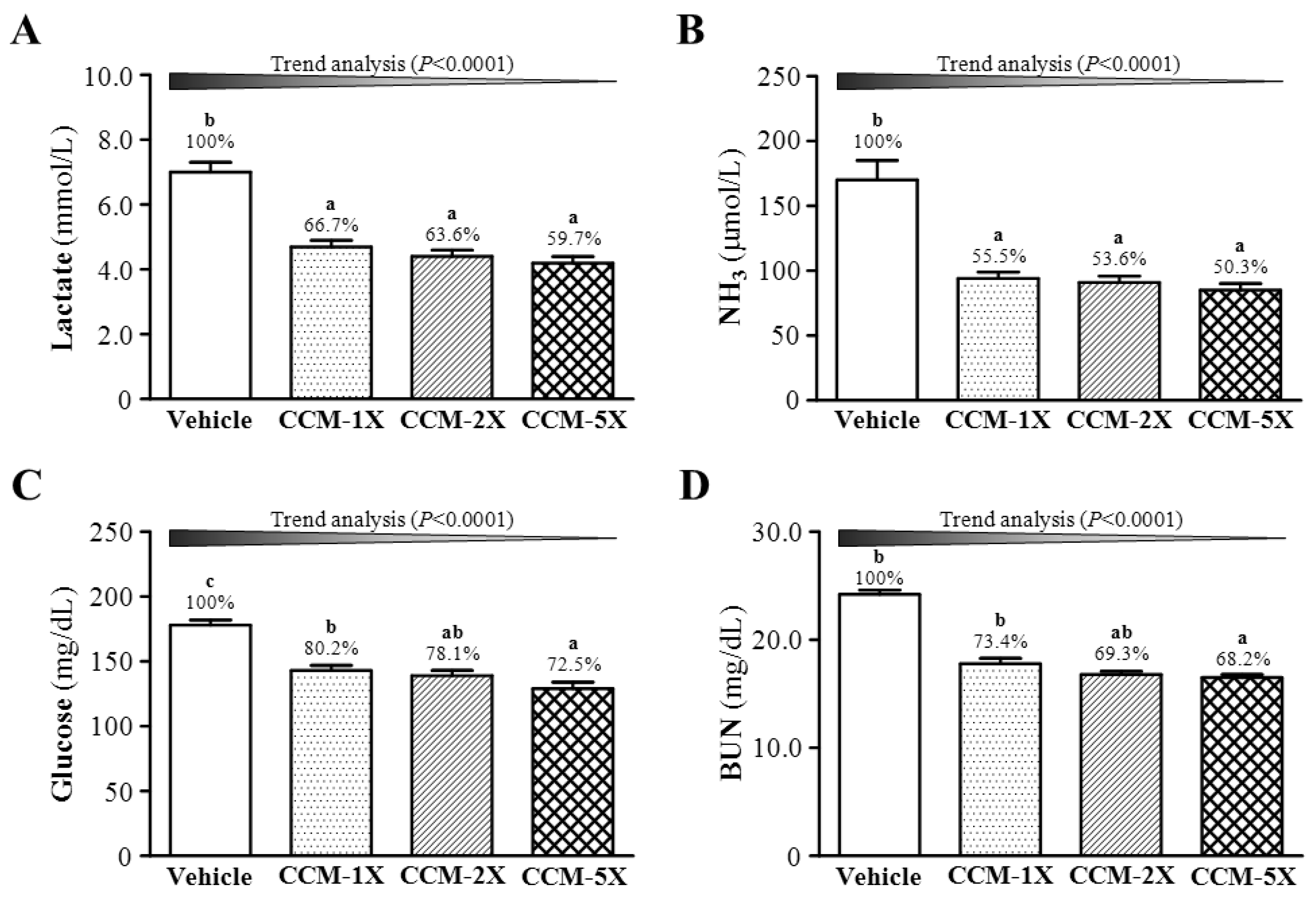
3.5. Effect of CCM Supplementation on Exercise-Induced Injury Indicators after Acute Exercise Challenge

3.6. Effect of CCM Supplementation on Hepatic and Muscular Glycogen Level
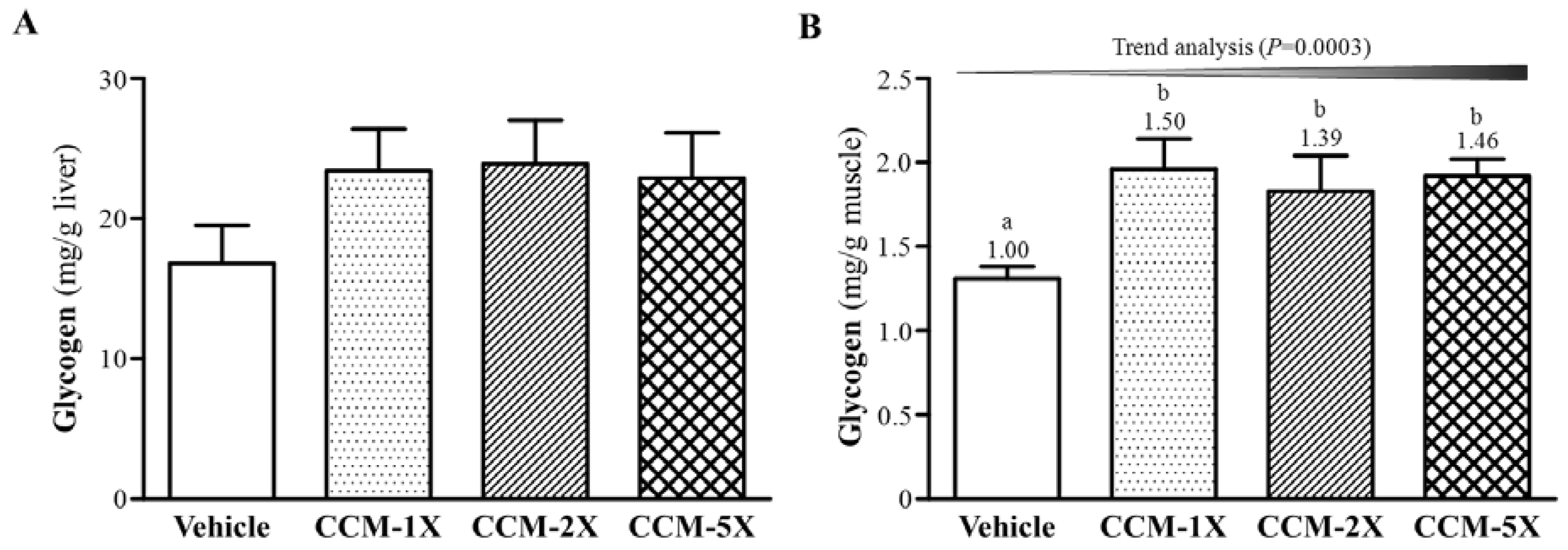
3.7. Subacute Toxicity of CCM Supplementation with General Characteristics

| Characteristic | Vehicle | CCM-1X | CCM-2X | CCM-5X | Trend Analysis |
|---|---|---|---|---|---|
| Initial BW (g) | 39 ± 0.6 | 39 ± 0.5 | 38 ± 0.4 | 39 ± 0.5 | 0.9655 |
| Final BW (g) | 42 ± 0.5 | 41 ± 0.9 | 41 ± 0.7 | 41 ± 0.8 | 0.2490 |
| Food intake (g/day) | 7.3 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.6 ± 0.1 | 0.1680 |
| Water intake (mL/day) | 9.3 ± 0.2 | 9.4 ± 0.3 | 9.4 ± 0.3 | 9.3 ± 0.3 | 0.8886 |
| Liver (g) | 2.28 ± 0.05 b | 2.06 ± 0.06 a | 2.09 ± 0.05 a | 2.15 ± 0.05 a,b | 0.1192 |
| Muscle (g) | 0.39 ± 0.01 | 0.40 ± 0.01 | 0.39 ± 0.01 | 0.41 ± 0.01 | 0.3369 |
| Kidney (g) | 0.73 ± 0.03 | 0.73 ± 0.02 | 0.71 ± 0.01 | 0.73 ± 0.04 | 0.6473 |
| Heart (g) | 0.24 ± 0.01 b | 0.24 ± 0.01 b | 0.21 ± 0.01 a | 0.21 ± 0.01 a | 0.0014 |
| Lung (g) | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.3283 |
| EFP (g) | 0.70 ± 0.05 b | 0.47 ± 0.04 a | 0.54 ± 0.04 a | 0.42 ± 0.04 a | 0.0016 |
| Relative liver weight (%) | 5.55 ± 0.14 b | 4.98 ± 0.08 a | 5.15 ± 0.18 a,b | 5.47 ± 0.15 b | 0.7800 |
| Relative muscle weight (%) | 0.93 ± 0.03 | 0.97 ± 0.03 | 0.97 ± 0.02 | 0.99 ± 0.02 | 0.0410 |
| Relative kidney weight (%) | 1.74 ± 0.07 | 1.80 ± 0.05 | 1.69 ± 0.06 | 1.78 ± 0.08 | 0.9644 |
| Relative heart weight (%) | 0.58 ± 0.03 b | 0.59 ± 0.03 b | 0.53 ± 0.02 a,b | 0.51 ± 0.01 a | 0.0060 |
| Relative lung weight (%) | 0.55 ± 0.02 | 0.54 ± 0.01 | 0.53 ± 0.02 | 0.55 ± 0.01 | 0.9823 |
| Relative EFP weight (%) | 1.65 ± 0.08 c | 1.13 ± 0.09 a,b | 1.33 ± 0.11 b | 1.03 ± 0.09 a | 0.0019 |
3.8. Subacute Toxicity of CCM Supplementation with Biochemistry and Histopathology Evaluation
| Parameter | Vehicle | CCM-1X | CCM-2X | CCM-5X | Trend Analysis |
|---|---|---|---|---|---|
| AST (U/L) | 74 ± 4 b | 68 ± 4 a,b | 64 ± 3 a,b | 61 ± 3 a | 0.0077 |
| ALT (U/L) | 50 ± 3 b | 44 ± 2 a | 42 ± 2 a | 42 ± 2 a | 0.0507 |
| LDH (U/L) | 316 ± 23 | 304 ± 33 | 284 ± 18 | 278 ± 16 | 0.2681 |
| Albumin (g/dL) | 3.50 ± 0.05 | 3.51 ± 0.04 | 3.46 ± 0.04 | 3.55 ± 0.04 | 0.7972 |
| TP (g/dL) | 4.80 ± 0.02 | 4.76 ± 0.04 | 4.76 ± 0.04 | 4.83 ± 0.03 | 0.6559 |
| BUN (mg/dL) | 24.9 ± 0.6 c | 22.2 ± 0.5 b | 20.9 ± 0.5 a,b | 20.6 ± 0.4 a | <0.0001 |
| Creatinine (mg/dL) | 0.23 ± 0.01 b | 0.19 ± 0.01 a | 0.18 ± 0.01 a | 0.20 ± 0.01 a | 0.0392 |
| CK (U/L) | 173 ± 15 | 170 ± 22 | 150 ± 29 | 151 ± 19 | 0.1268 |
| UA (mg/dL) | 1.58 ± 0.10 c | 1.36 ± 0.07 b,c | 1.27 ± 0.07 a,b | 1.12 ± 0.07 a | <0.0001 |
| TC (mg/dL) | 159 ± 5 b | 145 ± 5 a,b | 139 ± 7 a | 140 ± 5 a | 0.0043 |
| TG (mg/dL) | 141 ± 11 b | 129 ± 9 a,b | 129 ± 13 a,b | 108 ± 6 a | 0.0012 |
| Glucose (mg/dL) | 179 ± 6 | 168 ± 3 | 170 ± 4 | 179 ± 5 | 0.7645 |
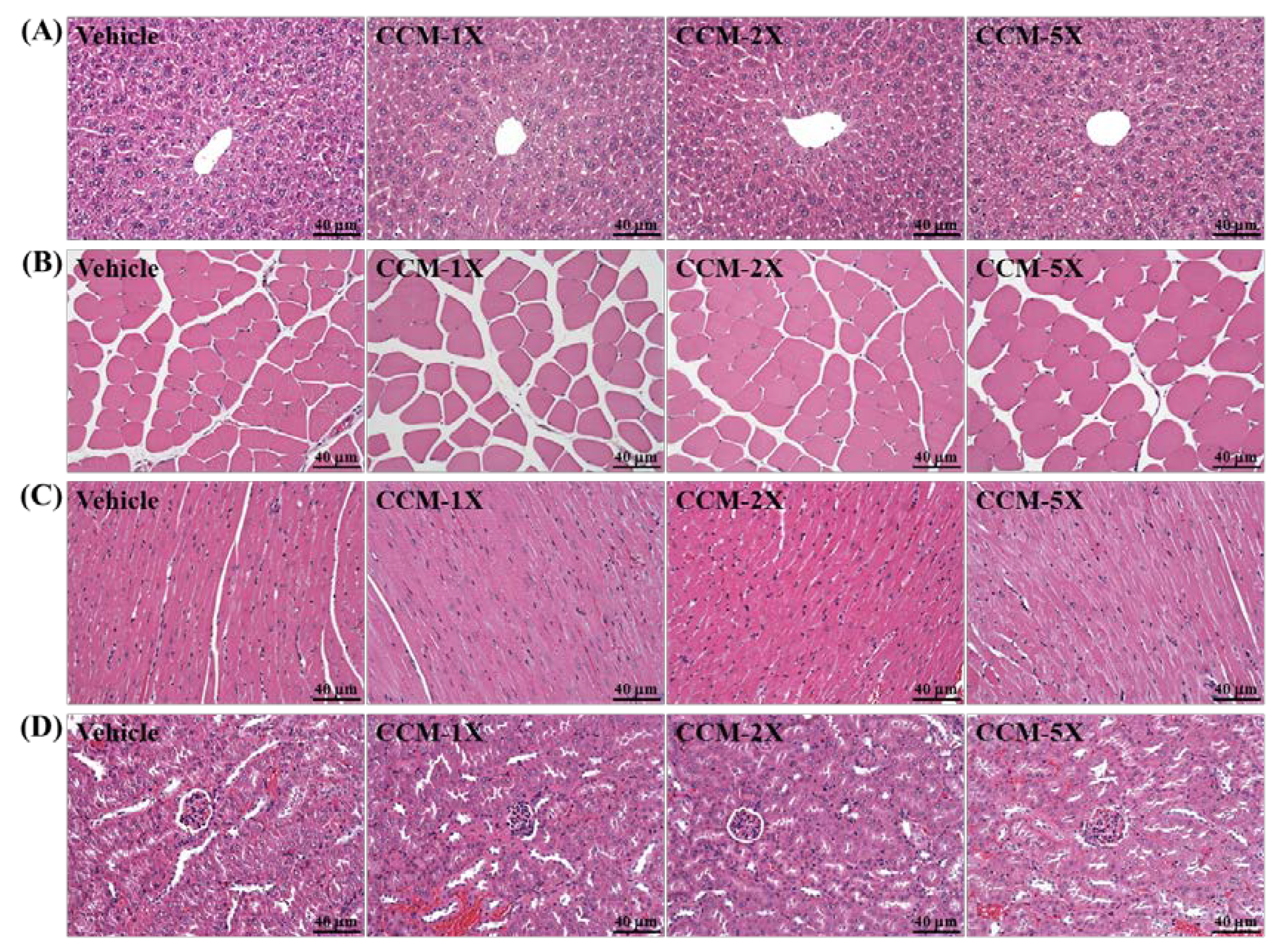
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ament, W.; Verkerke, G.J. Exercise and fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef] [PubMed]
- Zwarts, M.J.; Bleijenberg, G.; van Engelen, B.G. Clinical neurophysiology of fatigue. Clin. Neurophysiol. 2008, 119, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Azechi, H.; Board, M. Essential role of excessive tryptophan and its neurometabolites in fatigue. Can. J. Neurol. Sci. 2012, 39, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, J.M.; Brkic, M.; Simsch, C.; Nething, K.; Kresz, A.; Prokopchuk, O.; Liu, Y. Thyroid hormones, cytokines, physical training and metabolic control. Horm. Metab. Res. 2005, 37, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, D.; Maffiuletti, N.A.; Kayser, B.; Place, N. Mechanisms of fatigue and task failure induced by sustained submaximal contractions. Med. Sci. Sports Exerc. 2012, 44, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Portal, S.; Zadik, Z.; Rabinowitz, J.; Pilz-Burstein, R.; Adler-Portal, D.; Meckel, Y.; Cooper, D.M.; Eliakim, A.; Nemet, D. The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: A prospective randomized, double-blind, placebo-controlled study. Eur. J. Appl. Physiol. 2011, 111, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Altern. Med. 2012, 2012, 364741. [Google Scholar] [CrossRef]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.I.; Tsi, D.; Tan, A.C.; Wang, S.W.; Hsu, M.C. Effects of postexercise supplementation of chicken essence on the elimination of exercise-induced plasma lactate and ammonia. Chin. J. Physiol. 2005, 48, 187–192. [Google Scholar] [PubMed]
- Warren, G.L.; Ingalls, C.P.; Lowe, D.A.; Armstrong, R.B. Excitation-contraction uncoupling: Major role in contraction-induced muscle injury. Exerc. Sport Sci. Rev. 2001, 29, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lin, C.I.; Chiu, C.C.; Lin, Y.T.; Huang, W.K.; Huang, H.Y.; Huang, C.C. Chicken essence improves exercise performance and ameliorates physical fatigue. Nutrients 2014, 6, 2681–2696. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Tsai, S.C.; Lin, W.T. Potential ergogenic effects of L-arginine against oxidative and inflammatory stress induced by acute exercise in aging rats. Exp. Gerontol. 2008, 43, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Gao, T.; Wang, H.; Du, Y.; Li, J.; Li, C.; Wei, L.; Bi, H. Anti-fatigue activity of polysaccharides from the fruits of four Tibetan plateau indigenous medicinal plants. J. Ethnopharmacol. 2013, 150, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shi, Q.; Xu, S.; Du, C.; Liang, L.; Wu, K.; Wang, K.; Wang, X.; Chang, L.S.; He, D.; et al. Curcumin promotes KLF5 proteasome degradation through downregulating YAP/TAZ in bladder cancer cells. Int. J. Mol. Sci. 2014, 15, 15173–15187. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Niu, T.; Yang, Y.; Guo, Q.; Luo, F.; Qian, Z. Preparation of curcumin-loaded poly(ester amine) nanoparticles for the treatment of anti-angiogenesis. J. Biomed. Nanotechnol. 2014, 10, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Sukumaran, V.; Lakshmanan, A.P.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin decreases renal triglyceride accumulation through AMPK-SREBP signaling pathway in streptozotocin-induced type 1 diabetic rats. J. Nutr. Biochem. 2013, 24, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.E.; Chen, Y.C.; Chen, I.S.; Hsieh, S.C.; Chen, S.S.; Chiu, C.H. Curcumin protects against thioacetamide-induced hepatic fibrosis by attenuating the inflammatory response and inducing apoptosis of damaged hepatocytes. J. Nutr. Biochem. 2012, 23, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.S.; Abouelella, A.M.; Shahein, Y.E. Curcumin protection activities against γ-rays-induced molecular and biochemical lesions. BMC Res. Notes 2013, 6, 375. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, G.; Sivalingam, N. Molecular mechanism of TGF-β signaling pathway in colon carcinogenesis and status of curcumin as chemopreventive strategy. Tumour Biol. 2014, 35, 7295–7305. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.G. Curcumin and obesity. Biofactors 2013, 39, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Panzhinskiy, E.; Hua, Y.; Lapchak, P.A.; Topchiy, E.; Lehmann, T.E.; Ren, J.; Nair, S. Novel curcumin derivative CNB-001 mitigates obesity-associated insulin resistance. J. Pharmacol. Exp. Ther. 2014, 349, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Ringenbach, D.R.; Snow, M. Treadmill walking effects on grip strength in young men with Down syndrome. Res. Dev. Disabil. 2014, 35, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Krook, A.; Wallberg-Henriksson, H.; Zierath, J.R. Sending the signal: Molecular mechanisms regulating glucose uptake. Med. Sci. Sports Exerc. 2004, 36, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, S.; Al-Sayegh, N.; Nadar, M. Smoking impact on grip strength and fatigue resistance: Implications for exercise and hand therapy practice. J. Phys. Act. Health 2014, 11, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.; Goswami, A.; Mishra, A.; Chatterjee, M.; Jana, N.R. Curcumin induces stress response, neurite outgrowth and prevent NF-kappaB activation by inhibiting the proteasome function. Neurotox. Res. 2006, 9, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Tsuge, N.; Sawada, H.; Masamura, N.; Yamada, S.; Satomi, S.; Higashi, Y. A single consumption of curry improved postprandial endothelial function in healthy male subjects: A randomized, controlled crossover trial. Nutr. J. 2014, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Suzuki, K.; Kim, H.K.; Otsuka, Y.; Imaizumi, A.; Miyashita, M.; Sakamoto, S. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 2014, 35, 469–475. [Google Scholar] [PubMed]
- Benammi, H.; El Hiba, O.; Romane, A.; Gamrani, H. A blunted anxiolytic like effect of curcumin against acute lead induced anxiety in rat: Involvement of serotonin. Acta Histochem. 2014, 16, 920–925. [Google Scholar]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Zielinski, M.R.; Groschwitz, C.M.; Brown, A.S.; Gangemi, J.D.; Ghaffar, A.; Mayer, E.P. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, 2168–2173. [Google Scholar] [CrossRef]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Banister, E.W.; Cameron, B.J. Exercise-induced hyperammonemia: Peripheral and central effects. Int. J. Sports Med. 1990, 11, 129–142. [Google Scholar] [CrossRef]
- Boozed, M.A.; Hammouda, O.; Matran, R.; Robin, S.; Fabre, C. Changes in oxidative stress markers and biological markers of muscle injury with aging at rest and in response to an exhaustive exercise. PLoS One 2014, 9, e90420. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Bach, C.W.; Baur, D.A. Pre-exercise nutrition: The role of macronutrients, modified starches and supplements on metabolism and endurance performance. Nutrients 2014, 6, 1782–1808. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.W.; Yoo, K.Y.; Lee, S.H.; Jeong, H.J.; Lee, C.S.; Kim, S.J. Curcumin protects against regional myocardial ischemia/reperfusion injury through activation of RISK/GSK-3β and inhibition of p38 MAPK and JNK. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Dadhaniya, P.; Patel, C.; Muchhara, J.; Bhadja, N.; Mathuria, N.; Vachhani, K.; Soni, M.G. Safety assessment of a solid lipid curcumin particle preparation: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2011, 49, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Öner-İyidoğan, Y.; Koçak, H.; Seyidhanoğlu, M.; Gürdöl, F.; Gülçubuk, A.; Yildirim, F.; Çevik, A.; Uysal, M. Curcumin prevents liver fat accumulation and serum fetuin-A increase in rats fed a high-fat diet. J. Physiol. Biochem. 2013, 69, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Sun, D.; Zeng, X.; Yao, N.; Huang, X.; Huang, D.; Chen, Y. Piperine potentiates the hypocholesterolemic effect of curcumin in rats fed on a high fat diet. Exp. Ther. Med. 2014, 8, 260–266. [Google Scholar] [PubMed]
- Soetikno, V.; Sari, F.R.; Lakshmanan, A.P.; Arumugam, S.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the NRF2-KEAP1 pathway. Mol. Nutr. Food Res. 2013, 57, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Chiu, W.-C.; Chuang, H.-L.; Tang, D.-W.; Lee, Z.-M.; Wei, L.; Chen, F.-A.; Huang, C.-C. Effect of Curcumin Supplementation on Physiological Fatigue and Physical Performance in Mice. Nutrients 2015, 7, 905-921. https://doi.org/10.3390/nu7020905
Huang W-C, Chiu W-C, Chuang H-L, Tang D-W, Lee Z-M, Wei L, Chen F-A, Huang C-C. Effect of Curcumin Supplementation on Physiological Fatigue and Physical Performance in Mice. Nutrients. 2015; 7(2):905-921. https://doi.org/10.3390/nu7020905
Chicago/Turabian StyleHuang, Wen-Ching, Wan-Chun Chiu, Hsiao-Li Chuang, Deh-Wei Tang, Zon-Min Lee, Li Wei, Fu-An Chen, and Chi-Chang Huang. 2015. "Effect of Curcumin Supplementation on Physiological Fatigue and Physical Performance in Mice" Nutrients 7, no. 2: 905-921. https://doi.org/10.3390/nu7020905