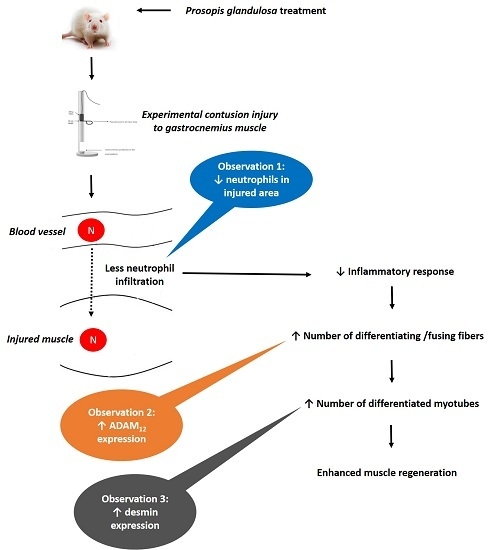
Chronic Prosopis Glandulosa Treatment Blunts Neutrophil Infiltration and Enhances Muscle Repair after Contusion Injury
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Animals
2.2. P. Glandulosa and Diclofenac Treatments
2.3. Induction of Experimental Muscle Contusion Injury and Sample Collection
2.4. Muscle Histology and Immunohistochemistry
2.5. Image Analysis
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Chronic P. Glandulosa Treatment Accelerates Repair of Muscle Ultrastructure
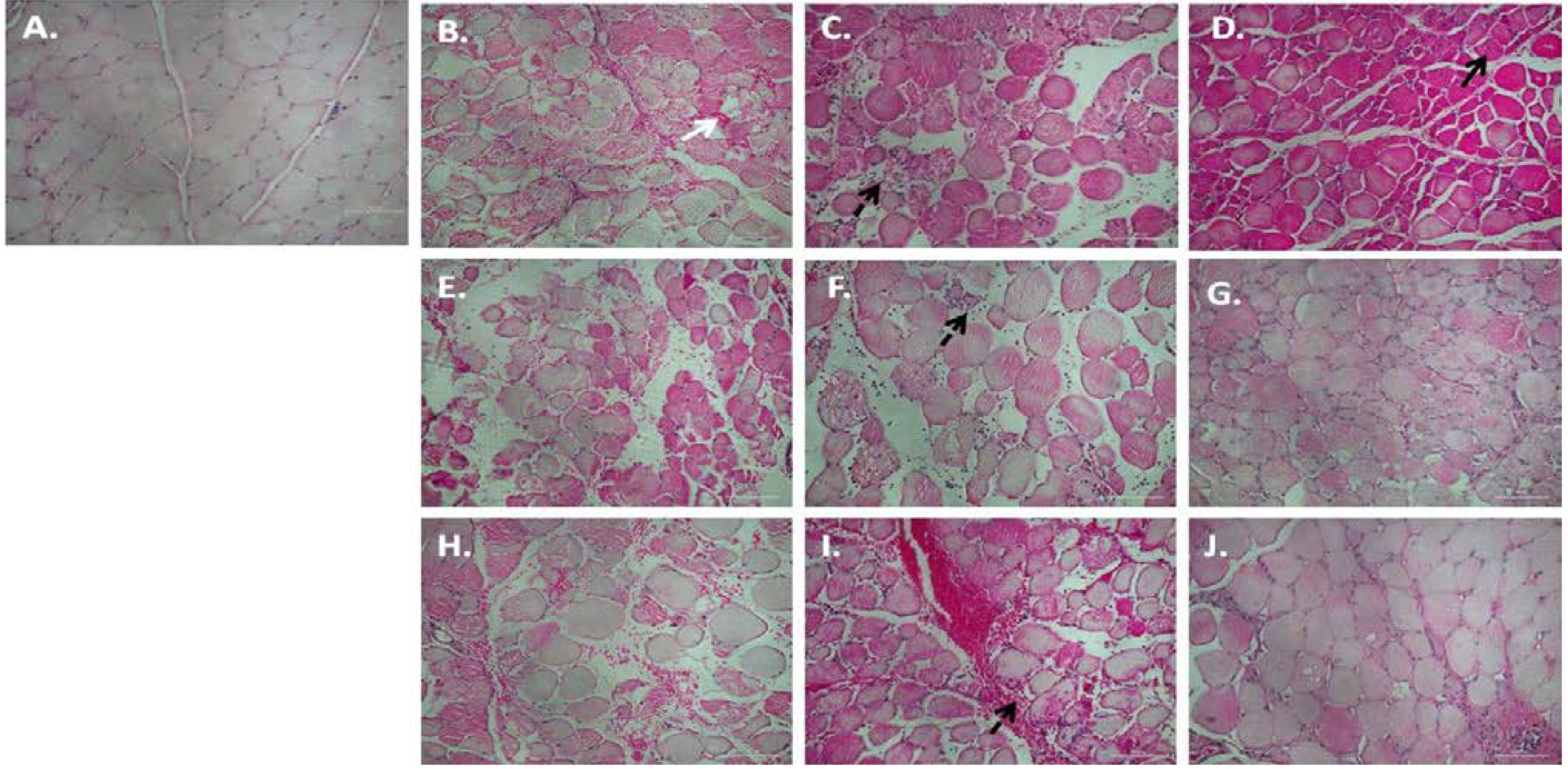
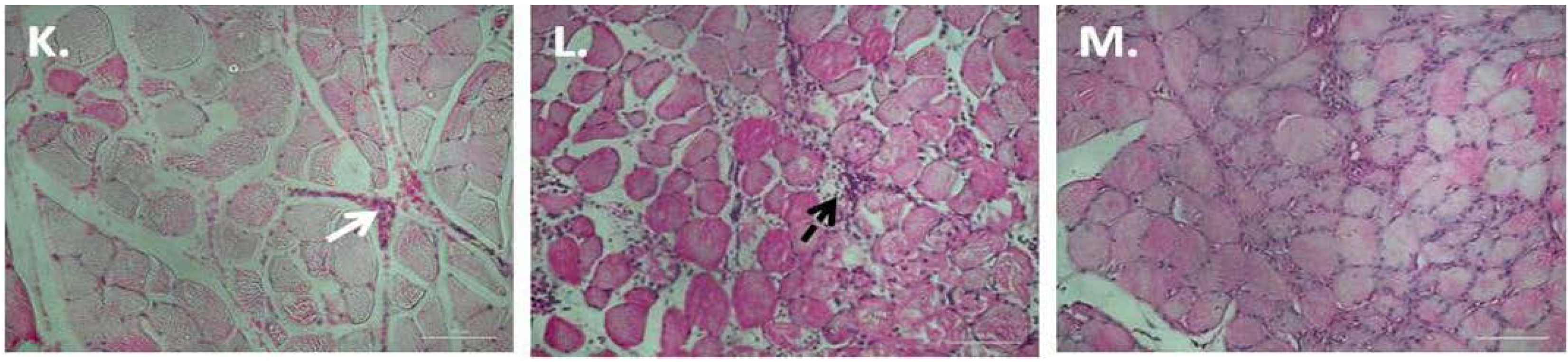
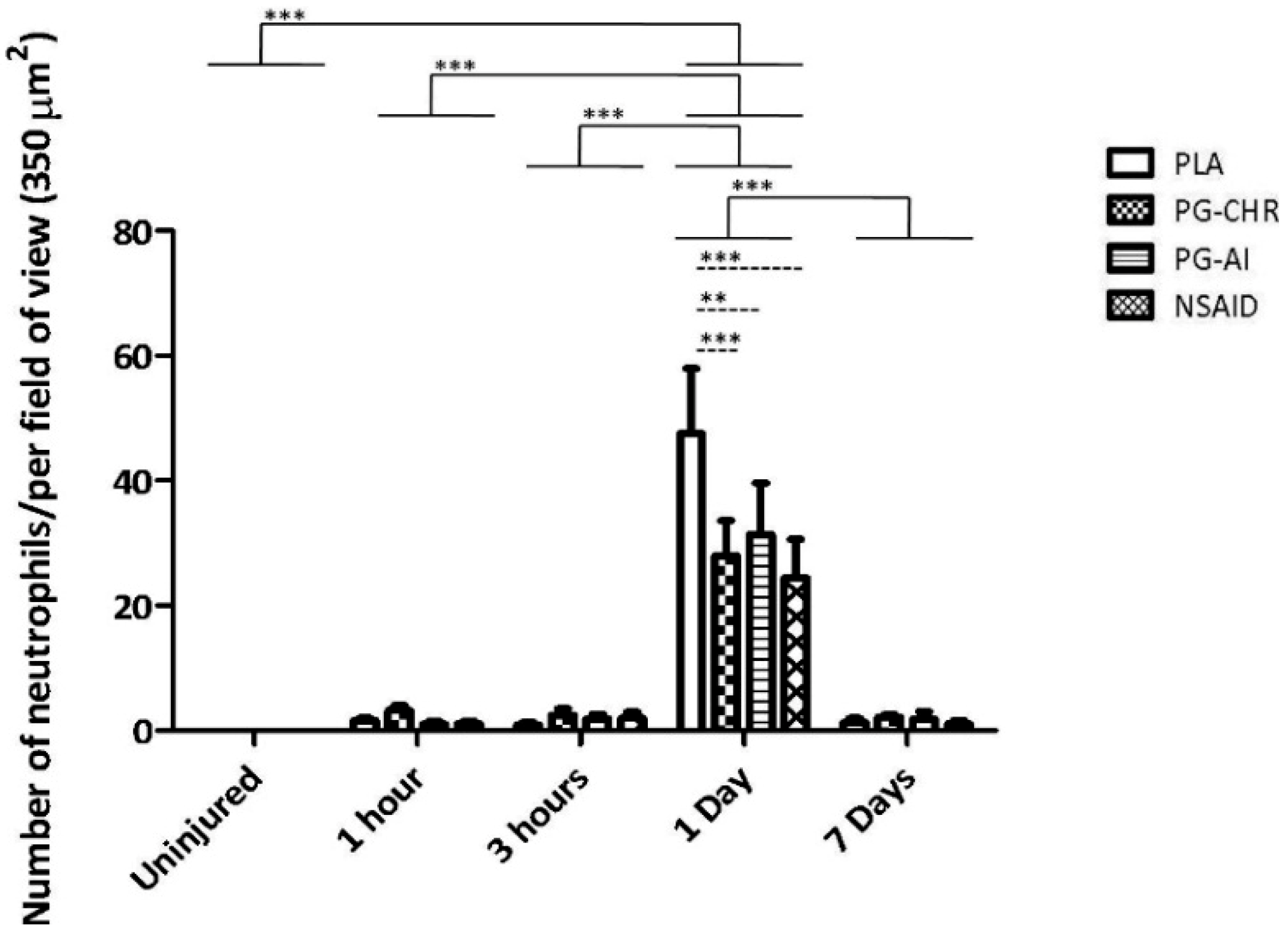
3.2. P. Glandulosa Treatment Blunted the Neutrophil Response to Contusion Injury
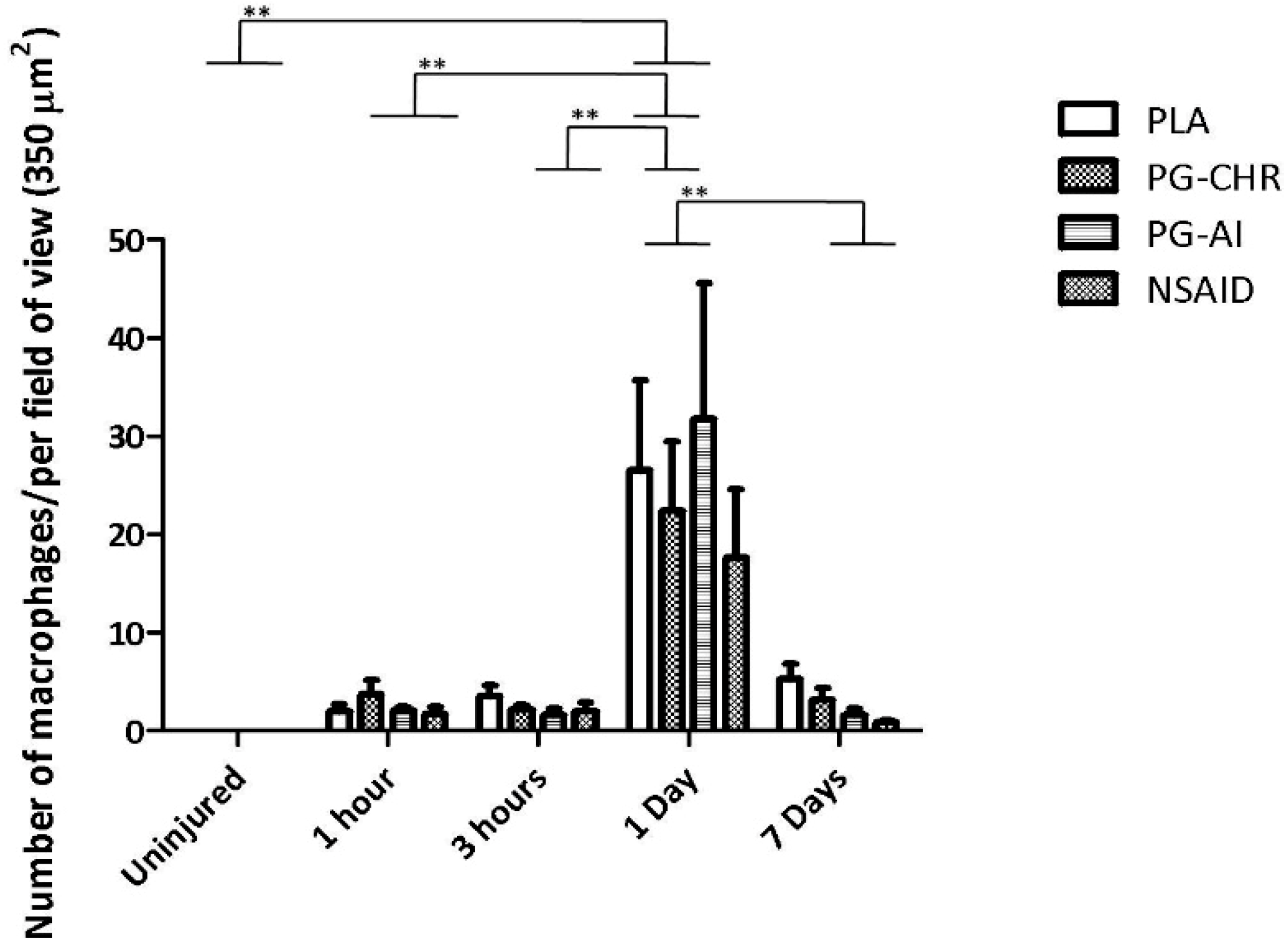
3.3. P. Glandulosa Treatment Did Not Affect Macrophage Response to Contusion Injury
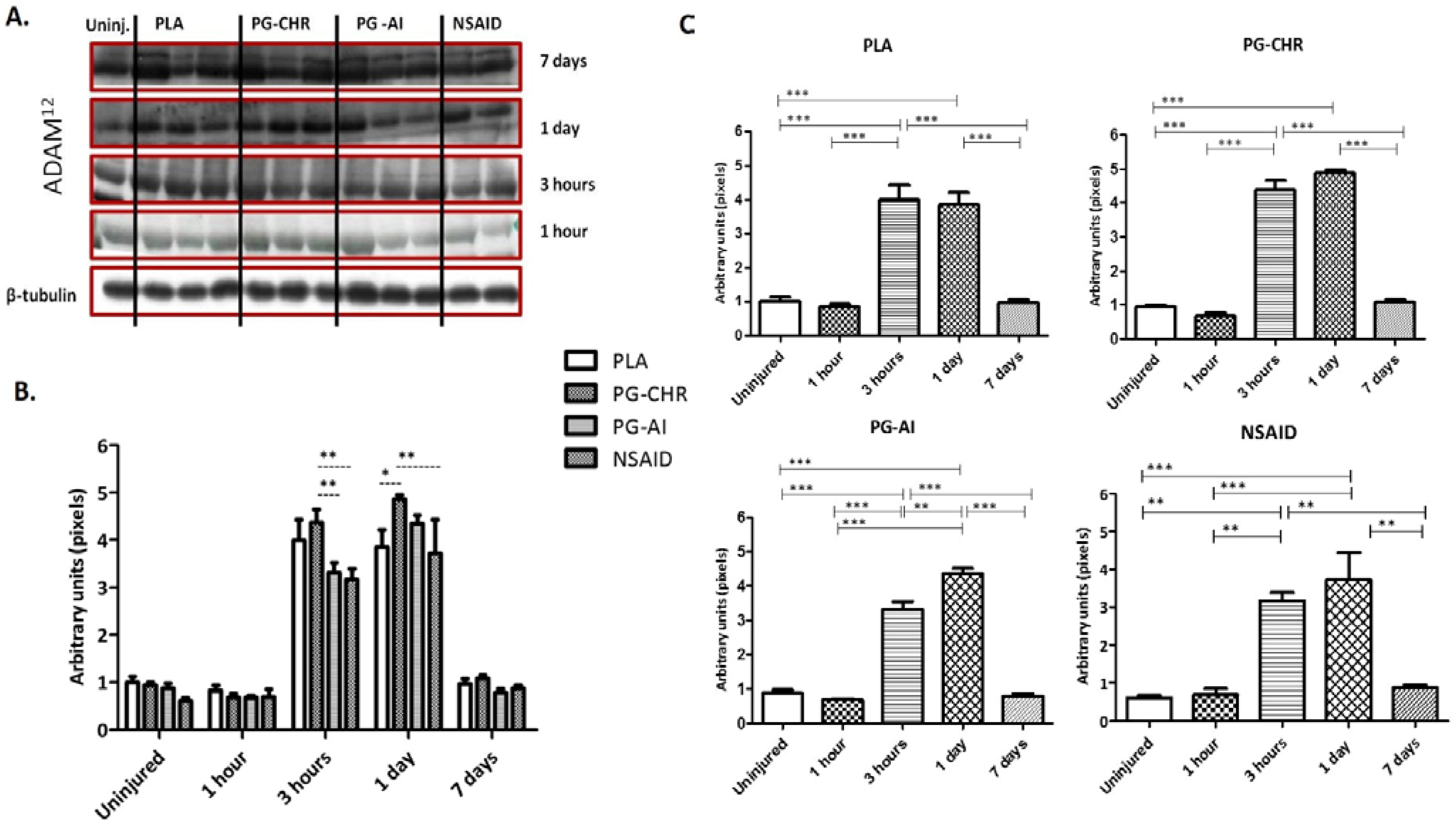
3.4. ADAM12 Expression is Enhanced in Response to Chronic P. Glandulosa Treatment
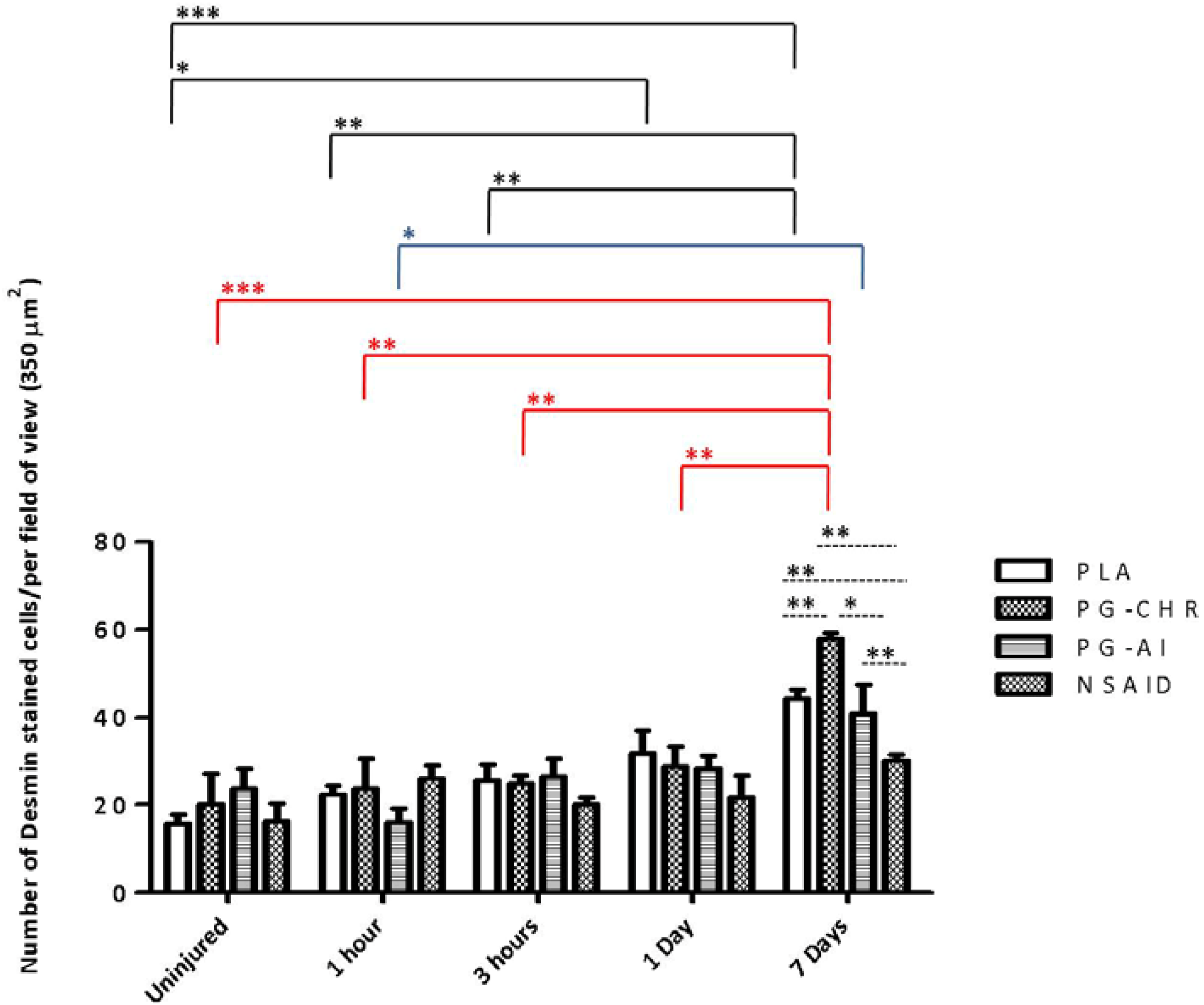
3.5. Desmin Expression is Increased in Response to Chronic P. Glandulosa Treatment
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Supplementary File 2Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quintero, A.J.; Wright, V.J.; Fu, F.H.; Haurd, J. Stem cells for the treatment of skeletal muscle injury. Clin. Sports Med. 2009, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lovering, R.M. Physical therapy and related interventions. In Skeletal Muscle Damage and Repair; Tiidus, P.M., Ed.; Human Kinetics: Champaign, IL, USA, 2008; pp. 219–230. [Google Scholar]
- Smith, C.; Kruger, M.J.; Smith, R.M.; Myburgh, K.H. The inflammatory response to skeletal muscle injury: Illuminating complexities. J. Sports Med. 2008, 38, 947–969. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Järvinen, T.L.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 345–353. [Google Scholar] [CrossRef]
- Arrington, E.D.; Miller, M.D. Skeletal muscle injuries. Orthop. Clin. North Am. 1995, 26, 411–422. [Google Scholar] [PubMed]
- Myburgh, K.H.; Kruger, M.J.; Smith, C. Accelerated skeletal muscle recovery after in vivo polyphenol administration. J. Nutr. Biochem. 2012, 23, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Summan, M.; Warren, G.L.; Mercer, R.R.; Chapman, R.; Hulderman, T.; van Rooijen, N.; Simeonova, P.P. Macrophages and skeletal muscle regeneration: A clodronate-containing liposome depletion study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 1488–1495. [Google Scholar] [CrossRef]
- Fielding, R.A.; Manfredi, T.J.; Ding, W.; Fiatarone, M.A.; Evans, W.J.; Cannon, J.G. Acute phase response in exercise. III. Neutrophil and IL-1β accumulation in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1993, 265, 166–172. [Google Scholar]
- Orimo, S.; Hiyamuta, E.; Arahata, K.; Sugita, H. Analysis of inflammatory cells and complement C3 in bupivacaine-induced myonecrosis. Muscle Nerve 1991, 14, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, 1173–1187. [Google Scholar] [CrossRef]
- Hurme, T.; Kalimo, H. Activation of myogenic precursor cells after muscle injury. Med. Sci. Sports Exerc. 1992, 24, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.L.; Rapizzi, E.; Cantini, M.; Libera, L.D.; Mazzoleni, F.; Arslan, P.; Carraro, U. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem. Biophys. Res. Commun. 1997, 235, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Prisk, V.; Foster, W.; Huard, J. Inhibited skeletal muscle healing in cyclooxygenase-2 gene-deficient mice: The role of PGE2 and PGF2alpha. J. Appl. Physiol. 2006, 101, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Friden, J.; Schmitz, M.C.; Lieber, R.L. Anti-inflammatory medication after muscle injury. A treatment resulting in short-term improvement but subsequent loss of muscle function. J. Bone Joint Surg. Am. 1995, 77, 1510–1519. [Google Scholar] [PubMed]
- Järvinen, M.; Lehto, M. The effect of early mobilization and immobilization on the healing process following muscle injuries. Sports Med. 1993, 15, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Deal, D.N.; Tipton, J.; Rosencrance, E.; Curl, W.W.; Smith, T.L. Ice reduces edema: A study of microvascular permeability in rats. J. Bone Joint Surg. Am. 2002, 84, 1573–1578. [Google Scholar] [PubMed]
- Hurme, T.; Rantanen, J.; Kalimo, H. Effects of early cryotherapy in experimental skeletal muscle injury. Scand. J. Med. Sci. Sports 1993, 3, 46–51. [Google Scholar] [CrossRef]
- Rantanen, J.; Thorsson, O.; Wollmer, P.; Hurme, T.; Kalimo, H. Effects of therapeutic ultrasound on the regeneration of skeletal muscle myofibers after experimental muscle injury. Am. J. Sports Med. 1999, 27, 54–59. [Google Scholar] [PubMed]
- Wilkin, L.D.; Merrick, M.A.; Kirby, T.E.; Devor, S.T. Influence of therapeutic ultrasound on skeletal muscle regeneration following blunt contusion. Int. J. Sports Med. 2004, 25, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Best, T.M.; Loitz-Ramage, B.; Corr, D.T.; Vanderby, R. Hyperbaric oxygen in the treatment of acute muscle stretch injuries. Results in an animal model. Am. J. Sports Med. 1998, 26, 367–372. [Google Scholar] [PubMed]
- Kasemkijwattana, C.; Menetrey, J.; Somogyi, G.; Moreland, M.S.; Fu, F.H.; Buranapanitkit, B.; Watkins, S.S.; Huard, J. Development of approaches to improve the healing following muscle contusion. Cell Transplant. 1998, 7, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Plafki, C.; Peters, P.; Almeling, M.; Welslau, W.; Busch, R. Complications and side effects of hyperbaric oxygen therapy. Aviat. Space Environ. Med. 2000, 71, 119–124. [Google Scholar] [PubMed]
- Kruger, M.J.; Myburgh, K.H.; Smith, C. Contusion injury with chronic in vivo polyphenol supplementation: Leukocyte responses. Med. Sci. Sport Exerc. 2014, 46, 225–231. [Google Scholar] [CrossRef]
- Gibson, S.; Hands, R.; Martinez, C. Medicinal Plants of the Southwest. New Mexico State University (NMSU). 2001. Available online: http://medplant.nmsu.edu/mesquite4.shtm (accessed on 5 September 2014).
- George, C.; Lochner, A.; Huisamen, B. The efficacy of Prosopis glandulosa as antidiabetic treatment in rat models of diabetes and insulin resistance. J. Ethnopharmacol. 2011, 137, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Huisamen, B.; George, C.; Dietrich, D.; Genade, S.; Lochner, A. Cardioprotective and anti-hypertensive effects of Prosopis glandulosa in rat model of pre-diabetes. Cardiovasc. J. Afr. 2013, 24, 31–37. [Google Scholar] [CrossRef]
- Stratton, S.A.; Heckmann, R.; Francis, R.S. Therapeutic ultrasound: Its effects on the integrity of a nonpenetrating wound. J. Orthop. Sports Phys. Ther. 1984, 5, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.D.; Yao, C.C.; Lee, M.Y.; Kok, L.F.; Wang, P.H.; Tyan, Y.S.; Han, C.P. True cytokeratin 8/18 immunohistochemistry is of no use in distinguishing between primary endocervical and endometrial adenocarcinomas in a tissue microarray study. Int. J. Gynecol. Pathol. 2010, 29, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Qian, J.; Easley, K.A.; Waldrop, S.M.; Cohen, C. Automated in situ hybridization and immunohistochemistry for cytomegalovirus detection in paraffin-embedded tissue sections. Appl. Immunohistochem. Mol. Morphol. 2009, 17, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.G., St.; Pierre, B.A. Cytokines in exertion-induced skeletal muscle injury. Mol. Cell. Biochem. 1998, 179, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.J.; Smith, C. Postcontusion polyphenol treatment alters inflammation and muscle regeneration. Med. Sci. Sports Exerc. 2012, 44, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.; Ito, K.; Russell, R.E.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: Molecular mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, 774–783. [Google Scholar] [CrossRef]
- Andrade, J.M.; Aboy, A.L.; Apel, M.A.; Raseira, M.C.; Pereira, J.F.; Henriques, A.T. Phenolic composition in different genotypes of Guabiju fruit (Myrcianthes pungens) and their potential as antioxidant and antichemotactic agent. J. Food Sci. 2011, 76, 1181–1187. [Google Scholar] [CrossRef]
- Fialkow, L.; Wang, Y.; Downey, G.P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007, 2, 153–164. [Google Scholar] [CrossRef]
- Lewis, M.S.; Whatley, R.E.; Cain, P.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J. Clin. Invest. 1988, 6, 2045–2055. [Google Scholar] [CrossRef]
- Judge, A.R.; Dodd, S.L. Oxidative damage to skeletal muscle following an acute bout of contractile claudication. Atherosclerosis 2003, 2, 219–224. [Google Scholar] [CrossRef]
- Mackey, A.L.; Kjaer, M.; Dandanell, S.; Mikkelsen, K.H.; Holm, L.; Dossing, S.; Kadi, F.; Koskinen, S.O.; Jensen, C.H.; Schroder, H.D.; et al. The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J. Appl. Physiol. 2007, 103, 425–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkelsen, U.R.; Langberg, H.; Helmark, I.C.; Skovgaard, D.; Andersen, L.L.; Kjaer, M.; Mackey, A.L. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J. Appl. Physiol. 2009, 107, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Vignaud, A.; Cebrian, J.; Martelly, I.; Caruelle, J.P.; Ferry, A. Effect of anti-inflammatory and antioxidant drugs on the long-term repair of severely injured mouse skeletal muscle. Exp. Physiol. 2005, 90, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Bornemann, A.; Kuschel, R.; Fujisawa-Sehara, A. Analysis for transcript expression of meltrin alpha in normal, regenerating, and denervated rat muscle. J. Muscle Res. Cell Motil. 2000, 21, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, B.J.; Loechel, F.; Mattei, M.G.; Engvall, E.; Albrechtsen, R.; Wewer, U.M. A novel, secreted form of human ADAM12 (meltrin alpha) provokes myogenesis in vivo. J. Biol. Chem. 1998, 273, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Yagami-Hiromasa, T.; Sato, T.; Kurisaki, T.; Kamijo, K.; Nabeshima, Y.; Fujisawa-Sehara, A. A metalloprotease-disintegrin participating in myoblast fusion. Nature 1995, 377, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, Z.; Gruszczynska-Biegala, J.; Zolkiewska, A. Role of metalloprotease disintegrin ADAM12 in determination of quiescent reserve cells during myogenic differentiation in vitro. Mol. Cell. Biol. 2003, 23, 6725–6738. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, J.; Hurme, T.; Lukka, R.; Heino, J.; Kalimo, H. Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: Evidence for two different populations of satellite cells. Lab. Invest. 1995, 72, 341–347. [Google Scholar] [PubMed]
- Vater, R.; Cullen, M.; Harris, J. The fate of desmin and titin during the degeneration and regeneration of the soleus muscle of the rat. Acta Neuropathol. 1992, 84, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Creuzet, S.; Lescaudron, L.; Li, Z.; Fontaine-Perus, J. MyoD, myogenin, and desmin-nls-lacZ transgene emphasize the distinct patterns of satellite cell activation in growth and regeneration. Exp. Cell Res. 1998, 243, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Przewoźniak, M.; Czaplicka, I.; Czerwińska, A.M.; Markowska-Zagrajek, A.; Moraczewski, J.; Stremińska, W.; Jańczyk-Ilach, K.; Ciemerych, M.A.; Brzoska, E. Adhesion proteins-an impact on skeletal myoblast differentiation. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Vaittinen, S.; Lukka, R.; Sahlgren, C.; Hurme, T.; Rantanen, J.; Lendahl, U.; Eriksson, J.E.; Kalimo, H. The expression of intermediate filament protein nestin as related to vimentin and desmin in regenerating skeletal muscle. J. Neuropathol. Exp. Neurol. 2001, 60, 588–597. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, C.; Smith, C.; Isaacs, A.W.; Huisamen, B. Chronic Prosopis Glandulosa Treatment Blunts Neutrophil Infiltration and Enhances Muscle Repair after Contusion Injury. Nutrients 2015, 7, 815-830. https://doi.org/10.3390/nu7020815
George C, Smith C, Isaacs AW, Huisamen B. Chronic Prosopis Glandulosa Treatment Blunts Neutrophil Infiltration and Enhances Muscle Repair after Contusion Injury. Nutrients. 2015; 7(2):815-830. https://doi.org/10.3390/nu7020815
Chicago/Turabian StyleGeorge, Cindy, Carine Smith, Ashwin W. Isaacs, and Barbara Huisamen. 2015. "Chronic Prosopis Glandulosa Treatment Blunts Neutrophil Infiltration and Enhances Muscle Repair after Contusion Injury" Nutrients 7, no. 2: 815-830. https://doi.org/10.3390/nu7020815