1. Introduction
Essence of chicken (EOC), a chicken meat extract, in Southeast Asia is traditionally consumed to improve cognition and reduce fatigue. EOC consists mainly of proteins, amino acids and di-peptides such as carnosine, balenine and anserine; peptides that are found in high concentrations in the human brain [
1,
2]. Previous research has found that drinking EOC for four weeks reduced subjective fatigue after a mental task [
3]. Recovery of serum cortisol levels following a stressor was also enhanced in those who consumed EOC for one week [
4]. More recently, Konagai
et al. [
5] found that seven days of consuming EOC reduced reaction times, improved working memory and decreased ratings of depression. The general pattern of findings suggests an improved mood and an enhanced ability to recover from a mental workload. The present study sought to replicate and extend these findings by examining the effects of EOC on mood, cortisol and heart rate variability (HRV), both at rest and in response to a cognitive challenge.
Heart rate variability (HRV) is a non-invasive method that monitors autonomic nervous system (ANS) activity and assesses its reactivity under different conditions [
6]. Individual differences in HRV, most notably high frequency (HF) power which is thought to reflect parasympathetic activity, have been shown to predict working memory, attention [
7] and a person’s ability to regulate emotions [
8]. However, little is known about how nutrition affects these processes. Carnosine has been found to inhibit neural activities of sympathetic efferent nerves innervating the adrenal gland and liver, and in rats, facilitates the activity of the vagal celiac nerve that innervates the pancreas [
9]. Similarly, after laparotomy an intraduodenal injection of anserine to anaesthetized rats suppressed sympathetic nerve activity and enhanced the activity of the vagal gastric efferent [
10]. These mechanisms are thought to mediate the anti-hyperglycaemic effects of carnosine and anserine [
11]; however, to our knowledge these effects have not been studied in humans. The primary objective of the present study was to investigate the effects of a supplement containing carnosine and anserine on autonomic nervous system activity in humans using HRV. A secondary objective was to test the effects of EOC on HRV, cortisol levels, cognition and mood.
3. Results
Table 1 reports the descriptive data for participants who drank either the EOC or the placebo drinks. The original sample consisted of 50 subjects of which 46 (92%) completed the study. Of the four subjects that did not return for their second visit, three were allocated to the placebo and one to the EOC condition (Chi2 (1,
n = 50) = 1.087 n.s.). To assess compliance to the experimental protocol subjects were also asked how many drinks they actually consumed; they were informed that this would not affect their payment. 65.2% (
n = 30) reported consuming all ten drinks as instructed; the number of subjects in each condition who reported consuming all ten drinks was equal (EOC
n = 15, Placebo
n = 15). Of those that did not consume all ten drinks, 21.7% (EOC
n = 5, Placebo
n = 5) consumed nine drinks, 8.7% (EOC
n = 2, Placebo
n = 2) consumed 8 drinks and 4.3% (EOC
n = 2, Placebo
n = 0) drank seven of the drinks. Therefore the number of drinks consumed did not depend on the condition to which the participants had been allocated to (Chi2 (3,
n = 46) = 1.087, n.s.). When asked if they thought they were taking an active ingredient, a placebo or don’t know, 19.6% (EOC
n = 2, Placebo
n = 7) said they were taking an active substance, 23.9% (EOC
n = 7, Placebo
n = 4) thought they took the placebo and 56.5% (EOC
n = 15, Placebo
n = 11) were unsure. The drink participants thought they consumed did not depend on the condition to which they had been allocated to (Chi2 (2,
n = 46) = 4.132, n.s.); therefore the blind was successful. When asked about any side effects 84.8% (EOC
n = 20, Placebo
n = 19) reported no side effects, one person, who took the placebo, reported an inability to concentrate and another reported sleeplessness. Of those taking the EOC treatment, one reported feeling more thirsty than usual, one reported decreased bowel movements, one reported feeling more tired and another thought they experienced slight insomnia. Again none of these effects depended upon which drink had been consumed (Chi2 (6,
n = 46) = 6.091, n.s.).
Baseline data are presented in
Table 2. At baseline participants who subsequently consumed the placebo were more clearheaded than those who consumed EOC. There were no other significant differences at baseline between those who subsequently consumed EOC or a placebo.
Table 2.
Baseline data (mean (SE)) for participants who consumed EOC/Placebo.
Table 2.
Baseline data (mean (SE)) for participants who consumed EOC/Placebo.
| | EOC | PLACEBO |
|---|
| Decision times | | 192.4 (9.9) | 172.9 (10.3) |
| Movement times | | 365.1 (12.6) | 337.4 (13.1) |
| Serial sevens RT | | 1895.9 (161.3) | 2033.3 (211.0) |
| Serial sevens errors | | 2.6 (0.4) | 1.8 (0.5) |
| Focused attention RT | Congruent | 567.5 (14.2) | 560.8 (14.8) |
| Neutral | 574.9 (16.3) | 556.1 (17.4) |
| Incongruent | 630.3 (16.2) | 626.9 (16.9) |
| Focused attention errors | Congruent | 0.84 (0.1) | 0.2 (0.1) |
| Neutral | 1.0 (0.6) | 0.1 (0.6) |
| Incongruent | 0.9 (0.2) | 0.7 (0.2) |
| Perceived stress | | 17.8 (1.3) | 17.3 (1.4) |
| General health questionnaire | Anxiety | 7.1 (0.8) | 7.8 (0.9) |
| Sleep | 2.6 (0.3) | 2.4 (0.3) |
| Depression | 5.0 (0.6) | 4.2 (0.6) |
| Social functioning | 5.1 (0.4) | 5.6 (0.4) |
| Total GHQ | 28.4 (2.4) | 30.2 (2.6) |
| POMS | Depressed/Elated | 4.5 (1.2) | 4.1 (1.3) |
| Anxious/Composed | 0.3 (1.3) | 0.5 (1.4) |
| Agreeable/Hostile | 7.9 (1.0) | 7.6 (1.1) |
| Confident/Unsure | 1.6 (1.3) | 0.5 (1.4) |
| Energetic/tired | −2.0 (1.2) | −3.0 (1.3) |
| Clearheaded/Confused | 1.6 (1.2) | 3.3 (1.4) |
| VAS | Depressed/Elated | 217.8 (8.5) | 219.3 (8.9) |
| Anxious/Composed | 221.5 (9.9) | 243.1 (10.2) |
| Agreeable/Hostile | 255.4 (7.5) | 260.9 (7.8) |
| Confident/Unsure | 212.0 (9.4) | 232.5 (9.8) |
| Energetic/Tired | 187.3 (9.4) | 179.4 (9.6) |
| Clearheaded/Confused | 222.8 (8.2) ** | 250.5 (8.6) ** |
| HRV | R-R | 761.9 (19.7) | 803.9 (20.5) |
| SDNN | 68.3 (5.1) | 70.4 (5.2) |
| rMSSD | 47.8 (5.3) | 50.0 (6.8) |
| LF power | 1464.2 (230.5) | 1454.3 (263.6) |
| HF power | 960.69 (160.3) | 961.4 (166.3) |
| LF/HF | 2.1 (0.3) | 1.9 (0.2) |
| Cortisol | | 0.21 (0.02) | 0.20 (0.02) |
3.1. Heart Rate Variability
The mean R-R interval was calculated over three five minute periods (Rest: prior to “stress” task; Active: during the task; Recovery: after the task). For each of the HRV indices data were analysed using a three way ANCOVA (Time × Gender × Variant (EOC or placebo). For each HRV index its respective “rest” measure on the first visit was used as the covariant.
3.1.1. Time—Domain Analysis
The interaction Time (Rest/Active/Recovery) × Variant (EOC/Placebo) was not significant (F(2,72) = 1.453, n.s.). However, there was a main effect of time (F(2,72) = 3.484, p < 0.03). The R-R interval during the task was shorter (Rest: 787.9 (15.7); Active: 735.3 (12.2); Recovery: 801.4 (15.0)). This effect shows that the “stressor” did, as planned, lead to an increase in heart rate and as such the participants were suitably “stressed” by the task. However, consuming EOC did not affect heart rate during the task. When SDNN was considered the interaction Time × Variant was again non-significant (F(2,74) = 0.660, n.s.). The main effect of time was significant (F(2,74) = 4.882, p < 0.01). SDNN reduced during the task (Rest: 67.2 (5.2); Active: 66.9 (4.6); Recovery: 77.0 (4.9). Again this effect shows that the “stressor” was effective. Similarly when rMSSD was considered the interaction Time × Variant was not significant (F(2,74) = 0.983, n.s.). The main effect of time was significant (F(2,74) = 4.058, p < 0.02). rMSSD reduced during the task (Rest: 45.9 (6.4); Active: 40.8 (4.5); Recovery: 48.9 (6.1) suggesting that the task was effective although EOC did not influence rMSSD. There were no effects involving gender for any of the time domain HRV indices.
3.1.2. Frequency—Domain Analysis
LF and HF power and their ratio were calculated over three five minute periods. When the influence of consuming EOC on LF power were considered there was no effect (Time × Variant (F(2,72) = 0.40, n.s.). Similarly, there were no effects of time or gender. When HF power was considered all effects were again non-significant; (Time × Variant (F(2,72) = 0.10, n.s.) Again there were no effects involving time or gender.
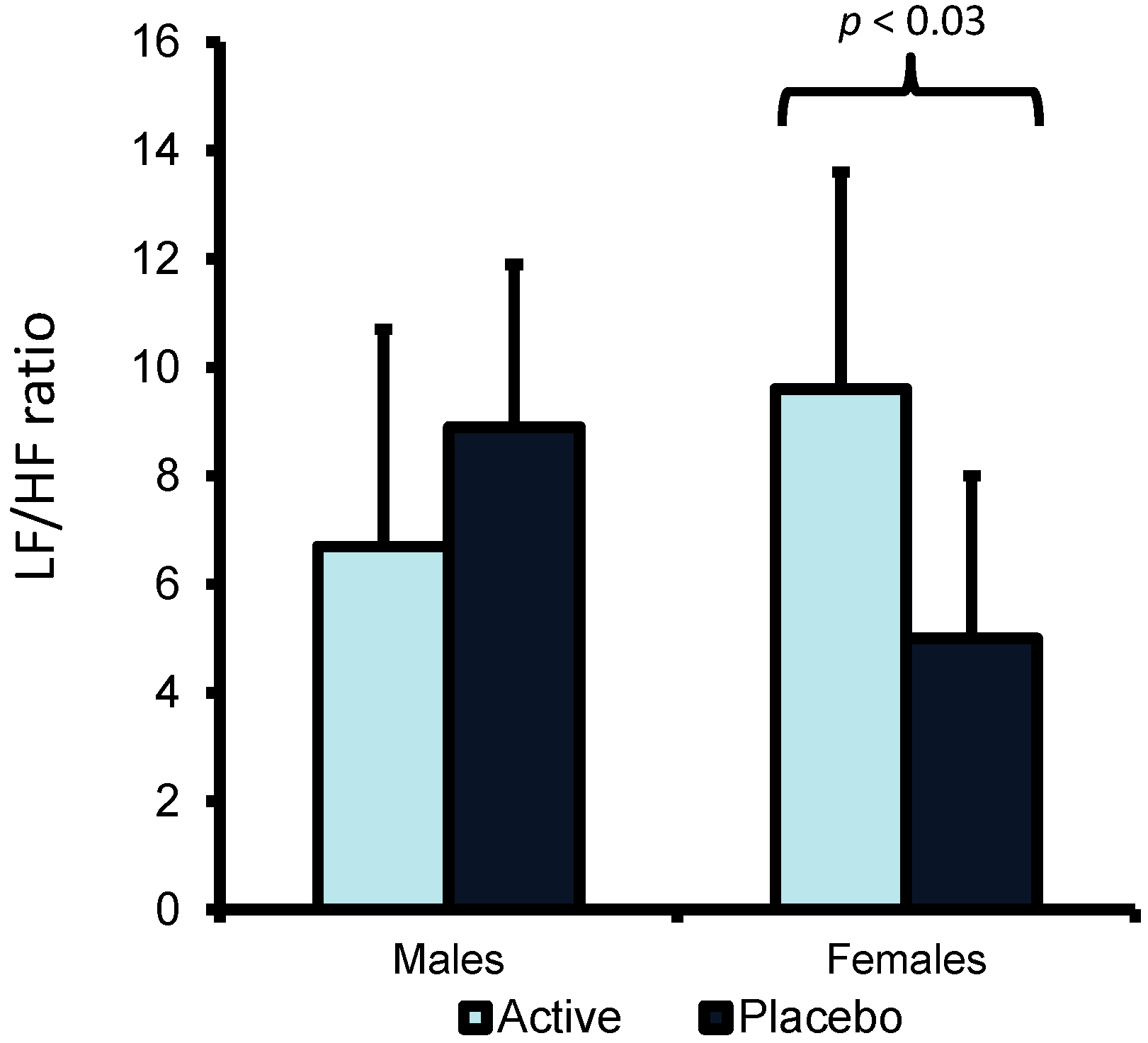
However, with the LF/HF ratio there was a Variant X Gender interaction (F(1,36) = 8.944,
p < 0.005). Follow up tests revealed that females but not males, who had drunk EOC rather than placebo, had a higher LF/HF ratio (
Figure 1).
3.2. Cortisol
Cortisol was measured when participants arrived at the laboratory (Time 1), immediately following the difficult task (Time 2), again after the cognitive battery (Time 3) and after a 45 min recovery period (Time 4). Data were considered using a three way ANCOVA (Time (1, 2, 3, and 4) × Gender × Variant (EOC/Placebo)). Baseline (Time 1) cortisol on the first visit was used as the covariate. The interaction Variant × Time (Time 1, 2, 3, 4) was not significant (F(3,123) = 0.908, n.s.). However, there was a main effect of time (F(3,123) = 13.344,
p < 0.001); participants cortisol levels were highest when they first arrived at the laboratory (Time 1) and steadily declined until the end of the procedure (Time 4). This finding suggests that the “stressor” did not activate the hypothalamic-pituitary-adrenal axis. To replicate the findings of Nagai
et al. [
4] change scores were calculated (Time 4 minus Time 3) to represent recovery from immediately after the test battery to forty five minutes later. There was a trend for those who consumed EOC to recover more quickly (−0.05 for EOC, −0.03 for Placebo) but this effect did not reach significance (F(1,42) = 1.969,
p < 0.1). There were no effects of gender.
Figure 1.
The effect of Essence of Chicken (EOC) on LF/HF ratio in males and females. Data are mean (SE) for Low Frequency/High Frequency (LF/HF) ratio. Females but not males, who drunk EOC rather than placebo, had a higher LF/HF ratio (p < 0.03).
Figure 1.
The effect of Essence of Chicken (EOC) on LF/HF ratio in males and females. Data are mean (SE) for Low Frequency/High Frequency (LF/HF) ratio. Females but not males, who drunk EOC rather than placebo, had a higher LF/HF ratio (p < 0.03).
3.3. Serial Sevens
3.3.1. Reaction Times
The interaction Variant (EOC/Placebo) × Gender was non-significant (F(1,42) = 0.020, n.s.), and neither was the main effect of variant (F(1,41) = 2.519, n.s.).
3.3.2. Accuracy
The interaction Variant (EOC/Placebo) × Gender was non-significant (F(1,42) = 1.795, n.s.) and neither was the main effect of variant (F(1,42) = 1.695, n.s.).
3.4. Focused Attention—Arrow Flankers
3.4.1. Congruent Stimuli
When reaction times were considered the interaction Variant (EOC/Placebo) × Gender was non-significant (F(1,41) = 0.162, n.s.) as were all other interactions. Similarly, when the number of incorrect responses were considered all effects were non-significant; Variant (EOC/Placebo) × Gender interaction was non-significant (F(1,41) = 2.468, n.s.).
3.4.2. Neutral Stimuli
When reaction times were considered the interaction Variant (EOC/Placebo) × Gender was non-significant (F(1,40) = 0.001, n.s.) as were all other interactions. With the number of incorrect responses, all effects were non-significant; Variant (EOC/Placebo) × Gender (F(1,40) = 0.098, n.s.).
3.4.3. Incongruent Stimuli
When reaction times were considered the interaction Variant (EOC/Placebo) × Gender was non-significant (F(1,41) = 0.789, n.s.) as were all other interactions. With the number of incorrect responses, all effects were non-significant; Variant (EOC/Placebo) × Gender (F(1,41) = 0.774, n.s.).
3.5. Reaction Times
3.5.1. Movement Times
Neither the Variant (EOC/Placebo) × Gender × Lamps (1, 2, 4, 8 lamps) (F(3,123) = 0.027, n.s.) nor any other interactions or main effects reached significance.
3.5.2. Decision Times
The interaction Variant (EOC/Placebo) × Gender × Lamps (1, 2, 4, 8 lamps) was non-significant (F(3,123) = 0.382, n.s.), however, the interaction Variant (EOC/Placebo) × Lamps (1, 2, 4, 8 lamps) reached significance (F(1,123) = 3.477, p < 0.01). Those who consumed EOC tended to have faster decision time on the 8 lamp task (174.7 (6.6) for EOC; 199.5 (6.9) for Placebo) and the 4 lamp task (167.4 (5.6) for EOC; 187.9 (5.8) for Placebo) but follow up tests did not reach significance.
3.6. Mood (VAS)
To assess the effects of treatment on mood and changes in the response to testing, a 3-way ANCOVA was conducted for each VAS scale: Variant (EOC/Placebo) × Gender × Time (0, 30, 60 min) with the initial (baseline) rating on day 1 as the covariant.
3.6.1. Energetic/Tired
The Variant (EOC/Placebo) × Gender × Time (0, 30, 60 min) was non-significant (F(2,86) = 0.063, n.s.), as was the main effect of Variant (F(1,41) = 0.130, n.s.) The interaction Variant × Time approached significance (F(2,86) = 2.684, p < 0.07); participants tended to be more energetic at the end of testing if they drunk EOC but as this effect did not reach significance it should be interpreted with caution.
3.6.2. Agreeable/Hostile
The Variant (EOC/Placebo) × Gender × Time (0, 30, 60 min) was not significant (F(2,82) = 0.380, n.s.), however, the main effect of Variant (EOC/Placebo) reached significance (F(1,41) = 8.190,
p < 0.007;
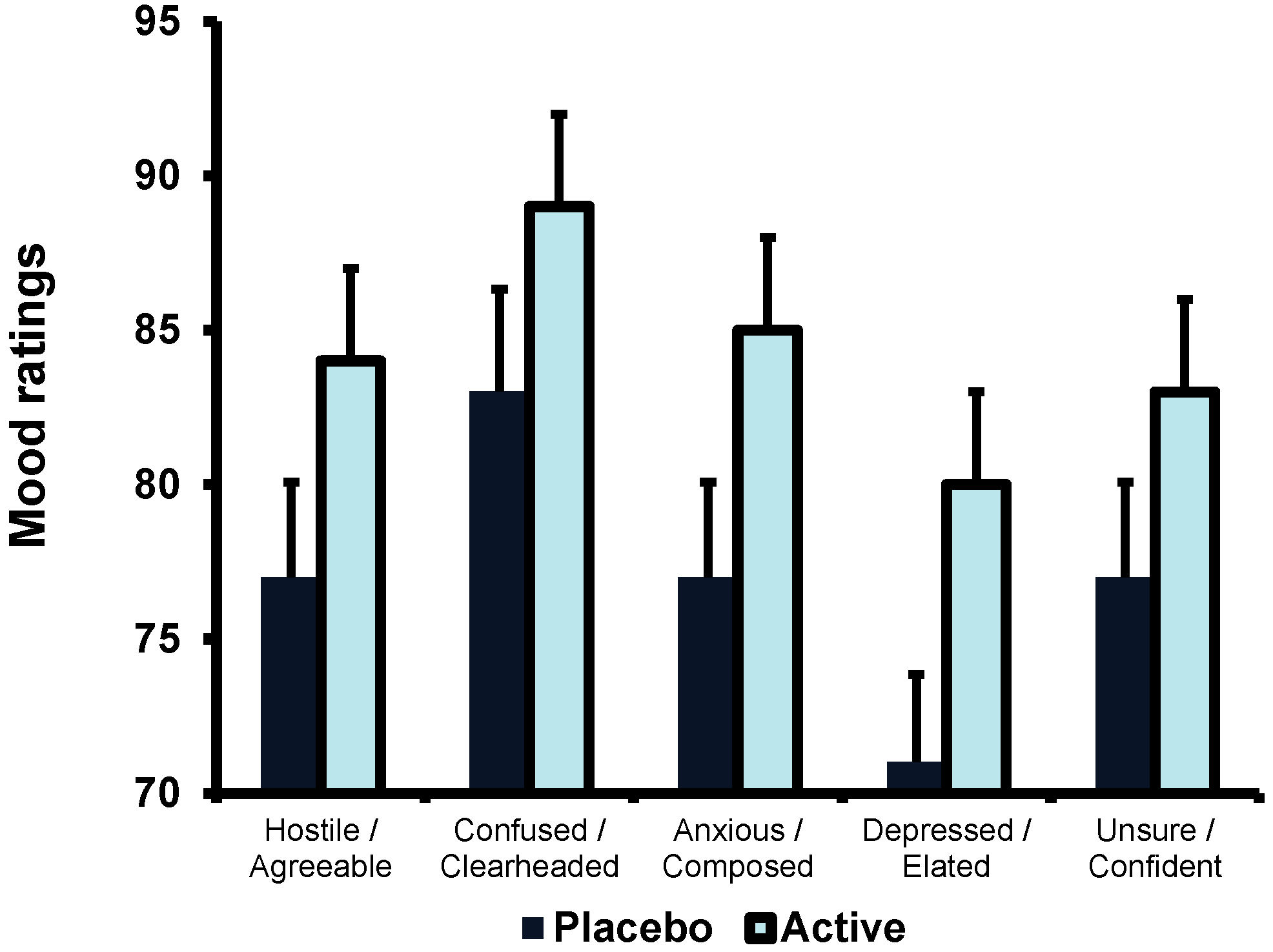
Figure 2). Participants who consumed EOC rated themselves as more agreeable than those who had drunk the placebo (89.3 (1.4) for EOC, 83.4 (1.4) for Placebo). As there was no effect of time neither the “stressful” task nor the cognitive test battery affected participants’ ratings of agreeableness. There was no effect of Gender.
Figure 2.
The effect of EOC on ratings of mood. Data are mean (SE) for the sum of all three time points on visit 2 for ratings of agreeableness, clear-headedness, anxiety, depression and confidence. Participants who had drunk EOC, rather than placebo, rated themselves more agreeable (p < 0.007), more clearheaded (p < 0.05), less anxious (p < 0.04), less depressed (p < 0.01) and more confident (p < 0.05).
Figure 2.
The effect of EOC on ratings of mood. Data are mean (SE) for the sum of all three time points on visit 2 for ratings of agreeableness, clear-headedness, anxiety, depression and confidence. Participants who had drunk EOC, rather than placebo, rated themselves more agreeable (p < 0.007), more clearheaded (p < 0.05), less anxious (p < 0.04), less depressed (p < 0.01) and more confident (p < 0.05).
3.6.3. Clearheaded/Confused
The Variant (EOC/Placebo) × Gender × Time (0, 30, 60 min) was not significant (F(2,82) = 0.515, n.s.), however, there was a main effect of Variant (EOC/Placebo) (F(1,43) = 3.808,
p < 0.05;
Figure 2). Participants who consumed EOC were significantly more clearheaded compared to those that consumed the placebo (85.0 (2.5) for EOC, 77.5 (2.7) for Placebo). There was also a main effect of gender (F(1,42) = 11.378,
p < 0.002); males reported feeling more clearheaded than females (Males: 85.8 (2.4); Females 74.6 (2.3)). As the effect of time was not significant completing the tasks did not influence participants’ ratings of confusion.
3.6.4. Composed/Anxious
The Variant (EOC/Placebo) × Gender × Time (0, 30, 60 min) was non-significant (F(2,80) = 353, n.s.), however, there was a main effect of Variant (EOC/Placebo) (F(1,40) = 3484,
p < 0.04;
Figure 2). Participants who consumed EOC were significantly less anxious than those who had drunk the placebo (84.9 (2.4) for EOC; 77.4 (2.5) for Placebo). Gender did not affect anxiety ratings and the effect of time was not significant.
3.6.5. Elated/Depressed
The Variant (EOC/Placebo) × Gender × Time (0, 30, 60 min) was non-significant (F(2,82) = 0.786, n.s.), however, there was a main effect of Variant (EOC/Placebo) (F(1,41) = 6.544,
p < 0.01;
Figure 2). Those who consumed the placebo rated themselves as more depressed than those who consumed EOC (79.8 (2.3) for EOC, 71.0 (2.4) for Placebo). Neither gender nor time influenced the ratings of depression.
3.6.6. Confident/Unsure
The Variant (EOC/Placebo) × Gender × Time (0, 30, 60 min) interaction was non-significant (F(2,82) = 0.075, n.s.), however, there was a main effect of Variant (EOC/Placebo) (F(1,41) = 3.987,
p < 0.05;
Figure 2). Subjects who consumed EOC were significantly more confident compared to who had drunk the placebo (83.0 (2.0) for EOC; 77.1 (2.1) for Placebo). There was no effect of Gender. In addition, as there was no effect of time, neither the “stressful” task nor the cognitive test battery affected confidence ratings.
3.7. Profile of Mood States
When ratings on the POMS were considered the findings were similar to those for the VAS. Participants were more confident (F(1,40) = 3.896, p < 0.05) and less depressed (F(1,39) = 5.639, p < 0.02) if they had drunk EOC. However, no effects of EOC on anxious/calm, clearheaded/confused, agreeable/hostile, or energetic/tired were observed.
3.8. Perceived Stress Scale
The interaction Variant (EOC/Placebo) × Gender was not significant (F(1,40) = 0.028, n.s.) and neither was the main effect of Variant (F(1,40) = 0.872, n.s.).
3.9. General Health Questionnaire
Neither the interaction Variant (EOC/Placebo) × Gender (F(1,38) = 0.002, n.s.) nor the main effect of variant (F(1,38) = 0.958, n.s.) were significant. Similarly, there were no effects of treatment on any of the individual subscales; Anxiety, Quality of Sleep, Depression and Social functioning.
4. Discussion
The present study aimed to examine the influence of EOC on mood in general and also as a response to a challenging cognitive test battery. It was hypothesised that consuming EOC would increase energy levels and reduce ratings of anxiety and depression. Previous studies have found that taking EOC was associated with increased energy; a recent study by Yamano
et al. [
3] examined the effects of 28 days supplementation of EOC on daily ratings of fatigue and found that when participants consumed EOC, rather than a placebo, they rated themselves as less fatigued. Similarly, Nagai
et al. [
4] found that subjects’ energy levels declined less during a mental arithmetic if they drank EOC rather than a placebo. Although there was a trend towards increased energy levels after EOC in the present (
p < 0.07) study the effect did not reach significance. It is, however, possible that with a larger sample size this effect would have been significant supporting the findings of Yamano
et al. [
3] and Nagai
et al. [
4]. Also consistent with a beneficial effect of EOC on energy levels was the increased LF/HF ratio observed in the present study (
Figure 1). This may indicate that consuming EOC is associated with a general increase in arousal which may be perceived as producing greater levels of energy.
In support of our hypothesis, the present study found that participants who had drunk EOC reported feeling less depressed and anxious and more confident, clearheaded and agreeable (
Figure 2). Azhar
et al. [
16] reported that participants, who had drunk EOC, reported fewer mild psychiatric symptoms (GHQ) after two weeks. Similarly a measure of the quality of life improved (SF36 Health Status Survey) in those consuming EOC. However, we found no evidence of an association between EOC and total GHQ ratings or any of its subscales; Anxiety, Quality of Sleep, Depression and Social functioning. It is, however, interesting that in our study participants who consumed EOC rated themselves as less depressed and less anxious on both versions of the POMS (72 item questionnaire and VAS), but that these effects were not detectable on the GHQ depression or anxiety subscales. The GHQ was developed to detect in a community sample those who would benefit from seeing a psychiatrist [
21] and our sample scored very low on the depression (4.7 (3.1)/21) and anxiety (7.3 (4.5)/21) subscales. It is possible that a questionnaire designed to distinguish those who may and may not have an increased chance of depression may not be sensitive enough to detect short-term differences in well-functioning young adults. The average rating on the depression VAS was 73.2 (17.6) and on the anxiety VAS was 77.8 (21.8), suggesting that our sample was generally calm and happy, therefore, supplementing with EOC may improve mood in a non-depressed or anxious population, however, research is needed to establish whether there are any effects of EOC on mood in a clinical sample.
A further aim of the present study was to examine whether consuming EOC influences autonomic nervous system activity at rest and in response to a stressful task. Based on animal research, which found anserine and carnosine reduced sympathetic nervous system activity [
9], it was hypothesised that EOC would produce the same effects in humans. To our knowledge only one study has previously considered the effects of EOC on autonomic nervous system activity. Shin and Moritani [
26] compared the acute effects (1.5 h after a single dose) of consuming either EOC, a combination of capsaicin, green tea extract and EOC, or a placebo, on indices of autonomic nervous system activity (very low frequency (VLF), LF, HF and total power) in six healthy males. They found that the combination of capsaicin, green tea extract and EOC increased VLF, LF and total power but EOC taken alone had no effect [
26]. The present findings are consistent with those of Shin and Moritani [
26]; there was no evidence for an effect of EOC on indices of autonomic nervous system activity in males. However females who consumed EOC had a higher LF/HF ratio compared to those who consumed the placebo (
Figure 1). The finding would suggest either an increase in sympathetic nervous system activity or a decrease in vagal activity after drinking EOC. This is in contrast to animal research which suggested that carnosine/anserine reduced sympathetic and increased parasympathetic nervous system activity [
9,
10]. However, this research was conducted using anaesthetised rats and it is possible that different results would have occurred when awake. Gender differences in cardiovascular reactivity have been frequently documented [
27,
28] which may explain why consuming EOC only influenced HRV in females. Although there was a significant effect of EOC on HRV it did not influence the participants’ response to a mental challenge: rather there was a general increase in HRV regardless of whether subjects were active or at rest. Given that HRV is related to mood and in particular depression [
29] it is possible that the effect of EOC on mood may be related to changes in HRV. Further research is needed to elucidate in humans further the effects of EOC on HRV indices.
As a further index of subjects’ stress levels we measured salivary cortisol at baseline, in response to the tasks and after a recovery period. It has been previously reported that after a mental workload the speed of recovery of serum cortisol levels was enhanced in those who had consumed EOC [
4]. Given that elevated cortisol levels are associated with poorer cognition [
30] and mood [
31], the enhanced cortisol recovery may partially explain the beneficial effects of EOC on cognition and mood. However, the present study was unable to replicate these findings possibly due to the smaller sample size. In addition, we did not find an increase in cortisol in response to the challenging task; rather participants’ cortisol was highest upon arrival and steadily declined thereafter. This is perhaps not surprising given the half-life of cortisol is about one hour and our challenging task latest only five minutes. Nonetheless if EOC were to influence hypothalamic-pituitary axis (HPA) activity we might have observed a significant main effect of EOC but we did not. It should also be considered that the “stressful task” used in the present study was designed to be mild; that is to represent daily challenges. These findings may not generalise to more severe stressors that have a more profound influence on mood and cortisol responses, for example, the “Trier social stress test”. Therefore, the present study does not support a beneficial effect of EOC on HPA activity in general but further research is required to determine the effect of EOC on HPA activity in response to more prolonged “stressors”.
Previous research has also suggested that EOC may improve aspects of cognition, in particular working memory [
4,
16] and attention [
3,
5]. Unfortunately, the present study was unable to replicate these findings; drinking EOC was not associated with any aspect cognitive performance. A possible explanation for this is the smaller sample size that was used in the present study; nonetheless more research is needed to elucidate the effects of EOC.
Since EOC contains many different components it is difficult to identify the active ingredient that may enhance mood; EOC consists mainly of proteins, peptides and free amino acids [
15]. Among the peptides carnosine and anserine are also present at relatively high concentrations in the human brain [
2] and may contribute toward neuronal protection [
32], possibly via their antioxidant [
2,
33] or antiglycation [
34] activities. For example, rats supplemented with a chicken breast extract or carnosine showed significantly reduced depression like symptoms (measured by the forced swimming test) and had decreased 3-methoxy-4-hydroxyphenylglycol (a major metabolite of norepinephrine) levels in their hippocampus [
35]. Furthermore, supplementing chicken extract has been shown to increase carnosine and anserine levels in animals’ brains [
36]. This suggests that the anti-depressant like effects of chicken extract effect may be due, in part, to its major components, carnosine and anserine.
The mechanisms mediating the beneficial effects EOC or carnosine are not, however, understood. It is possible that EOC, through virtue of its histidine containing peptides, may exert its effects via the histaminergic system in the brain [
15]. Histidine is the precursor to histamine and therefore has an important role in the maintenance of wakefulness [
37], possibly reducing fatigue. There is also some evidence that consuming EOC may modulate cerebral levels of 5-HT. Xu and Sim [
38] found that EOC markedly increased the level of 5-hydroxyindoleacetic acid (5-HIAA) in the cerebrospinal fluid, the main metabolite of 5-HT, a finding consistent with our data showing reduced feelings of depression and anxiety. Recently, Tsuruoka
et al. [
39] isolated a Diketopiperazine (cyclo(
l-Phe-
l-Phe)) from EOC and found that it was a serotonin transport inhibitor that increased cerebral monoamine levels and significantly improved depressive behaviour in mice. These findings suggest that the benefits of EOC on mood may be mediated by serotonergic mechanisms although more research is needed to confirm this hypothesis.
There remain many unanswered questions: further research is needed to establish whether the beneficial effects of EOC are observed after a single acute dose or whether longer term dietary supplementation is required. In the studies to date EOC has been consumed for between seven [
4] and twenty eight days [
3] with participants returning for testing on the last day of supplementation. Given that subjects will have taken EOC only hours before testing, the use of this approach makes it difficult to disentangle acute from longer term benefits. It should also be considered that the large number of statistical tests carried out in the present study may have raised the possibility of type 1 errors. However, the present findings are in line with previously observed benefits of EOC, in particular its effect on mood [
2,
3]. Nonetheless, the present findings should be regarded as exploratory and are in need of replication.