Hypolipidemic Effect of Tomato Juice in Hamsters in High Cholesterol Diet-Induced Hyperlipidemia
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials, Animals, and Experiment Design
| Nutrition Facts | Content |
|---|---|
| /g KOT (Lyophilized Powder) | |
| Protein | 0.12 g |
| Fat | 0 |
| Saturated fat | 0 |
| Trans fat | 0 |
| Carbohydrate | 0.81 g |
| Sugar | 0.53 g |
| Sodium | 1.19 mg |
| Total calories | 3.47 Kcal |
| Dietary fiber | 0.12 g |
| Lycopene | 1.44 mg |
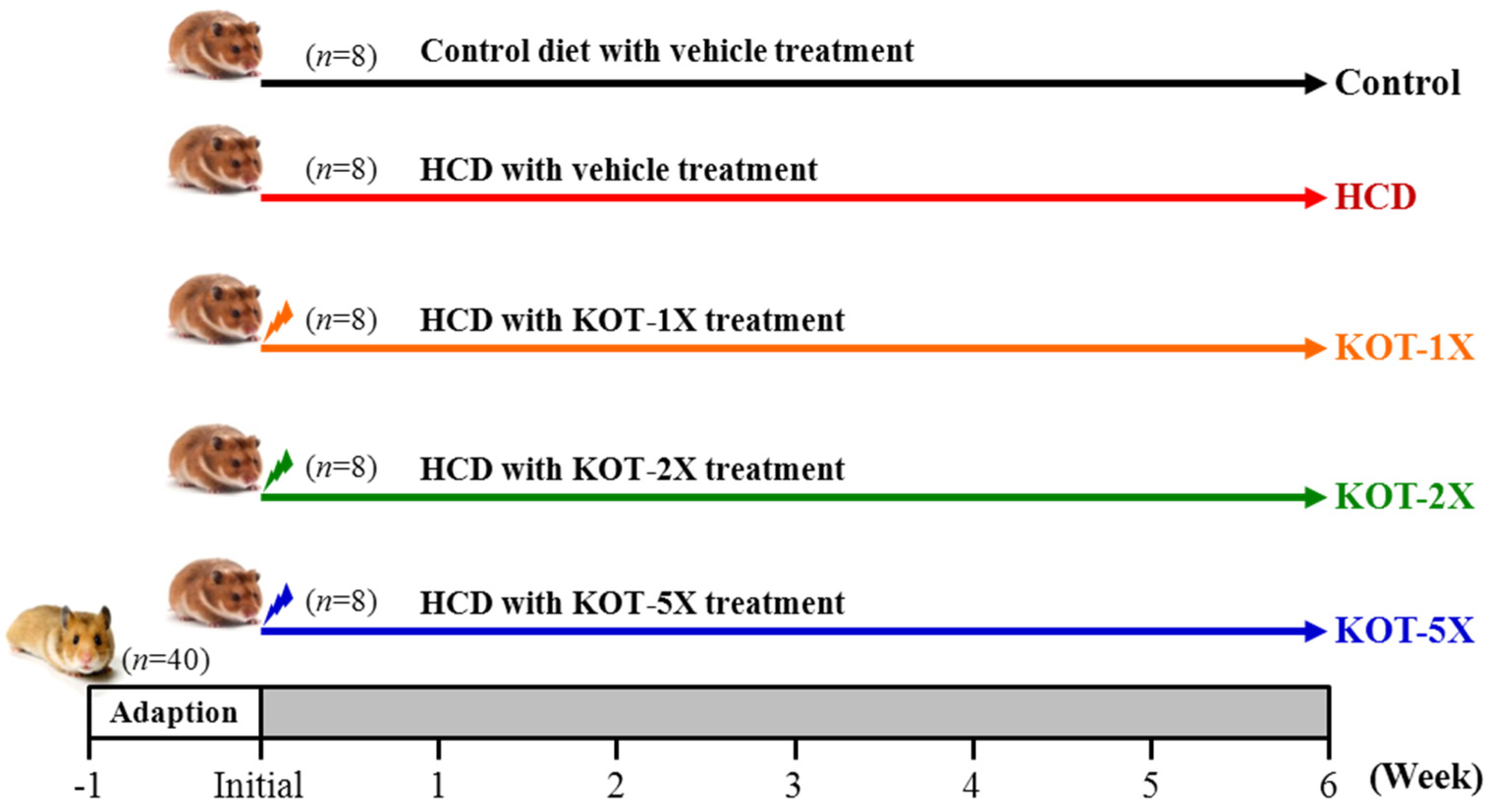
2.2. HCD Composition
2.3. Clinical Biochemical Profiles
2.4. Liver and Fecal Lipid Analysis
2.5. Histological Staining of Tissues
2.6. Statistical Analysis
3. Results and Discussion
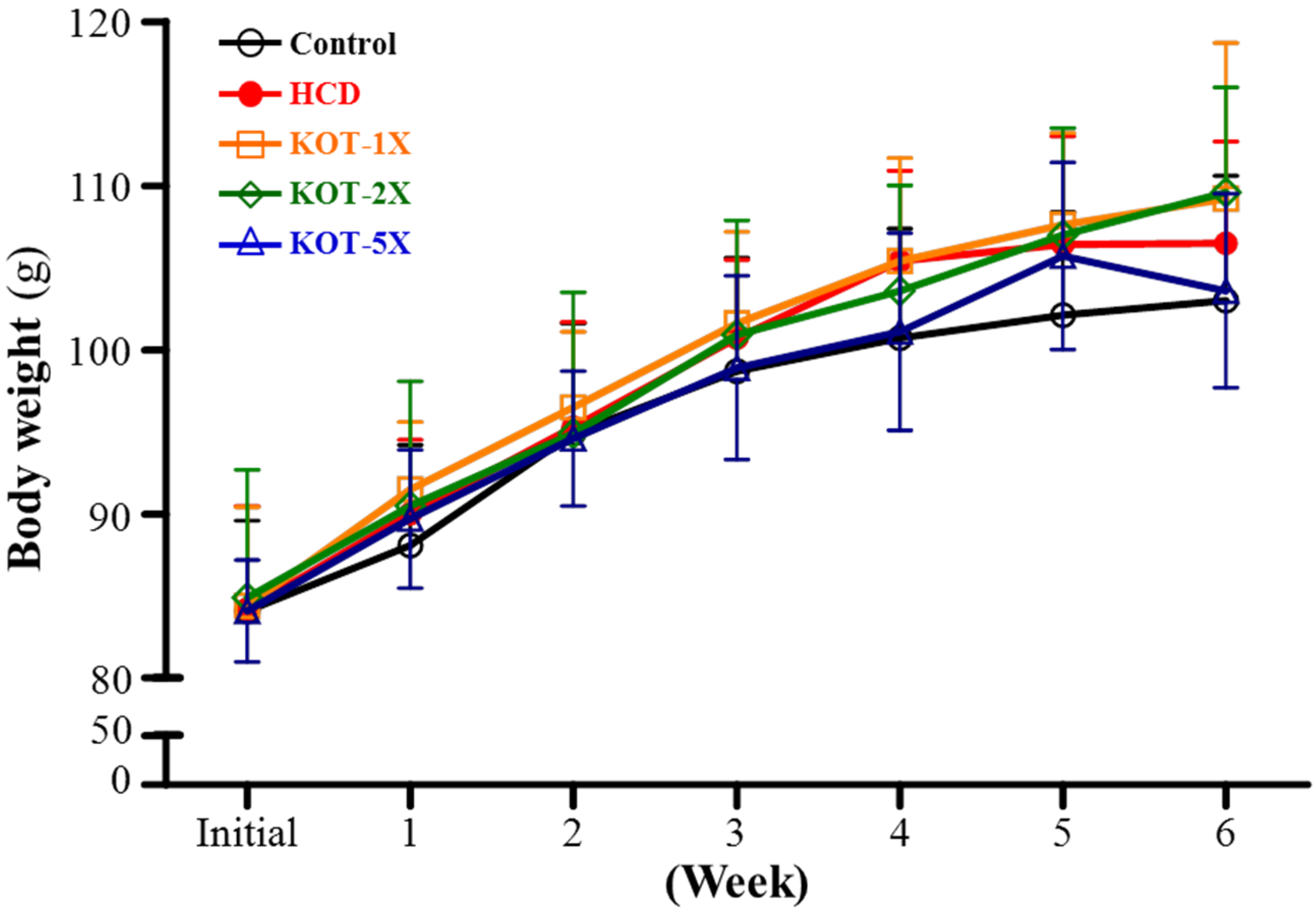
3.1. Hamster BW and Daily Intake
| Characteristics | Control | HCD | KOT-1X | KOT-2X | KOT-5X | Trend Analysis |
|---|---|---|---|---|---|---|
| Initial BW (g) | 84.1 ± 5.5 | 84.2 ± 6.3 | 84.4 ± 6.0 | 84.9 ± 7.8 | 84.1 ± 3.1 | 0.8023 |
| Final BW (g) | 103.0 ± 7.6 | 106.5 ± 6.2 | 109.2 ± 9.5 | 109.6 ± 6.4 | 103.6 ± 5.9 | 0.6573 |
| Diet intake (g/hamster/day) | 8.59 ± 0.57 | 8.40 ± 0.79 | 8.31 ± 0.70 | 8.29 ± 0.73 | 8.21 ± 0.65 | 0.2794 |
| Water intake (g/hamster/day) | 9.35 ± 1.25 c | 8.20 ± 0.80 b | 8.13 ± 0.83 a,b | 7.67 ± 0.66 a,b | 7.25 ± 0.80 a | 0.9522 |
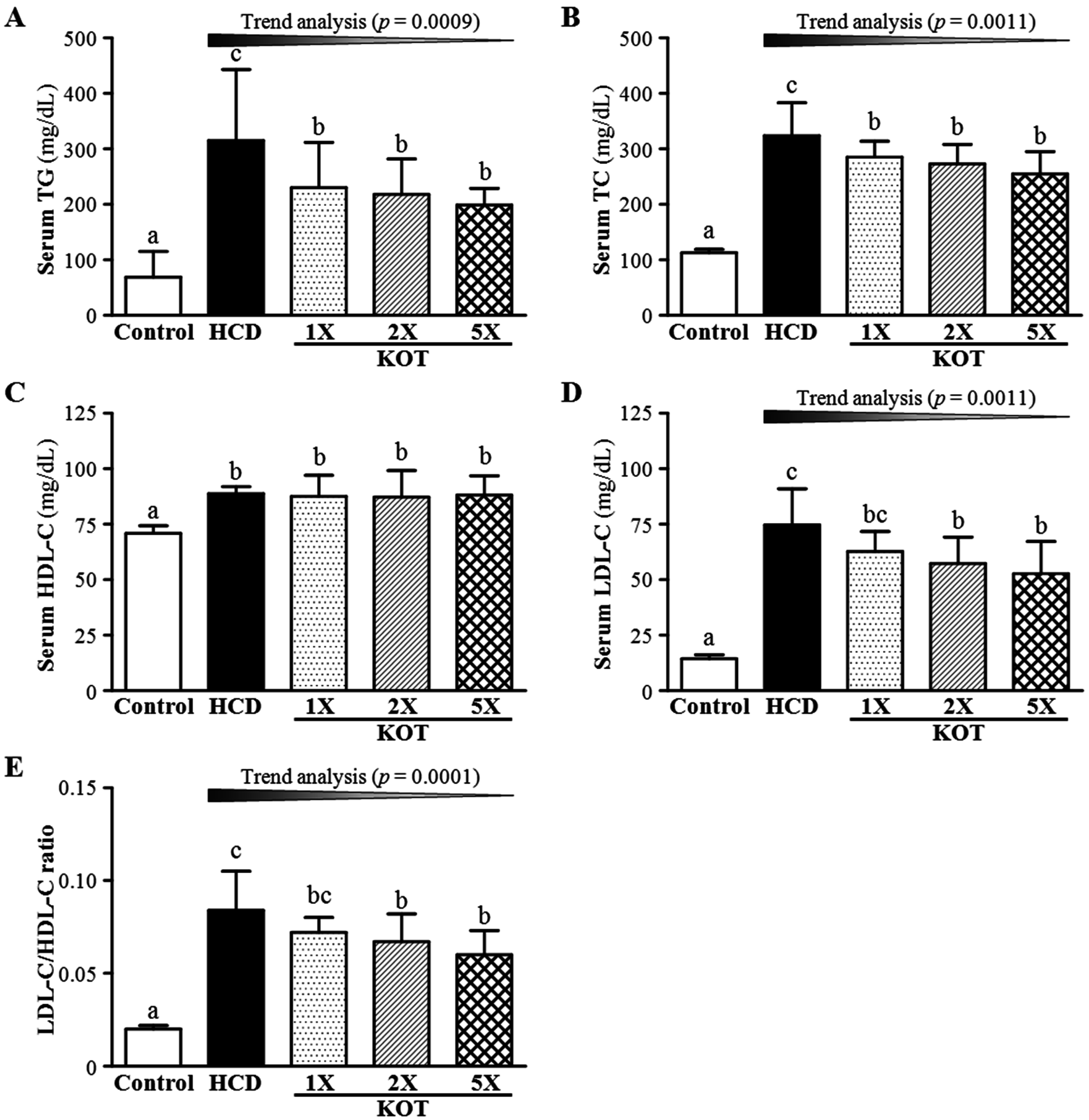
3.2. Effect of Six-Week KOT Supplementation on Serum Lipid Levels and LDL-C/HDL-C Ratio in Hamsters
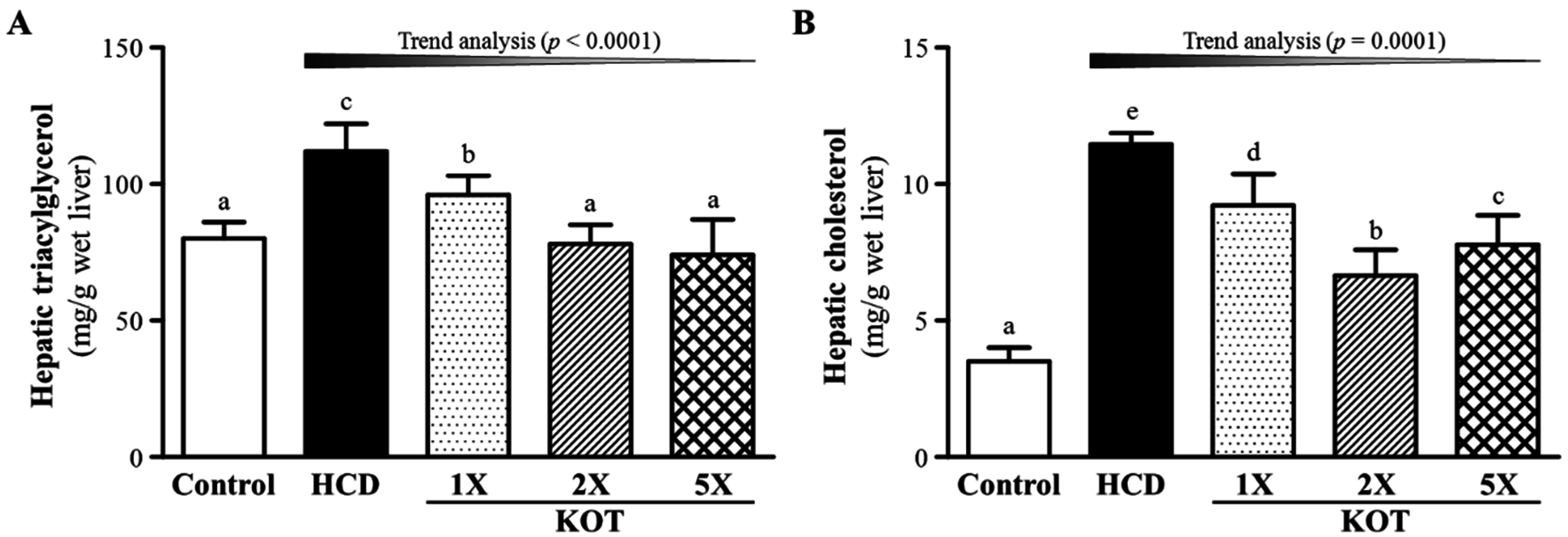
3.3. Effect of Six-Week KOT Supplementation on Hepatic TG and TC Levels in Hyperlipidemic Hamsters
3.4. Effect of Six-Week Supplementation with KOT on Fecal TG and TC Levels in Hyperlipidemic Hamsters
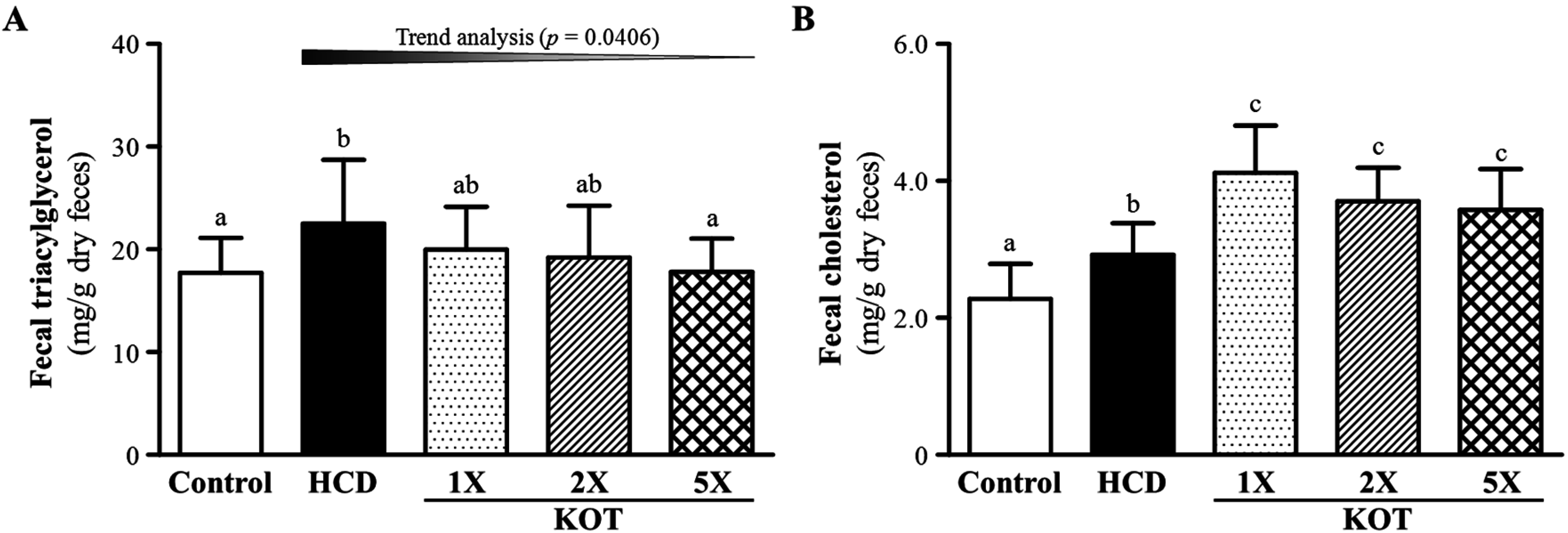
3.5. Effect of KOT Supplementation on Tissue Weight at the End of the Experiment
| Organ Weight | Control | HCD | KOT-1X | KOT-2X | KOT-5X | Trend Analysis |
|---|---|---|---|---|---|---|
| Liver (g) | 2.92 ± 0.21 a | 4.52 ± 0.62 c | 4.22 ± 0.36 b,c | 4.09 ± 0.32 b | 3.89 ± 0.26 b | 0.0004 |
| Kidney (g) | 1.00 ± 0.06 | 1.01 ± 0.06 | 1.04 ± 0.07 | 1.01 ± 0.05 | 0.99 ± 0.04 | 0.3862 |
| Heart (g) | 0.48 ± 0.05 | 0.50 ± 0.04 | 0.47 ± 0.04 | 0.46 ± 0.03 | 0.49 ± 0.05 | 0.1733 |
| EFP (g) | 1.75 ± 0.23 a | 2.42 ± 0.55 b | 2.04 ± 0.23 a | 1.94 ± 0.20 a | 1.78 ± 0.17 a | <0.0001 |
| Relative liver (%) | 2.85 ± 0.32 a | 4.25 ± 0.52 c | 3.89 ± 0.49 b,c | 3.74 ± 0.32 b | 3.76 ± 0.24 b | 0.0172 |
| Relative kidney (%) | 0.98 ± 0.12 | 0.95 ± 0.08 | 0.96 ± 0.08 | 0.92 ± 0.08 | 0.95 ± 0.03 | 0.8067 |
| Relative heart (%) | 0.47 ± 0.06 | 0.47 ± 0.05 | 0.44 ± 0.05 | 0.43 ± 0.05 | 0.47 ± 0.03 | 0.9285 |
| Relative EFP (%) | 1.71 ± 0.27 a | 2.26 ± 0.48 b | 1.88 ± 0.29 a | 1.77 ± 0.14 a | 1.71 ± 0.09 a | 0.0004 |
3.6. Effect of KOT Supplementation on Biochemical Analyses at the End of the Experiment
| Parameters | Control | HCD | KOT-1X | KOT-2X | KOT-5X | Trend Analysis |
|---|---|---|---|---|---|---|
| AST (U/L) | 48 ± 7 a | 62 ± 23 b | 37 ± 4 a | 38 ± 4 a | 37 ± 6 a | 0.0031 |
| ALT (U/L) | 69 ± 9 a | 80 ± 13 b | 63 ± 4 a | 61 ± 7 a | 61 ± 7 a | 0.0006 |
| LDH (U/L) | 151 ± 22 a,b | 155 ± 38 b | 151 ± 31 a,b | 145 ± 21 a,b | 127 ± 15 a | 0.0689 |
| Albumin (g/dL) | 3.2 ± 0.1 | 3.3 ± 0.1 | 3.3 ± 0.1 | 3.2 ± 0.1 | 3.2 ± 0.1 | 0.2660 |
| TP (g/dL) | 5.3 ± 0.1 | 5.4 ± 0.2 | 5.5 ± 0.2 | 5.4 ± 0.1 | 5.4 ± 0.1 | 0.7738 |
| BUN (mg/dL) | 18.3 ± 1.2 | 17.9 ± 1.5 | 17.5 ± 1.4 | 17.3 ± 1.4 | 17.4 ± 2.1 | 0.4757 |
| Creatinine (mg/dL) | 0.16 ± 0.03 | 0.17 ± 0.05 | 0.18 ± 0.01 | 0.16 ± 0.03 | 0.18 ± 0.02 | 0.9734 |
| Glucose (mg/dL) | 157 ± 28 | 157 ± 28 | 162 ± 39 | 158 ± 41 | 168 ± 28 | 0.4517 |
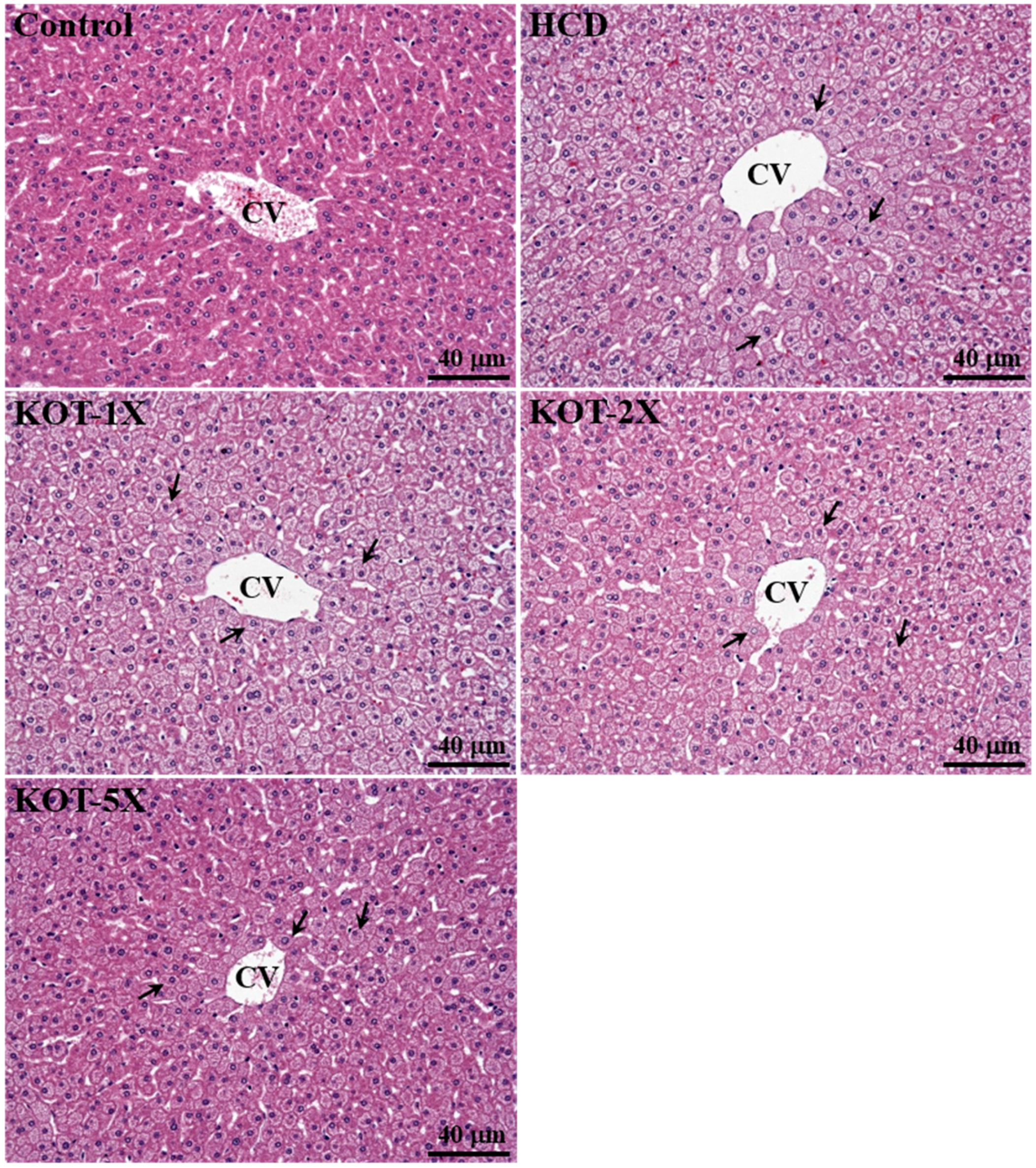
3.7. Effect of KOT Supplementation on Histology at the End of the Experiment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beecher, G.R. Nutrient content of tomatoes and tomato products. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef]
- Rickman, J.C.; Bruhn, C.M.; Barrett, D.M. Nutritional comparison of fresh, frozen, and canned fruits and vegetables II. Vitamin A and carotenoids, vitamin E, minerals and fiber. J. Sci. Food Agric. 2007, 87, 1185–1196. [Google Scholar] [CrossRef]
- Friedman, M. Tomato glycoalkaloids: Role in the plant and in the diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Giovanelli, G.; Paradiso, A. Stability of dried and intermediate moisture tomato pulp during storage. J. Agric. Food Chem. 2002, 50, 7277–7281. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, C.; Ambrosino, P.; Esposito, F.; Fogliano, V. Antioxidative activity and carotenoid and tomatine contents in different typologies of fresh consumption tomatoes. J. Agric. Food Chem. 2000, 48, 4723–4727. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Verheul, M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill.) cultivars. J. Sci. Food Agric. 2009, 89, 1255–1270. [Google Scholar] [CrossRef]
- Takagi, K.; Toyoda, M.; Shimizu, M.; Satoh, T.; Saito, Y. Determination of tomatine in foods by liquid chromatography after derivatization. J. Chromatogr. A 1994, 659, 127–131. [Google Scholar] [CrossRef]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.J.; Cheong, S.H. The effect of fermented milk supplement with tomato (Lycopersion esculentum) on blood lipid profiles in ovariectomy-induced hyperlipidemic female rats. FASEB J. 2007, 21, 1086. [Google Scholar]
- Hsu, Y.M.; Lai, C.H.; Chang, C.Y.; Fan, C.T.; Chen, C.T.; Wu, C.H. Characterizing the lipid- lowering effects and antioxidant mechanisms of tomato paste. Biosci. Biotechnol. Biochem. 2008, 72, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.; Periago, M.J.; Böhm, V.; Berruezo, G.R. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br. J. Nutr. 2008, 99, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Page, D.; van Stratum, E.; Degrou, A.; Renard, C.M.G.C. Kinetics of temperature increase during tomato processing modulate the bioaccessibility of lycopene. Food Chem. 2012, 135, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). USDA National Nutrient Database for Standard Reference, Release 25, 2012. Available online: http://ndb.nal.usda.gov/ndb/search/list (accessed on 23 May 2015).
- Bernal, C.; Martín-Pozuelo, G.; Lozano, A.B.; Sevilla, Á.; García-Alonso, J.; Canovas, M.; Periago, M.J. Lipid biomarkers and metabolic effects of lycopene from tomato juice on liver of rats with induced hepatic steatosis. J. Nutr. Biochem. 2013, 24, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, I.; Pérez-Sánchez, H.; Martín-Pozuelo, G.; García-Alonso, J.; Periago, M.J. The inhibitory effects of bioactive compounds of tomato juice binding to hepatic HMGCR: In vivo study and molecular modelling. PLoS ONE 2014, 9, e83968. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Martínez-Huélamo, M.; Vallverdu-Queralt, A.; Valderas-Martinez, P.; Illán, M.; Sacanella, E.; Escribano, E.; Estruch, R.; Lamuela-Raventos, R.M. Influence of olive oil on carotenoid absorption from tomato juice and effects on postprandial lipemia. Food Chem. 2015, 168, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Colle, I.J.; Lemmens, L.; van Buggenhout, S.; Met, K.; van Loey, A.M.; Hendrickx, M.E. Processing tomato pulp in the presence of lipids: The impact on lycopene bioaccessibility. Food Res. Int. 2013, 51, 32–38. [Google Scholar] [CrossRef]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Schwartz, S.J. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [PubMed]
- Ahuja, K.D.; Pittaway, J.K.; Ball, M.J. Effects of olive oil and tomato lycopene combination on serum lycopene, lipid profile, and lipid oxidation. Nutrition 2006, 22, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Obaaid, M.A.; Sedairy, S.A.; Assaf, A.H.; Zhang, J.J.; Lei, K.Y. Tomato powder is more protective than lycopene supplement against lipid peroxidation in rats. Nutr. Res. 2010, 30, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Jeong, Y.J.; Park, Y.B.; Lee, M.K.; Jeon, S.M.; McGregor, R.A.; Choi, M.S. Dose dependent effects of lycopene enriched tomato-wine on liver and adipose tissue in high-fat diet fed rats. Food Chem. 2012, 130, 42–48. [Google Scholar] [CrossRef]
- Kim, Y.I.; Hirai, S.; Goto, T.; Ohyane, C.; Takahashi, H.; Tsugane, T.; Kawada, T. Potent PPARα activator derived from tomato juice, 13-oxo-9, 11-octadecadienoic acid, decreases plasma and hepatic triglyceride in obese diabetic mice. PLoS ONE 2012, 7, e31317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sima, A.; Bulla, A.; Simionescu, N. Experimental obstructive coronary atherosclerosis in the hyperlipidemic hamster. J. Submicrosc. Cytol. Pathol. 1990, 22, 1–16. [Google Scholar] [PubMed]
- Sullivan, M.P.; Cerda, J.J.; Robbins, F.L.; Burgin, C.W. The gerbil, hamster, and guinea pig as rodent models for hyperlipidemia. Lab. Anim. Sci. 1993, 43, 575–578. [Google Scholar] [PubMed]
- Stancu, C.S.; Sanda, G.M.; Deleanu, M.; Sima, A.V. Probiotics determine hypolipidemic and antioxidant effects in hyperlipidemic hamsters. Mol. Nutr. Food Res. 2014, 58, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chen, Y.M.; Kan, N.W.; Ho, C.S.; Wei, L.; Chan, C.H.; Huang, C.C. Hypolipidemic effects and safety of Lactobacillus reuteri 263 in a hamster model of hyperlipidemia. Nutrients 2015, 7, 3767–3782. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Hsu, Y.J.; Chen, Y.M.; Huang, W.C.; Huang, C.C.; Hsu, M.C. Effects of combined extract of cocoa, coffee, green tea and garcinia on lipid profiles, glycaemic markers and inflammatory responses in hamsters. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Simonen, P. Phytosterols, phytostanols, and lipoprotein metabolism. Nutrients 2015, 7, 7965–7977. [Google Scholar] [CrossRef] [PubMed]
- Go, G.-W. Low-Density Lipoprotein Receptor-Related Protein 6 (LRP6) is a novel nutritional therapeutic target for hyperlipidemia, non-alcoholic fatty liver disease, and atherosclerosis. Nutrients 2015, 7, 4453–4464. [Google Scholar] [CrossRef] [PubMed]
- Ooi, E.M.; Watts, G.F.; Ng, T.W.; Barrett, P.H.R. Effect of dietary fatty acids on human lipoprotein metabolism: A comprehensive update. Nutrients 2015, 7, 4416–4425. [Google Scholar] [CrossRef] [PubMed]
- Boekholdt, S.M.; Arsenault, B.J.; Mora, S.; Pedersen, T.R.; LaRosa, J.C.; Nestel, P.J.; Kastelein, J.J. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: A meta-analysis. JAMA 2012, 307, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kennedy, D.J.; Ramakrishnan, D.P.; Yang, M.; Huang, W.; Li, Z.; Silverstein, R.L. Oxidized LDL-bound CD36 recruits an Na+/K+-ATPase-Lyn complex in macrophages that promotes atherosclerosis. Sci. Signal. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Tamada, M.; Makita, S.; Abiko, A.; Naganuma, Y.; Nagai, M.; Nakamura, M. Low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a useful marker for early-stage carotid atherosclerosis. Metabolism 2010, 59, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.M.; Zhang, D.W. Hypercholesterolemia, low density lipoprotein receptor and proprotein convertase subtilisin/kexin-type 9. J. Biomed. Res. 2015, 29, 356–361. [Google Scholar] [PubMed]
- Friedman, M.; Fitch, T.E.; Yokoyama, W.E. Lowering of plasma LDL cholesterol in hamsters by the tomato glycoalkaloid tomatine. Food Chem. Toxicol. 2000, 38, 549–553. [Google Scholar] [CrossRef]
- Enomoto, M.; Adachi, H.; Hirai, Y.; Fukami, A.; Satoh, A.; Otsuka, M.; Imaizumi, T. LDL-C/HDL-C ratio predicts carotid intima-media thickness progression better than HDL-C or LDL-C alone. J. Lipids 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sun, Z.; Li, Y.; Ai, J.; Sun, Q.; Tian, Y. The correlation between serum lipid profile with carotid intima-media thickness and plaque. BMC Cardiovasc. Disord. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kamakari, K.; Goto, T.; Hara, H.; Mohri, S.; Suzuki, H.; Shibata, D.; Nakata, R.; Inoue, H.; Takahashi, N.; et al. 9-Oxo-10(E),12(Z),15(Z)-octadecatrienoic acid activates peroxisome proliferator-activated receptor α in Hepatocytes. Lipids 2015, 50, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Hara, H.; Goto, T.; Kamakari, K.; Wataru, N.; Mohri, S.; Takahashi, N.; Suzuki, H.; Shibata, D.; Kawada, T. 13-Oxo-9(Z),11(E),15(Z)-octadecatrienoic acid activates peroxisome proliferator-activated receptor γ in adipocytes. Lipids 2015, 50, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dvir, I.; Stark, A.H.; Chayoth, R.; Madar, Z.; Arad, S.M. Hypocholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp in rats. Nutrients 2009, 1, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Jensen, M.G.; Aarestrup, J.; Petersen, K.E.; Søndergaard, L.; Mikkelsen, M.S.; Astrup, A. Flaxseed dietary fibers lower cholesterol and increase fecal fat excretion, but magnitude of effect depend on food type. Nutr. Metab. 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Mizranita, V.; Pratisto, E.H. Statin-associated ocular disorders: The FDA and ADRAC data. Int. J. Clin. Pharm. 2015, 37, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Gilat, T.; Leikin-Frenkel, A.; Goldiner, I.; Juhel, C.; Lafont, H.; Gobbi, D.; Konikoff, F.M. Prevention of diet-induced fatty liver in experimental animals by the oral administration of a fatty acid bile acid conjugate (FABAC). Hepatology 2003, 38, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Kiyota, N.; Tsurushima, K.; Yoshitomi, M.; Horlad, H.; Ikeda, T.; Nagai, R. Tomatidine, a tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in apoE-deficient mice by inhibiting acyl-CoA: Cholesterol acyl-transferase (ACAT). J. Agric. Food Chem. 2012, 60, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Rudel, L.L.; Lee, R.G.; Cockman, T.L. Acyl coenzyme A: Cholesterol acyltransferase types 1 and 2: Structure and function in atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 121–127. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.-C.; Wei, L.; Huang, W.-C.; Hsu, Y.-J.; Chen, Y.-M.; Huang, C.-C. Hypolipidemic Effect of Tomato Juice in Hamsters in High Cholesterol Diet-Induced Hyperlipidemia. Nutrients 2015, 7, 10525-10537. https://doi.org/10.3390/nu7125552
Lee L-C, Wei L, Huang W-C, Hsu Y-J, Chen Y-M, Huang C-C. Hypolipidemic Effect of Tomato Juice in Hamsters in High Cholesterol Diet-Induced Hyperlipidemia. Nutrients. 2015; 7(12):10525-10537. https://doi.org/10.3390/nu7125552
Chicago/Turabian StyleLee, Li-Chen, Li Wei, Wen-Ching Huang, Yi-Ju Hsu, Yi-Ming Chen, and Chi-Chang Huang. 2015. "Hypolipidemic Effect of Tomato Juice in Hamsters in High Cholesterol Diet-Induced Hyperlipidemia" Nutrients 7, no. 12: 10525-10537. https://doi.org/10.3390/nu7125552






