Review: The Potential of the Common Bean (Phaseolus vulgaris) as a Vehicle for Iron Biofortification
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Iron in Beans
3.1.1. Genetic Variability of Iron Concentrations in Beans
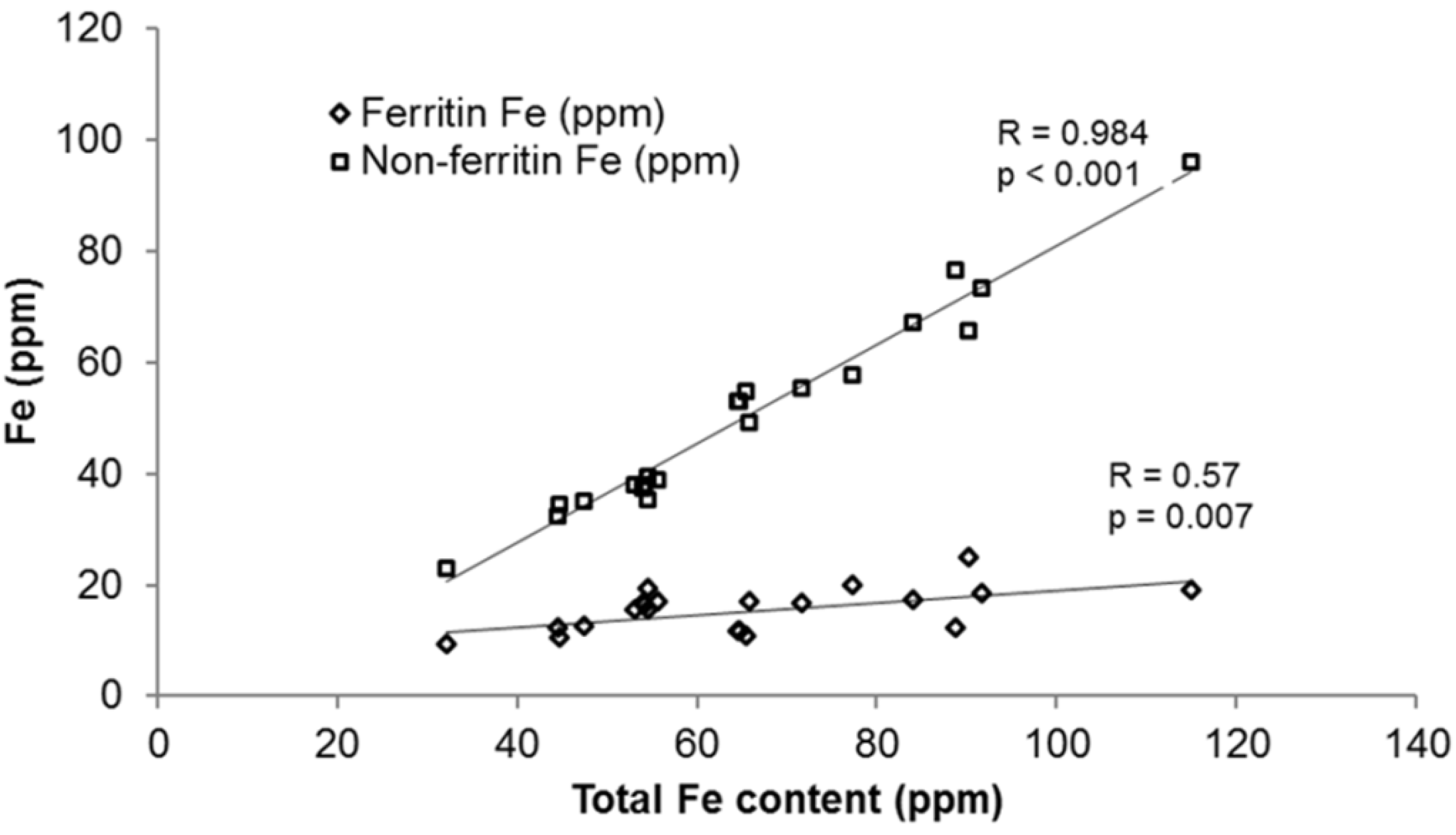
3.1.2. Iron Speciation in Beans
3.1.3. Progress in Bean Iron Biofortification
3.2. Compounds Influencing Iron Absorption from Beans
3.2.1. Polyphenols-Impact on Iron Absorption and Human Health
3.2.2. Occurrence of Different PP in Common Beans

3.2.3. Phytic Acid-Impact on Iron Absorption and Human Health
3.2.4. Phytic Acid in Common Beans

3.2.5. Proteins-Impact on Iron Absorption
3.2.6. Proteins in Common Beans
3.3. Bean Iron Bioavailability
3.3.1. The Impact of Bean PP on Human Iron Absorption
3.3.2. The Impact of Bean-PA on Human Iron Absorption

3.4. Can Iron-Biofortified Beans be an Efficacious Intervention to Combat Iron Deficiency?
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Debouck, D.G.; Toro, O.; Paredes, O.M.; Johnson, W.C.; Gepts, P. Genetic diversity and ecological distribution of Phaseolus vulgaris (Fabaceae) in northwestern South America. Econ. Bot. 1993, 47, 408–423. [Google Scholar] [CrossRef]
- HarvestPlus. Iron-bean, 2009. Available online: http://www.harvestplus.org/sites/default/files/HarvstPlus_Bean_Strategy.pdf (accessed on 26 January 2015).
- Blair, M.; Gonzales, L.F.; Kimani, P.M.; Butare, L. Genetic diversity, inter-gene pool introgression and nutritional quality of common beans (Phaseolus vulgaris L.) from central africa. Theor. Appl. Genet. 2010, 121, 237–248. [Google Scholar] [CrossRef]
- Broughton, W.J.; Hernandez, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)-model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar]
- Welch, R.M.; House, W.A.; Beebe, S.; Cheng, Z. Genetic selection for enhanced bioavailable levels of iron in bean (Phaseolus vulgaris L.) seeds. J. Agric. Food Chem. 2000, 48, 3576–3580. [Google Scholar] [CrossRef]
- Pennington, J.A.T.; Young, B. Iron zinc copper manganese selenium and iodine in foods from the United States total diet study. Food Compost. Anal. 1990, 3, 166–184. [Google Scholar] [CrossRef]
- Souci, S.W.; Fachmann, W.; Kraut, H. Food Composition and Nutrition Tables; Medpharm: Stuttgart, Germary, 1994; Volume 5. [Google Scholar]
- CGIAR. Common Bean, 2012. Available online: http://www.cgiar.org/our-research/crop-factsheets/beans/ (accessed on Day January 2015).
- FAO. Food Balance Sheet, 2012. Available online: http://faostat3.fao.org/faostat-gateway/go/to/download/FB/FBS/E (accessed on Day January 2015).
- Jones, A.L. Phaseolus Bean: Post-Harvest Operations, 1999. Available online: http://www.fao.org/fileadmin/user_upload/inpho/docs/Post_Harvest_Compendium_-_Phaesolus_beans.pdf (accessed on 26 January 2015).
- Hotz, C.; McClafferty, B. From harvest to health: Challenges for developing biofortified staple foods and determining their impact on micronutrient status. Food Nutr. Bull. 2007, 28, S271–S279. [Google Scholar] [PubMed]
- HarvestPlus. Rwanda, 2009. Available online: http://www.harvestplus.org/content/iron-beans-rwanda (accessed on 26 January 2015).
- HarvestPlus. DRC, 2009. Available online: http://www.harvestplus.org/content/iron-beans-dr-congo (accessed on 26 January 2015).
- Beebe, S.; Gonzalez, A.V.; Rengifo, J. Research on trace minerals in the common bean. Food Nutr. Bull. 2000, 21, 387–391. [Google Scholar]
- Ortiz-Monasterio, J.I.; Palacios-Rojas, N.; Meng, E.; Pixley, K.; Trethowan, R.; Pena, R.J. Enhancing the mineral and vitamin content of wheat and maize through plant breeding. J. Cereal. Sci. 2007, 46, 293–307. [Google Scholar] [CrossRef]
- Gregorio, G.B. Progress in breeding for trace minerals in staple crops. J. Nutr. 2002, 500S–502S. [Google Scholar]
- Bänziger, M.; Long, J. The potential for increasing the iron and zinc density through plant-breeding. Food Nutr. Bull. 2000, 21, 397–400. [Google Scholar]
- Bouis, H.E. The potential of genetically modified food crops to improve human nutrition in developing countries. J. Dev. Stud. 2007, 43, 79–96. [Google Scholar] [CrossRef]
- WHO. Guidelines on Food Fortification with Micronutrients. WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Baynes, R.D.; Bothwell, T.H. Iron-deficiency. Annu. Rev. Nutr. 1990, 10, 133–148. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant. Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Qaim, M.; Stein, A.J.; Meenakshi, J.V. Economics of biofortification. Agric. Econ. Blackwell 2007, 37, 119–133. [Google Scholar] [CrossRef]
- Gilligan, D.O. Biofortification, agricultural technology adoption, and nutrition policy: Some lessons and emerging challenges. CESifo Econ. Stud. 2012, 58, 405–421. [Google Scholar] [CrossRef]
- Oury, F.X.; Leenhardt, F.; Remesy, C.; Chanliaud, E.; Duperrier, B.; Balfourier, F.; Charmet, G. Genetic variability and stability of grain magnesium, zinc and iron concentrations in bread wheat. In Proceedings of the International Workshop on Modelling Quality Traits and Their Genetic Variability for Wheat, Clermont-Ferrand, France, 18–21 July 2004; Elsevier Science Bv: Clermont-Ferrand, France, 2004; pp. 177–185. [Google Scholar]
- Blair, M.W.; Izquierdo, P. Use of the advanced backcross-QTL method to transfer seed mineral accumulation nutrition traits from wild to Andean cultivated common beans. Theor. Appl. Genet. 2012, 125, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; Monserrate, F.; Beebe, S.E.; Restrepo, J.; Flores, J.O. Registration of high mineral common bean germplasm lines NUA35 and NUA56 from the red-mottled seed class. J. Plant. Regist. 2010, 4, 55–59. [Google Scholar] [CrossRef]
- HarvestPlus. Crop Development And Delivery Schedule Iron Bean DRC, 2014. Available online: http://www.harvestplus.org/sites/default/files/Delivery%20Schedule_Iron%20Bean%20DR%20Congo.pdf (accessed on 26 January 2015).
- Bouis, H.E.; Welch, R.M. Biofortification—A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010, 50, S20–S32. [Google Scholar] [CrossRef]
- Anton, A.; Ross, K.; Beta, T.; Fulcher, R.; Arntfield, S. Effect of pre-dehulling treatments on some nutritional and physical properties of navy and pinto beans (Phaseolus vulgaris L.). LWT-Food Sci. Technol. 2008, 41, 771–778. [Google Scholar] [CrossRef]
- Ariza-Nieto, M.; Blair, M.W.; Welch, R.M.; Glahn, R.P. Screening of iron bioavailability patterns in eight bean (Phaseolus vulgaris L.) genotypes using the caco-2 cell in vitro model. J. Agric. Food Chem. 2007, 55, 7950–7956. [Google Scholar]
- Beninger, C.W.; Gu, L.W.; Prior, R.L.; Junk, D.C.; Vandenberg, A.; Bett, K.E. Changes in polyphenols of the seed coat during the after-darkening process in pinto beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2005, 53, 7777–7782. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Juillerat, M.A.; Reddy, M.B.; Lynch, S.R.; Dassenko, S.A.; Cook, J.D. Soy protein, phytate, and iron-absorption in humans. Am. J. Clin. Nutr. 1992, 56, 573–578. [Google Scholar] [PubMed]
- Hallberg, L.; Brune, M.; Rossander, L. Iron-absorption in man-ascorbic-acid and dose-dependent inhibition by phytate. Am. J. Clin. Nutr. 1989, 49, 140–144. [Google Scholar] [PubMed]
- Hurrell, R.F.; Reddy, M.B.; Juillerat, M.A.; Cook, J.D. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am. J. Clin. Nutr. 2003, 77, 1213–1219. [Google Scholar] [PubMed]
- Petry, N.; Egli, I.; Zeder, C.; Walczyk, T.; Hurrell, R. Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J. Nutr. 2010, 140, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [PubMed]
- Petry, N.; Egli, I.; Gahutu, J.B.; Tugirimana, P.L.; Boy, E.; Hurrell, R. Stable iron isotope studies in Rwandese women indicate that the common bean has limited potential as a vehicle for iron biofortification. J. Nutr. 2012, 142, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Egli, I.; Gahutu, J.B.; Tugirimana, P.L.; Boy, E.; Hurrell, R. Phytic acid concentration influences iron bioavailability from biofortified beans in Rwandese women with low iron status. J. Nutr. 2014, 144, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Bonsmann, S.S.G. Dietary Factors Infleuncing Non-Heme Iron Absorption. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2006. [Google Scholar]
- Hoppler, M. Content and Bioavailability of Ferritin-Bound Iron in Staple Food Crops. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2008. [Google Scholar]
- Petry, N. Biofortification: Optimizing Iron Absorption from Beans and Other Staple Foods. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2011. [Google Scholar]
- Blair, M.W.; Medina, J.I.; Astudillo, C.; Rengifo, J.; Beebe, S.E.; Machado, G.; Graham, R. QTL for seed iron and zinc concentration and content in a mesoamerican common bean (Phaseolus vulgaris L.) population. Theor. Appl. Genet. 2010, 121, 1059–1070. [Google Scholar] [CrossRef]
- CIAT. Genetic Resources Program-Bean Collection, 2011; International Center for Tropical Agriculture. Available online: http://isa.ciat.cgiar.org/urg/beancollection.do;jsessionid=3DD436BFD78E7AB8BC00871432B44819 (accessed on 26 January 2015).
- Islam, F.M.A.; Basford, K.E.; Jara, C.; Redden, R.J.; Beebe, S. Seed compositional and disease resistance differences among gene pools in cultivated common bean. Genet. Resour. Crop Evol. 2002, 49, 285–293. [Google Scholar] [CrossRef]
- Guzman-Maldonado, S.H.; Acosta-Gallegos, J.; Paredes-Lopez, O. Protein and mineral content of a novel collection of wild and weedy common bean (Phaseolus vulgaris L.). J. Sci. Food Agric. 2000, 80, 1874–1881. [Google Scholar] [CrossRef]
- Blair, M.; Astudillo, C.; Grusak, M.; Graham, R.; Beebe, S. Inheritance of seed iron and zinc concentrations in common bean (Phaseolus vulgaris L.). Mol. Breed. 2009, 23, 197–207. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. Ferritins: Molecular properties, iron storage function and cellular regulation. Bba Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Theil, E.C. Iron, ferritin, and nutrition. Annu. Rev. Nutr. 2004, 24, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Laulhere, J.P.; Lescure, A.M.; Briat, J.F. Purification and characterization of ferritins from maize, pea, and soybean seeds-distribution in various pea organs. J. Biol. Chem. 1988, 263, 10289–10294. [Google Scholar] [PubMed]
- Sczekan, S.R.; Joshi, J.G. Isolation and characterization of ferritin from soybeans (glycine-max). J. Biol. Chem. 1987, 262, 13780–13788. [Google Scholar] [PubMed]
- Wardrop, A.J.; Wicks, R.E.; Entsch, B. Occurrence and expression of members of the ferritin gene family in cowpeas. Biochem. J. 1999, 337, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Hoppler, M.; Zeder, C.; Walczyk, T. Quantification of ferritin-bound iron in plant samples by isotope tagging and species-specific isotope dilution mass spectrometry. Anal. Chem. 2009, 81, 7368–7372. [Google Scholar] [CrossRef] [PubMed]
- Hoppler, M.; Egli, I.; Petry, N.; Gille, D.; Zeder, C.; Walczyk, T.; Blair, M.W.; Hurrell, R.F. Iron speciation in beans (Phaseolus vulgaris) biofortified by common breeding. J. Food Sci. 2014, 79, C1629–C1634. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; Izquierdo, P.; Astudillo, C.; Grusak, M.A. A legume biofortification quandary: Variability and genetic control of seed coat micronutrient accumulation in common beans. Front. Plant Sci. 2013, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Derman, D.P.; Bothwell, T.H.; Torrance, J.D.; Macphail, A.P.; Bezwoda, W.R.; Charlton, R.W.; Mayet, F.G.H. Iron-absorption from ferritin and ferric hydroxide. Scand. J. Haematol. 1982, 29, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Walker, R.B.; Layrisse, M.; Clark, P.; Finch, C.A. Nutritive value of food iron. Am. J. Clin. Nutr. 1965, 16, 464–471. [Google Scholar] [PubMed]
- Layrisse, M.; Martineztorres, C.; Renzy, M.; Leets, I. Ferritin iron-absorption in man. Blood 1975, 45, 689–698. [Google Scholar] [PubMed]
- Martinez-Torres, C.; Leets, I.; Taylor, P.; Ramirez, J.; Camacho, M.D.; Layrisse, M. Heme, ferritin and vegetable iron-absorption in humans from meals denatured of heme iron during the cooking of beef. J. Nutr. 1986, 116, 1720–1725. [Google Scholar] [PubMed]
- Skikne, B.; Fonzo, D.; Lynch, S.R.; Cook, J.D. Bovine ferritin iron bioavailability in man. Eur. J. Clin. Nutr. 1997, 27, 228–233. [Google Scholar]
- Beard, J.L.; Burton, J.W.; Theil, E.C. Purified ferritin and soybean meal can be sources of iron for treating iron deficiency in rats. J. Nutr. 1996, 126, 154–160. [Google Scholar] [PubMed]
- Lonnerdal, B.; Bryant, A.; Liu, X.F.; Theil, E.C. Iron absorption from soybean ferritin in nonanemic women. Am. J. Clin. Nutr. 2006, 83, 103–107. [Google Scholar] [PubMed]
- Davila-Hicks, P.; Theil, E.C.; Lonnerdal, B. Iron in ferritin or in salts (ferrous sulfate) is equally bioavailable in nonanemic women. Am. J. Clin. Nutr. 2004, 80, 936–940. [Google Scholar] [PubMed]
- Theil, E.C.; Chen, H.J.; Miranda, C.; Janser, H.; Elsenhans, B.; Nunez, M.T.; Pizarro, F.; Schumann, K. Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J. Nutr. 2012, 142, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Kalgaonkar, S.; Lonnerdal, B. Effects of dietary factors on iron uptake from ferritin by caco-2 cells. J. Nutr. Biochem. 2008, 19, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kalgaonkar, S.; Lonnerdal, B. Receptor-mediated uptake of ferritin-bound iron by human intestinal caco-2 cells. J. Nutr. Biochem. 2009, 20, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.D.S.; Garri, C.; Pizarro, F.; Walter, T.; Theil, E.C.; Nunez, M.T. Caco-2 intestinal epithelial cells absorb soybean ferritin by mu2 (AP2)-dependent endocytosis. J. Nutr. 2008, 138, 659–666. [Google Scholar] [PubMed]
- Cook, J.D.; Finch, C.A.; Walker, R.; Martinez, C.; Layrisse, M.; Monsen, E. Food iron absorption measured by an extrinsic tag. Clin. Investig. 1972, 51, 805–815. [Google Scholar] [CrossRef]
- Bejjani, S.; Pullakhandam, R.; Punjal, R.; Nair, K.M. Gastric digestion of pea ferritin and modulation of its iron bioavailability by ascorbic and phytic acids in caco-2 cells. World J. Gastroenterol. 2007, 13, 2083–2088. [Google Scholar] [PubMed]
- Hoppler, M.; Schonbachler, A.; Meile, L.; Hurrell, R.F.; Walczyk, T. Ferritin-iron is released during boiling and in vitro gastric digestion. J. Nutr. 2008, 138, 878–884. [Google Scholar] [PubMed]
- Jin, F.X.; Frohman, C.; Thannhauser, T.W.; Welch, R.M.; Glahn, R.P. Effects of ascorbic acid, phytic acid and tannic acid on iron bioavailability from reconstituted ferritin measured by an in vitro digestion-caco-2 cell model. Br. J. Nutr. 2009, 101, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Makower, R.U. Extraction and determination of phytic acid in beans (Phaseolus vulgaris). Cereal Chem. 1970, 47, 288–296. [Google Scholar]
- Ribeiro, N.D.; Domingues, L.D.; Zemolin, A.E.M.; Possobom, M.T.D. Selection of common bean lines with high agronomic performance and high calcium and iron concentrations. Pesqui. Agropecu. Bras. 2013, 48, 1368–1375. [Google Scholar] [CrossRef]
- Donangelo, C.M.; Woodhouse, L.R.; King, S.M.; Toffolo, G.; Shames, D.M.; Viteri, F.E.; Cheng, Z.; Welch, R.M.; King, J.C. Iron and zinc absorption from two bean (Phaseolus vulgaris L.) genotypes in young women. J. Agric. Food Chem. 2003, 51, 5137–5143. [Google Scholar] [CrossRef]
- Blair, M.; Astudillo, C.; Beebe, S.; Roa, I.; Kimani, P.M.; Chirwa, R.M. Biofortification Breeding of Common Bean (Phaseolus vulgaris L.), 2009. Available online: http://www.biokemi.org/biozoom/issues/525/articles/2397 (accessed on 26 January 2015).
- Shehu, H.E.; Jamala, G.Y. Available Zn distribution, response and uptake of rice (Oriza sativa) to applied zn along a topose quence of lake gerio fadama soils at Yola, North-eastern Nigeria. J. Am. Sci. 2010, 6, 1013–1016. [Google Scholar]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Zhao, F.J. Strategies for increasing the selenium content of wheat. J. Cereal Sci. 2007, 46, 282–292. [Google Scholar] [CrossRef]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. Biofortification of durum wheat with zinc and iron. Cereal Chem. 2010, 87, 10–20. [Google Scholar] [CrossRef]
- Hirschi, K.D. Nutrient biofortification of food crops. Annu. Rev. Nutr. 2009, 29, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z.; Batten, G.D.; Crowley, D.E. Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crop Res. 1999, 60, 27–40. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Schulin, R.; Chaney, R.L.; Daneshbakhsh, B.; Afyuni, M. Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture. A review. Agron. Sustain. Dev. 2010, 30, 83–107. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Novillo, J.; Obrador, A.; Lopez-Valdivia, L.M. Mobility and leachability of zinc in two soils treated with six organic zinc complexes. J. Agric. Food Chem. 2001, 49, 3833–3840. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.; Obrador, A.; Alvarez, J.M. Behavior of zinc from six organic fertilizers applied to a navy bean crop grown in a calcareous soil. J. Agric. Food Chem. 2007, 55, 7084–7092. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Yazici, A.; Gokmen, O.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef] [Green Version]
- Boonchuay, P.; Cakmak, I.; Rerkasem, B.; Prom-U-Thai, C. Effect of different foliar zinc application at different growth stages on seed zinc concentration and its impact on seedling vigor in rice. Soil Sci. Plant Nutr. 2013, 59, 180–188. [Google Scholar] [CrossRef]
- Aciksoz, S.B.; Yazici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Shi, R.L.; Rezaul, K.M.; Zhang, F.S.; Zou, C.Q. Iron and zinc concentrations in grain and flour of winter wheat as affected by foliar application. J. Agric. Food Chem. 2010, 58, 12268–12274. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Friedman, M. Chemistry, biochemistry, and dietary role of potato polyphenols. A review. J. Agric. Food Chem. 1997, 45, 1523–1540. [Google Scholar] [CrossRef]
- Sisa, M.; Bonnet, S.L.; Ferreira, D.; van der Westhuizen, J.H. Photochemistry of flavonoids. Molecules 2010, 15, 5196–5245. [Google Scholar] [CrossRef] [PubMed]
- Brune, M.; Rossander, L.; Hallberg, L. Iron-absorption and phenolic-compounds-importance of different phenolic structures. Eur. J. Clin. Nutr. 1989, 43, 547–558. [Google Scholar] [PubMed]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Method Enzymol. 2001, 335, 190–203. [Google Scholar]
- Brune, M.; Hallberg, L.; Skanberg, A.B. Determination of iron-binding phenolic groups in foods. J. Food Sci. 1991, 56, 128–131. [Google Scholar] [CrossRef]
- Purawatt, S.; Siripinyanond, A.; Shiowatana, J. Flow field-flow fractionation-inductively coupled optical emission spectrometric investigation of the size-based distribution of iron complexed to phytic and tannic acids in a food suspension: Implications for iron availability. Anal. Bioanal. Chem. 2007, 389, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Rossander, L. Absorption of iron from western-type lunch and dinner meals. Am. J. Clin. Nutr. 1982, 35, 502–509. [Google Scholar] [PubMed]
- Disler, P.B.; Lynch, S.R.; Charlton, R.W.; Torrance, J.D.; Bothwell, T.H.; Walker, R.B.; Mayet, F. Effect of tea on iron-absorption. Gut 1975, 16, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Reddy, M.B.; Hurrell, R.F. The effect of red and white wines on nonheme-iron absorption in humans. Am. J. Clin. Nutr. 1995, 61, 800–804. [Google Scholar] [PubMed]
- Gillooly, M.; Bothwell, T.H.; Torrance, J.D.; Macphail, A.P.; Derman, D.P.; Bezwoda, W.R.; Mills, W.; Charlton, R.W.; Mayet, F. The effects of organic-acids, phytates and polyphenols on the absorption of iron from vegetables. Br. J. Nutr. 1983, 49, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Tuntipopipat, S.; Judprasong, K.; Zeder, C.; Wasantwisut, E.; Winichagoon, P.; Charoenkiatkul, S.; Hurrell, R.; Walczyk, T. Chili, but not turmeric, inhibits iron absorption in young women from an iron-fortified composite meal. J. Nutr. 2006, 136, 2970–2974. [Google Scholar] [PubMed]
- Gillooly, M.; Bothwell, T.H.; Charlton, R.W.; Torrance, J.D.; Bezwoda, W.R.; Macphail, A.P.; Derman, D.P.; Novelli, L.; Morrall, P.; Mayet, F. Factors affecting the absorption of iron from cereals. Br. J. Nutr. 1984, 51, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cercamondi, C.I.; Egli, I.M.; Zeder, C.; Hurrell, R.F. Sodium iron edta and ascorbic acid, but not polyphenol oxidase treatment, counteract the strong inhibitory effect of polyphenols from brown sorghum on the absorption of fortification iron in young women. Br. J. Nutr. 2014, 111, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s. [Google Scholar]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Taubert, D.; Berkels, R.; Roesen, R.; Klaus, W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA J. Am. Med. Assoc. 2003, 290, 1029–1030. [Google Scholar] [CrossRef]
- Rein, D.; Paglieroni, T.G.; Wun, T.; Pearson, D.A.; Schmitz, H.H.; Gosselin, R.; Keen, C.L. Cocoa inhibits platelet activation and function. Am. J. Clin. Nutr. 2000, 72, 30–35. [Google Scholar] [PubMed]
- Heiss, C.; Dejam, A.; Kleinbongard, P.; Schewe, T.; Sies, H.; Kelm, M. Vascular effects of cocoa rich in flavan-3-ols. JAMA J. Am. Med. Assoc. 2003, 290, 1030–1031. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Croce, G.; Tiberti, S.; Aggio, A.; Ferri, C. Flavonoids, vascular function and cardiovascular protection. Curr. Pharm. Design. 2009, 15, 1072–1084. [Google Scholar] [CrossRef]
- Sesso, H.D.; Gaziano, J.M.; Buring, J.E.; Hennekens, C.H. Coffee and tea intake and the risk of myocardial infarction. Am. J. Epidemiol. 1999, 149, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.J.; Maclure, M.; Muller, J.E.; Sherwood, J.B.; Mittleman, M.A. Tea consumption and myocardial mortality after acute infarction. Circulation 2002, 105, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. European Food and Safelty Authority. Available online: http://www.efsa.europa.eu/en/search/doc/2809.pdf (accessed on 26 January 2015).
- Njike, V.Y.; Faridi, Z.; Shuval, K.; Dutta, S.; Kay, C.D.; West, S.G.; Kris-Etherton, P.M.; Katz, D.L. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int. J. Cardiol. 2011, 149, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Schewe, T.; Heiss, C.; Kelm, M. Cocoa polyphenols and inflammatory mediators. Am. J. Clin. Nutr. 2005, 81, 304s–312s. [Google Scholar] [PubMed]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.A.B.E.; van Balen, G.P.; van den Berg, D.J.; Bast, A.; van der Vijgh, W.J.F. Influence of iron chelation on the antioxidant activity of flavonoids. Biochem. Pharmacol. 1998, 56, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Murakami, K. Interaction of iron with polyphenolic compounds: Application to antioxidant characterization. Anal. Biochem. 1998, 257, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Shukla, S.K.; Kumar, I.P.; Namita, I.; Afrin, F.; Sharma, R.K. Radioprotective properties of apple polyphenols: An in vitro study. Mol. Cell. Biochem. 2006, 288, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Caldas, G.V.; Blair, M.W. Inheritance of seed condensed tannins and their relationship with seed-coat color and pattern genes in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2009, 119, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Escarpa, A.; Gonzalez, M.C. An overview of analytical chemistry of phenolic compounds in foods. Crit. Rev. Anal. Chem. 2001, 31, 57–139. [Google Scholar] [CrossRef]
- Tajeri Foman, J. Evaluation potentieller Strategien zur Eisenbiofortifizierung von Phaseolus vulgaris. ETH Zurich: Zurich, Switzerland, 2006. [Google Scholar]
- Reddy, N.R.; Pierson, M.D.; Sathe, S.K.; Salunkhe, D.K. Dry bean tannins: A review of nutritional implications. J. Am. Oil Chem. Soc. 1985, 62, 541–549. [Google Scholar] [CrossRef]
- Diaz, A.M.; Caldas, G.V.; Blair, M.W. Concentrations of condensed tannins and anthocyanins in common bean seed coats. Food Res. Int. 2010, 43, 595–601. [Google Scholar] [CrossRef]
- Maldonado, S.H.G.; MarinJarillo, A.; Castellanos, J.Z.; DeMejia, E.G.; AcostaGallegosc, J.A. Relationship between physical and chemical characteristics and susceptibility to Zabrotes subfasciatus (Boh.) (Coleoptera:Bruchidae) and Acanthoscelides obtectus (Say) in common bean (Phaseolus vulgaris L.) varieties. J. Stored Prod. Res. 1996, 32, 53–58. [Google Scholar]
- Aparicio-Fernandez, X.; Yousef, G.G.; Loarca-Pina, G.; de Mejia, E.; Lila, M.A. Characterization of polyphenolics in the seed coat of black jamapa bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2005, 53, 4615–4622. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.W.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [PubMed]
- Lin, L.; Harnly, J.; Pastor-Corrales, M.; Luthria, D. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Alonso, L.G.; Lygin, A.; Widholm, J.M.; Valverde, M.E.; Paredes-Lopez, O. Polyphenols in wild and weedy mexican common beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 4436–4444. [Google Scholar] [CrossRef] [PubMed]
- Choung, M.G.; Choi, B.R.; An, Y.N.; Chu, Y.H.; Cho, Y.S. Anthocyanin profile of Korean cultivated kidney bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2003, 51, 7040–7043. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Dao, L.T.; Full, G.H.; Wong, R.Y.; Harden, L.A.; Edwards, R.H.; Berrios, J.D. Characterization of black bean (Phaseolus vulgaris L.) anthocyanins. J. Agric. Food Chem. 1997, 45, 3395–3400. [Google Scholar] [CrossRef]
- Wu, X.L.; Prior, R.L. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: Vegetables, nuts, and grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Glahn, R.P.; Miller, D.D. Bioaccessibility of phenols in common beans (Phaseolus vulgaris L.) and iron (Fe) availability to caco-2 cells. J. Agric. Food Chem. 2008, 56, 10999–11005. [Google Scholar]
- Raboy, V. Myo-inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry 2003, 64, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Cheryan, M. Phytic acid interactions in food systems. CRC Crit. Rev. Food Sci. Nutr. 1980, 13, 297–335. [Google Scholar] [CrossRef]
- O’dell, B.L.; de Boland, A.R.; Koirtyohann, S.R. Distribution of phytate and nutritionally important elements among morphological components of cereal grains. J. Agric. Food Chem. 1972, 20, 718–721. [Google Scholar]
- Schlemmer, U.; Frolich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.R.; Sathe, S.K. Food Phytates; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ.Sci. B 2008, 9, 165–191. [Google Scholar] [CrossRef]
- Murphy, A.M.; Otto, B.; Brearley, C.A.; Carr, J.P.; Hanke, D.E. A role for inositol hexakisphosphate in the maintenance of basal resistance to plant pathogens. Plant J. 2008, 56, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Chaouki, N.; Hurrell, R.F. Iron deficiency due to consumption of a habitual diet low in bioavailable iron: A longitudinal cohort study in moroccan children. Am. J. Clin. Nutr. 2005, 81, 115–121. [Google Scholar] [PubMed]
- Navert, B.; Sandstrom, B.; Cederblad, A. Reduction of the phytate content of bran by leavening in bread and its effect on zinc-absorption in man. Br. J. Nutr. 1985, 53, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Heaney, R.P.; Martin, B.R.; Fitzsimmons, M.L. Human calcium-absorption from whole-wheat products. J. Nutr. 1991, 121, 1769–1775. [Google Scholar] [PubMed]
- Bohn, T.; Davidsson, L.; Walczyk, T.; Hurrell, R.F. Phytic acid added to white-wheat bread inhibits fractional apparent magnesium absorption in humans. Am. J. Clin. Nutr. 2004, 79, 418–423. [Google Scholar] [PubMed]
- Davidsson, L.; Almgren, A.; Juillerat, M.A.; Hurrell, R.F. Manganese absorption in humans: The effect of phytic acid and ascorbic acid in soy formula. Am. J. Clin. Nutr. 1995, 62, 984–987. [Google Scholar] [PubMed]
- Brune, M.; Rossander, L.; Hallberg, L. Iron-absorption-no intestinal adaptation to a high-phytate diet. Am. J. Clin. Nutr. 1989, 49, 542–545. [Google Scholar] [PubMed]
- Hunt, J.R.; Roughead, Z.K. Adaptation of iron absorption in men consuming diets with high or low iron bioavailability. Am. J. Clin. Nutr. 2000, 71, 94–102. [Google Scholar] [PubMed]
- Armah, S.M.; Carriquiry, A.; Sullivan, D.; Cook, J.D.; Reddy, M.B. A complete diet-based algorithm for predicting nonheme iron absorption in adults. J. Nutr. 2013, 143, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Siegenberg, D.; Baynes, R.D.; Bothwell, T.H.; Macfarlane, B.J.; Lamparelli, R.D.; Car, N.G.; Macphail, P.; Schmidt, U.; Tal, A.; Mayet, F. Ascorbic-acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am. J. Clin. Nutr. 1991, 53, 537–541. [Google Scholar] [PubMed]
- Troesch, B.; Egli, I.; Zeder, C.; Hurrell, R.F.; de Pee, S.; Zimmermann, M.B. Optimization of a phytase-containing micronutrient powder with low amounts of highly bioavailable iron for in-home fortification of complementary foods. Am. J. Clin. Nutr. 2009, 89, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Ranum, P.; de Pee, S.; Biebinger, R.; Hulthen, L.; Johnson, Q.; Lynch, S. Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food Nutr. Bull. 2010, 31, S7–S21. [Google Scholar] [PubMed]
- Hurrell, R.F. Phytic acid degradation as a means of improving iron absorption. Int. J. Vitam. Nutr. Res. 2004, 74, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.S. Bioavailability of minerals in legumes. Br. J. Nutr. 2002, 88, S281–S285. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461s–1467s. [Google Scholar] [CrossRef] [PubMed]
- Tuntawiroon, M.; Sritongkul, N.; Rossanderhulten, L.; Pleehachinda, R.; Suwanik, R.; Brune, M.; Hallberg, L. Rice and iron-absorption in man. Eur. J. Clin. Nutr. 1990, 44, 489–497. [Google Scholar] [PubMed]
- Hotz, C.; Gibson, R.S. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J. Nutr. 2007, 137, 1097–1100. [Google Scholar] [PubMed]
- Blair, M.W.; Herrera, A.L.; Sandoval, T.A.; Caldas, G.V.; Filleppi, M.; Sparvoli, F. Inheritance of seed phytate and phosphorus levels in common bean (Phaseolus vulgaris L.) and association with newly-mapped candidate genes. Mol. Breed. 2012, 30, 1265–1277. [Google Scholar]
- Egli, I.; Davidsson, L.; Juillerat, M.A.; Barclay, D.; Hurrell, R.F. The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. J. Food Sci. 2002, 67, 3484–3488. [Google Scholar] [CrossRef]
- Marero, L.M.; Payumo, E.M.; Aguinaldo, A.R.; Matsumoto, I.; Homma, S. Antinutritional factors in weaning foods prepared from germinated cereals and legumes. Food Sci. Technol. Leb. 1991, 24, 177–181. [Google Scholar]
- Sharma, A.; Kapoor, A.C. Levels of antinutritional factors in pearl millet as affected by processing treatments and various types of fermentation. Plant Food Hum. Nutr. 1996, 49, 241–252. [Google Scholar] [CrossRef]
- Shamsuddin, A.M. Anti-cancer function of phytic acid. Int. J. Food Sci. Tech. 2002, 37, 769–782. [Google Scholar] [CrossRef]
- Kelly, C. Can excess iron increase the risk for coronary heart disease and cancer? Nutr. Bull. 2002, 27, 165–179. [Google Scholar] [CrossRef]
- Radulescu, S.; Brookes, M.J.; Salgueiro, P.; Ridgway, R.A.; McGhee, E.; Anderson, K.; Ford, S.J.; Stones, D.H.; Iqbal, T.H.; Tselepis, C.; et al. Luminal iron levels govern intestinal tumorigenesis after apc loss in vivo. Cell Rep. 2012, 2, 270–282. [Google Scholar]
- Coradini, D.; Pellizzaro, C.; Marimpietri, D.; Abolafio, C.; Daidone, M.G. Sodium butyrate modulates cell cycle-related proteins in HT29 human colonic adenocarcinoma cells. Cell Proliferat. 2000, 33, 139–146. [Google Scholar] [CrossRef]
- Saied, H.T.; Shamsuddin, A.M. Up-regulation of the tumor suppressor gene p53 and WAF1 gene expression by IP6 in HT-29 human colon carcinoma cell line. Anticancer Res. 1998, 18, 1479–1484. [Google Scholar] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Ohkawara, Y.; Bamba, M.; Nakai, I.; Kinka, S.; Masuda, M. Absorption of iron from human large intestine. Gastroenterology 1963, 44, 611–614. [Google Scholar] [PubMed]
- Crichton, R.R. Solution Chemistry of Iron in Biological Media. In Inorganic Biochemistry of Iron Metabolism: From Molecular Mechanisms to Clinical Consequences, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Barampama, Z.; Simard, R.E. Nutrient composition, protein-quality and antinutritional factors of some varieties of dry beans (Phaseolus vulgaris) grown in Burundi. Food Chem. 1993, 47, 159–167. [Google Scholar] [CrossRef]
- Campion, B.; Sparvoli, F.; Doria, E.; Tagliabue, G.; Galasso, I.; Fileppi, M.; Bollini, R.; Nielsen, E. Isolation and characterisation of an lpa (low phytic acid) mutant in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2009, 118, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Afiukwa, C.A.; Igwenyi, I.O.; Ogah, O.; Offor, C.E.; Ugwu, O.O. Variations in seed phytic and oxalic acid contents among nigerian cowpea accessions and their relationship with grain yield. Cont. J. Food Sci. Technol. 2011, 5, 40–48. [Google Scholar]
- Barrier-Giullot, B.; Casado, P.; Maupetit, P.; Jondreville, C.; Gatel, F. Wheat phosphorus availability: 2-in vitro study in broilers and pigs; relationship with endogenous phytasic acitivity and phytic phosphorus content in wheat. J. Sci. Food Agric. 1996, 70, 69–74. [Google Scholar] [CrossRef]
- Panzeri, D.; Cassani, E.; Doria, E.; Tagliabue, E.; Forti, L.; Campion, B.; Bollini, R.; Brearley, C.A.; Pilu, R.; Nielsen, E.; et al. A defective abc transporter of the MRP family, responsible for the bean lpa1 mutation, affects the regulation of the phytic acid pathway, reduces seed myo-inositol and alters aba sensitivity. New Phytol. 2011, 1, 70–83. [Google Scholar]
- Raboy, V. The abcs of low-phytate crops. Nat. Biotechnol. 2007, 25, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Egli, I.; Campion, B.; Nielsen, E.; Hurrell, R. Genetic reduction of phytate in common bean (Phaseolus vulgaris L.) seeds increases iron absorption in young women. J. Nutr. 2013, 143, 1219–1224. [Google Scholar]
- Kristensen, M.B.; Hels, O.; Morberg, C.; Marving, J.; Bugel, S.; Tetens, I. Pork meat increases iron absorption from a 5-day fully controlled diet when compared to a vegetarian diet with similar vitamin C and phytic acid content. Br. J. Nutr. 2005, 94, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Bjornrasmussen, E.; Hallberg, L. Effect of animal proteins on the absorption of food iron in man. Nutr. Metab. 1979, 23, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Boech, S.B.; Hansen, M.; Bukhave, K.; Jensen, M.; Sorensen, S.S.; Kristensen, L.; Purslow, P.P.; Skibsted, L.H.; Sandstrom, B. Nonheme-iron absorption from a phytate-rich meal is increased by the addition of small amounts of pork meat. Am. J. Clin. Nutr. 2003, 77, 173–179. [Google Scholar] [PubMed]
- Layrisse, M.; Martineztorres, C.; Leets, I.; Taylor, P.; Ramirez, J. Effect of histidine, cysteine, glutathione or beef on iron-absorption in humans. J. Nutr. 1984, 114, 217–223. [Google Scholar] [PubMed]
- Taylor, P.G.; Martineztorres, C.; Romano, E.L.; Layrisse, M. The effect of cysteine-containing peptides released during meat digestion on iron-absorption in humans. Am. J. Clin. Nutr. 1986, 43, 68–71. [Google Scholar] [PubMed]
- Martinez-Torres, C.; Romano, E.; Layrisse, M. Effect of cysteine on iron-absorption in man. Am. J. Clin. Nutr. 1981, 34, 322–327. [Google Scholar] [PubMed]
- Layrisse, M.; Martinez, C.; Roche, M. Effect of interaction of various foods on iron absorption. Am. J. Clin. Nutr. 1968, 21, 1175–1183. [Google Scholar] [PubMed]
- Storcksdieck, S.; Bonsmann, G.; Hurrell, R.F. Iron-binding properties, amino acid composition, and structure of muscle tissue peptides from in vitro digestion of different meat sources. J. Food Sci. 2007, 72, S19–S29. [Google Scholar] [CrossRef]
- Laparra, J.M.; Tako, E.; Glahn, R.P.; Miller, D.D. Isolated glycosaminoglycans from cooked haddock enhance nonheme iron uptake by caco-2 cells. J. Agric. Food Chem. 2008, 56, 10346–10351. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Barbera, R.; Alegria, A.; Glahn, R.P.; Miller, D.D. Purified glycosaminoglycans from cooked haddock may enhance fe uptake via endocytosis in a caco-2 cell culture model. J. Food Sci. 2009, 74, H168–H173. [Google Scholar] [CrossRef] [PubMed]
- Bonsmann, S.S.G.; Walczyk, T.; Renggli, S.; Hurrell, R.F. Nonheme iron absorption in young women is not influenced by purified sulfated and unsulfated glycosaminoglycans. J. Nutr. 2007, 137, 1161–1164. [Google Scholar] [PubMed]
- Armah, C.N.; Sharp, P.; Mellon, F.A.; Pariagh, S.; Lund, E.K.; Dainty, J.R.; Teucher, B.; Fairweather-Tait, S.J. l-α-glycerophosphocholine contributes to meat's enhancement of nonheme iron absorption. J. Nutr. 2008, 138, 873–877. [Google Scholar] [PubMed]
- Cook, J.D.; Monsen, E.R. Food iron-absorption in human subjects 0.3. Comparison of effect of animal proteins on nonheme iron-absorption. Am. J. Clin. Nutr. 1976, 29, 859–867. [Google Scholar]
- Monsen, E.R.; Cook, J.D. Food iron-absorption in human-subjects .5. Effects of the major dietary constituents of a semi-synthetic meal. Am. J. Clin. Nutr. 1979, 32, 804–808. [Google Scholar]
- Hurrell, R.F.; Lynch, S.R.; Trinidad, T.P.; Dassenko, S.A.; Cook, J.D. Iron-absorption in humans as influenced by bovine-milk proteins. Am. J. Clin. Nutr. 1989, 49, 546–552. [Google Scholar] [PubMed]
- Cook, J.D.; Morck, T.A.; Lynch, S.R. The inhibitory effect of soy products on non-heme iron-absorption in man. Am. J. Clin. Nutr. 1981, 34, 2622–2629. [Google Scholar] [PubMed]
- Lynch, S.R.; Dassenko, S.A.; Cook, J.D.; Juillerat, M.A.; Hurrell, R.F. Inhibitory effect of a soybean-protein related moiety on iron-absorption in humans. Am. J. Clin. Nutr. 1994, 60, 567–572. [Google Scholar] [PubMed]
- Davidsson, L.; Dimitriou, T.; Walczyk, T.; Hurrell, R. Iron absorption from experimental infant formulas based on pea (Pisum sativum)-protein isolate: The effect of phytic acid and ascorbic acid. Br. J. Nutr. 2001, 85, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, L.; Galan, P.; Kastenmayer, P.; Cherouvrier, F.; Juillerat, M.A.; Hercberg, S.; Hurrell, R.F. Iron bioavailability studied in infants-the influence of phytic acid and ascorbic-acid in infant formulas based on soy isolate. Pediatr. Res. 1994, 36, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Hisayasu, S.; Orimo, H.; Migita, S.; Ikeda, Y.; Satoh, K.; Shinjo, S.; Hirai, Y.; Yoshino, Y. Soybean protein isolate and soybean lectin inhibit iron-absorption in rats. J. Nutr. 1992, 122, 1190–1196. [Google Scholar] [PubMed]
- Evans, R.J.; Bandemer, S.L. Nutritive value of legume seed proteins. J. Agric. Food Chem. 1967, 15, 439–443. [Google Scholar] [CrossRef]
- Mundi, S.; Aluko, R.E. Physicochemical and functional properties of kidney bean albumin and globulin protein fractions. Food Res. Int. 2012, 48, 299–306. [Google Scholar] [CrossRef]
- Montoya, C.A.; Lalles, J.P.; Beebe, S.; Leterme, P. Phaseolin diversity as a possible strategy to improve the nutritional value of common beans (Phaseolus vulgaris). Food Res. Int. 2010, 43, 443–449. [Google Scholar] [CrossRef]
- Carrasco-Castilla, J.; Hernandez-Alvarez, A.J.; Jimenez-Martinez, C.; Jacinto-Hernandez, C.; Alaiz, M.; Giron-Calle, J.; Vioque, J.; Davila-Ortiz, G. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem. 2012, 135, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Castilla, J.; Hernandez-Alvarez, A.J.; Jimenez-Martinez, C.; Jacinto-Hernandez, C.; Alaiz, M.; Giron-Calle, J.; Vioque, J.; Davila-Ortiz, G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. Var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 2012, 131, 1157–1164. [Google Scholar]
- Brown, E.; Hopper, J. Red cell, plasma, and blood volume in the healthy women measured by radiochromium cell-labeling and hematocrit. Clin. Invest. 1962, 41, 2182–2190. [Google Scholar] [CrossRef]
- Consaul, J.R.; Lee, K. Extrinsic tagging in iron bioavailability research: A critical-review. J. Agric. Food Chem. 1983, 31, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Björn-Rasmussen, E.; Hallberg, L.; Walker, R.B. Food iron-absorption in man 0.2. Isotopic-exchange of iron between labeled foods and between a food and an iron salt. Am. J. Clin. Nutr. 1973, 26, 1311–1319. [Google Scholar]
- Layrisse, M.; Martineztorres, C.; Renzi, M.; Velez, F.; Gonzalez, M. Sugar as a vehicle for iron fortification. Am. J. Clin. Nutr. 1976, 29, 8–18. [Google Scholar] [PubMed]
- Cook, J.D.; Dassenko, S.A.; Lynch, S.R. Assessment of the role of nonheme-iron availability in iron balance. Am. J. Clin. Nutr. 1991, 54, 717–722. [Google Scholar] [PubMed]
- Tidehag, P.; Hallmans, G.; Wing, K.; Sjostrom, R.; Agren, G.; Lundin, E.; Zhang, J.X. A comparison of iron absorption from single meals and daily diets using radiofe (Fe-55, Fe-59). Br. J. Nutr 1996, 75, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.R.; Beard, J.L.; Dassenko, S.A.; Cook, J.D. Iron absorption from legumes in humans. Am. J. Clin. Nutr. 1984, 40, 42–47. [Google Scholar] [PubMed]
- Beiseigel, J.M.; Hunt, J.R.; Glahn, R.P.; Welch, R.M.; Menkir, A.; Maziya-Dixon, B.B. Iron bioavailability from maize and beans: A comparison of human measurements with caco-2 cell and algorithm predictions. Am. J. Clin. Nutr. 2007, 86, 388–396. [Google Scholar] [PubMed]
- FAO/WHO. Vitamin and Mineral Requirements in Human Nutrition. FAO/WHO: Geneva, Swizerland, 2004. [Google Scholar]
- Hallberg, L.; Hulthen, L.; Garby, L. Iron stores in man in relation to diet and iron requirements. Eur. J. Clin. Nutr. 1998, 52, 623–631. [Google Scholar] [CrossRef] [PubMed]
- De Moura, F.; Palmer, A.; Finkelstein, J.; Haas, J.; Murray-Kolb, L.; Wenger, M.J.; Birol, E.; Boy, E.; Peña-Rosas, J. Are biofortified staple food crops improving vitamin A and iron status in women and children? New evidence from efficacy trials. Adv. Nutr. 2014, 5, 568–570. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petry, N.; Boy, E.; Wirth, J.P.; Hurrell, R.F. Review: The Potential of the Common Bean (Phaseolus vulgaris) as a Vehicle for Iron Biofortification. Nutrients 2015, 7, 1144-1173. https://doi.org/10.3390/nu7021144
Petry N, Boy E, Wirth JP, Hurrell RF. Review: The Potential of the Common Bean (Phaseolus vulgaris) as a Vehicle for Iron Biofortification. Nutrients. 2015; 7(2):1144-1173. https://doi.org/10.3390/nu7021144
Chicago/Turabian StylePetry, Nicolai, Erick Boy, James P. Wirth, and Richard F. Hurrell. 2015. "Review: The Potential of the Common Bean (Phaseolus vulgaris) as a Vehicle for Iron Biofortification" Nutrients 7, no. 2: 1144-1173. https://doi.org/10.3390/nu7021144





