The Nutraceutical Properties of Ovotransferrin and Its Potential Utilization as a Functional Food
Abstract
:1. Introduction
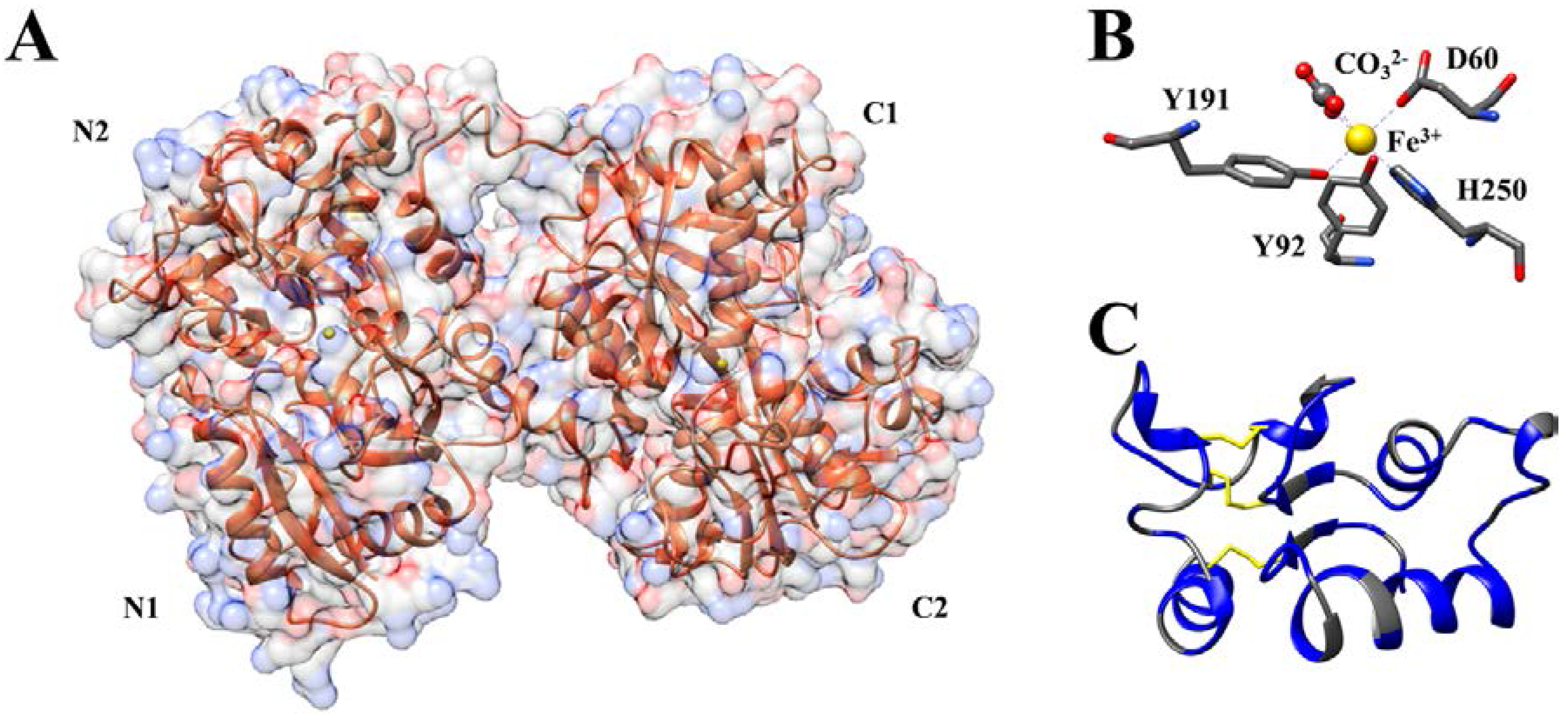
2. Ovotransferrin Synthesis and Structure
3. Antibacterial Activity of Ovotransferrin and Its Peptides
4. Antiviral Activity of Ovotransferrin and Its Peptides
5. Antioxidant Activity of Ovotransferrin and Its Peptides
6. Anti-Inflammatory Activities of Ovotransferrin and Its Peptides
7. Other Protective Activities of Ovotransferrin and Its Peptides
| PROTEIN OR PEPTIDE | ACTIVITY | MECHANISM/S | REFERENCES |
|---|---|---|---|
| Ovotransferrin (whole molecule) | ANTIMICROBIAL | ||
| BACTERIAL SENSITIVITY (BACTERIOSTATIC) |
| [25,27,30,32] | |
| BACTERIAL SENSITIVITY (BACTERICIDAL) |
| [7,25,32] | |
| BACTERIAL RESISTANCE |
| [33] | |
| ANTIBACTERIAL (FOOD PRESERVATIVE) |
| [43] | |
| ANTIVIRAL |
| [45] | |
| ANTIOXIDANT |
| [49,50] | |
| FLOGOSIS MARKER |
| [61,63,64,66] | |
| IMMUNE/ANTI-INFLAMMATION |
| [49,63,64] | |
| PROTEOLYTIC |
| [80] | |
| GROWTH FACTOR: | |||
| CARTILAGE NEOVASCULARIZATION |
| [59] | |
| CHONDROGENESIS AND OSTEOGNESIS REGULATION |
| [77,78] | |
| MYOTROPHIC |
| [74] | |
| NEUROTROPHYC |
| [79] | |
| CARRIER FOR DRUG DELIVERY |
| [83] | |
| Otrf peptide OTAP-92 | ANTIMICROBIAL |
| [41] |
| Otrf peptides219–227; 269–301; 633–638 | ANTIVIRAL |
| [46] |
| Reduced autocleaved Otrf (rac-Otrf) | ANTICANCER/ANTIPROLIFERATION |
| [71] |
| ANTIOXIDANT |
| [55] | |
| Otrf Peptides (IRW or IQW) | ANTINFLAMMATORY |
| [68] |
| Otrf peptide (KVREGT) | ANTIHYPERTENSIVE |
| [72,73] |
| Otrf Peptides (DLLFKDSAIMLK) (FFSASCVPGATIE) | ANTIOXIDANT |
| [54] |
| Otrf peptides (mix obtained using: protamex, or alkalase, or trypsin, or α-chymotrypsin) | ANTIOXIDANT |
| [52,53] |
8. Conclusions
Author Contributions
Conflicts of Interests
References
- Giansanti, F.; Leboffe, L.; Pitari, G.; Ippoliti, R.; Antonini, G. Physiological roles of ovotransferrin. Biochim. Biophys. Acta 2012, 1820, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Deeming, D.C. Behavior patterns during incubation. In Avian Incubation: Behaviour, Environment, and Evolution; Deeming, D.C., Ed.; Oxford University Press: Oxford, UK, 2002; pp. 63–87. [Google Scholar]
- Saxena, I.; Tayyab, S. Protein proteinase inhibitors from avian egg whites. Cell. Mol. Life Sci. 1997, 53, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Wesierska, E.; Saleh, Y.; Trziska, T.; Kopec, W.; Sierwinski, M.; Korzekwa, K. Antimicrobial activity of chicken egg white cystatin. World J. Microbiol. Biotechnol. 2005, 21, 59–64. [Google Scholar] [CrossRef]
- Miyagawa, S.; Matsumaoto, K.; Kamata, R.; Okamura, R.; Maeda, H. Spreading of Serratia marcescens in experimental keratitis and growth suppression by chicken egg white ovomacroglobulin. Jpn. J. Ophthalmol. 1991, 35, 402–410. [Google Scholar] [PubMed]
- Board, P.A.; Fuller, R. Non-specific antimicrobial defences of the avian egg, embryo and neonate. Biol. Rev. Camb. Philos. Soc. 1974, 49, 15–49. [Google Scholar] [CrossRef] [PubMed]
- Wellman-Labadie, O.; Picman, J.; Hinke, M.T. Comparative antibacterial activity of avian egg white protein extracts. Br. Poult. Sci. 2008, 49, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Sieber, R. Bioactive proteins and peptides in foods. Int. J. Vitam. Nutr. Res. 2011, 81, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, J.M.; Chambon, P. The complete nucleotide sequence of the chicken ovotransferrin mRNA. Eur. J. Biochem. 1982, 122, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Elleman, T.C.; Kingston, I.B.; Wilkins, A.G.; Kuhn, K.A. The primary structure of hen ovotransferrin. Eur. J. Biochem. 1982, 122, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.L.; Geynet, C.; Binart, N.; Catelli, M.G.; Schmelck, P.H.; Mester, J.; Lebeau, M.C.; Baulieu, E.E. Steroid receptors and effects of oestradiol and progesterone on chick oviduct proteins. Eur. J. Biochem. 1980, 107, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Dierich, A.; Gaub, M.P.; LePennec, J.P.; Astinotti, D.; Chambon, P. Cell-specificity of the chicken ovalbumin and conalbumin promoters. EMBO J. 1987, 6, 2305–2312. [Google Scholar] [PubMed]
- Williams, J. A comparison of glycopeptides from the ovotransferrin and serum transferrin of the hen. Biochem. J. 1968, 108, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Iwase, H.; Hotta, K. Ovotransferrin subfractionation dependent upon chain differences. J. Biol. Chem. 1977, 252, 5437–5443. [Google Scholar] [PubMed]
- Jacquinot, P.M.; Leger, D.; Wieruszeski, J.M.; Coddeville, B.; Montreuil, J.; Spik, G. Change in glycosylation of chicken transferrin glycans biosynthesized during embryogenesis and primary culture of embryo hepatocytes. Glycobiology 1994, 4, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Mikami, B.; Hirose, M. Crystal structure of diferric hen ovotransferrin at 2.4 Å resolution. J. Mol. Biol. 1995, 254, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Dewan, J.C.; Mikami, B.; Sacchettini, J.C.; Hirose, M. Crystal structure of hen apo-ovotransferrin: Both lobes adopt an open conformation upon loss of iron. J. Biol. Chem. 1999, 274, 28445–28452. [Google Scholar] [CrossRef] [PubMed]
- Thakurta, G.P.; Choudhury, D.; Dasgupta, R.; Dattagupta, J.K. Structure of diferric hen serum transferrin at 2.8 Å resolution. Acta Crystallogr. Sect. D 2003, 59, 1773–1781. [Google Scholar] [CrossRef]
- Williams, J. The evolution of transferrins. Trends Biochem. Sci. 1982, 7, 394–397. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Moreton, K.; Goodearl, A.D. Selective reduction of a disulphide bridge in hen ovotransferrin. Biochem. J. 1985, 228, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Yamashita, H.; Kurokawa, H.; Mikami, B.; Mikami, B. Alternative structural state of transferrin. The crystallographic analysis of iron-loaded but domain-opened ovotransferrin N-lobe. J. Biol. Chem. 1999, 274, 10190–10194. [Google Scholar] [CrossRef] [PubMed]
- Lindley, P.F.; Bajaj, M.; Evans, R.W.; Garatt, R.C.; Hasnain, S.S.; Jhoti, H.; Kuser, P.; Neu, M.; Patel, K.; Sarra, R.; et al. The mechanism of iron uptake by transferrins: The structure of an 18 kDa NII-domain fragment from duck ovotransferrin at 2.3 Å resolution. Acta Crystallogr. Sect. D 1993, 49, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Kuser, P.; Hall, D.R.; Haw, M.L.; Neu, M.; Evans, R.W.; Lindley, P.F. The mechanism of iron uptake by transferrins: The X-ray structures of the 18 kDa NII domain fragment of duck ovotransferrin and its nitrilotriacetate complex. Acta Crystallogr. Sect. D 2002, 58, 777–783. [Google Scholar] [CrossRef]
- Alderton, G.; Ward, W.H.; Fevold, H.L. Identification of the bacteria-inhibiting iron-binding protein of egg white as conalbumin. Arch. Biochem. 1946, 11, 9–13. [Google Scholar] [PubMed]
- Bullen, J.J.; Rogers, H.J.; Griffiths, E. Role of iron in bacterial infection. Curr. Top. Microbiol. Immunol. 1978, 80, 1–35. [Google Scholar] [PubMed]
- Antonini, E.; Orsi, N.; Valenti, P. Effetto delle transferrine sulla patogenicità delle Enterobacteriaceae. G. Mal. Infett. Parassit. 1977, 29, 481–489. [Google Scholar]
- Valenti, P.; de Stasio, A.; Mastromarino, P.; Seganti, L.; Sinibaldi, L.; Orsi, N. Influence of bicarbonate and citrate on the bacteriostatic action of ovotransferrin towards staphylococci. FEMS Microbiol. Lett. 1981, 10, 77–79. [Google Scholar] [CrossRef]
- MacGillivray, R.T.; Moore, S.A.; Chen, J.; Anderson, B.F.; Baker, H.; Luo, Y.; Bewley, M.; Smith, C.A.; Murphy, M.E.; Wang, Y.; et al. Two high-resolution crystal structures of the recombinant N-lobe of human transferrin reveal a structural change implicated in iron release. Biochemistry 1998, 37, 7919–7928. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Antonini, G.; Fanelli, M.R.; Orsi, N.; Antonini, E. Antibacterial activity of matrixbound ovotransferrin. Antimicrob. Agents Chemother. 1982, 21, 840–841. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.Y.; Mendonca, A.F.; Ahn, D.U. Effect of ethylenediaminetetraacetate and lysozyme on the antimicrobial activity of ovotransferrin against Listeria monocytogenes. Poult. Sci. 2008, 87, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Antonini, G.; von Hunolstein, C.; Visca, P.; Orsi, N.; Antonini, E. Studies of the antimicrobial activity of ovotransferrin. Int. J. Tissue React. 1983, 5, 97–105. [Google Scholar] [PubMed]
- Alcantara, J.; Schryvers, A.B. Transferrin binding protein two interacts with both the N-lobe and C-lobe of ovotransferrin. Microb. Pathog. 1996, 20, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Visca, P.; Antonini, G.; Orsi, N. Antifungal activity of ovotransferrin toward genus Candida. Mycopathologia 1985, 89, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, O.; Quiros, L.M.; Fierro, J.F. Transferrins selectively cause ion efflux through bacterial and artificial membranes. FEBS Lett. 2003, 548, 5–10. [Google Scholar] [CrossRef]
- Superti, F.; Ammendolia, M.G.; Berlutti, F.; Valenti, P. Ovotransferrin. In Bioactive Egg Compounds; Huopalahti, R., Lopez-Fandino, R., Eds.; Springer-Verlag: Berlin, Germany, 2007; pp. 43–48. [Google Scholar]
- Zhou, Z.R.; Smith, D.L. Assignment of disulfide bonds in proteins by partial acid hydrolysis and mass spectrometry. J. Protein Chem. 1990, 9, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Strahilevitz, J.; Mor, A.; Nicolas, P.; Shai, Y. Spectrum of antimicrobial activity and assembly of dermaseptin-β and its precursor form in phospholipid membranes. Biochemistry 1994, 33, 10951–10960. [Google Scholar] [CrossRef] [PubMed]
- Ehret-Sabatier, L.; Loew, D.; Goyffon, M.; Fehlbaum, P.; Hoffmann, J.A.; Dorsselaer, A.V.; Bulet, P. Characterization of novel cysteine-rich antimicrobial peptides from scorpion blood. J. Biol. Chem. 1996, 271, 29537–29544. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Iwamori, E.; Sugimoto, Y.; Aoki, T. Identification of a distinct antibacterial domain within the N-lobe of Ovotransferrin. Biochim. Biophys. Acta 1998, 1401, 289–303. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Sugimoto, Y.; Aoki, T. Ovotransferrin antimicrobial peptide (OTAP-92) kill bacteria through a membrane damage mechanism. Biochim. Biophys. Acta 2000, 1523, 196–205. [Google Scholar] [CrossRef]
- Galvez, A.; Gimenez-Gallego, G.; Reuben, J.P.; Roy-Contancin, L.; Feigenbaum, P.; Kaczorowski, G.J.; Garcia, M.L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J. Biol. Chem. 1990, 265, 11083–11090. [Google Scholar] [PubMed]
- Ko, K.Y.; Mendoncam, A.F.; Ismail, H.; Ahn, D.U. Ethylenediaminetetraacetate and lysozyme improves antimicrobial activities of ovotransferrin against Escherichia coli O157:H7. Poult. Sci. 2009, 88, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Seol, K.H.; Lim, D.G.; Jang, A.; Jo, C.; Lee, M. Antimicrobial effect of kappa carrageenan-based edible film containing ovotransferrin in fresh chicken breast stored at 5 °C. Meat Sci. 2009, 83, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Rossi, P.; Massucci, M.T.; Botti, D.; Antonini, G.; Valenti, P.; Seganti, L. Antiviral activity of ovotransferrin discloses an evolutionary strategy for the defensive activities of lactoferrin. Biochem. Cell Biol. 2002, 80, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Massucci, M.T.; Giardi, M.F.; Nozza, F.; Pulsinelli, E.; Nicolini, C.; Botti, D.; Antonini, G. Antiviral activity of ovotransferrin derived peptides. Biochem. Biophys. Res. Commun. 2005, 331, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, R.; Rega, B.; Marchetti, M.; Seganti, L.; Antonini, G.; Valenti, P. Bovine lactoferrin peptidic fragments involved in inhibition of Herpes simplex virus type 1 infection. Biochem. Biophys. Res. Commun. 1999, 264, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Haraguchi, T.; Aoki, T. Ovotransferrin is a redox-dependent autoprocessing protein incorporating four consensus self-cleaving motifs flanking the two kringles. Biochim. Biophys. Acta 2006, 1760, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Hoq, M.I.; Aoki, T. Ovotransferrin possesses SOD-like superoxide anion scavenging activity that is promoted by copper and manganese binding. Int. J. Biol. Macromol. 2007, 41, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Majumder, K.; Wu, J. Oxygen radical absorbance capacity of peptides from egg white protein ovotransferrin and their interaction with phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Kim, J.; Moon, S.H.; Ahn, D.U.; Paik, H.D.; Park, E. Antioxidant effects of ovotransferrin and its hydrolysates. Poult. Sci. 2012, 91, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Shen, S.; Nimalaratne, C.; Li, S.; Majumder, K.; Wu, J. Effects of addition of egg ovotransferrin-derived peptides on the oxygen Radical absorbance capacity of different teas. Food Chem. 2012, 135, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Lee, J.H.; Lee, Y.J.; Chang, K.H.; Paik, J.Y.; Ahn, D.U.; Paik, H.D. Screening for cytotoxic activity of ovotransferrin and its enzyme hydrolysates. Poult. Sci. 2013, 92, 424–434. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Luo, Y.; Wu, J. Conjugation of ovotransferrin with catechin shows improved antioxidant activity. J. Agric. Food Chem. 2014, 62, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Lee, J.H.; Ahnb, D.U.; Paika, H.D. In vitro antioxidant and mineral-chelating properties of natural and autocleaved ovotransferrin. J. Sci. Food Agric. 2015, 95, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.W.; Sofer, L.; Anderson, A.S.; Berneberg, E.L.; Cui, J.; Burnside, J. Induction of host gene expression following infection of chicken embryo fibroblasts with oncogenic Marek’s disease virus. J. Virol. 2001, 75, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Kushner, I.; Mackiewicz, A. The acute phase response: An overview. In Acute Phase Proteins: Molecular Biology, Biochemistry, and Clinical Applications; Kushner, I., Baumann, H., Mackiewicz, A., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 3–19. [Google Scholar]
- Gabay, C.; Kushner, I. Acute phase proteins and other systemic response to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [PubMed]
- Carlevaro, M.F.; Albini, A.; Ribatti, D.; Gentili, C.; Benelli, R.; Cermelli, S.; Cancedda, R.; Cancedda, F.D. Transferrin promotes endothelial cell migration and invasion: Implication in cartilage neovascularisation. J. Cell Biol. 1997, 136, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Hallquist, N.A.; Klasing, K.C. Serotransferrin, ovotransferrin and metallothionein levels during an immune response in chickens. Comp. Biochem. Physiol. B 1994, 108, 375–384. [Google Scholar] [CrossRef]
- Tohjo, H.; Miyoshi, F.; Uchida, E.; Niiyama, M. Polyacrylamide gel electrophoretic patterns of chicken serum in acute inflammation induced by intramuscular injection of turpentine. Poult. Sci. 1995, 74, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Chamanza, R.; Toussaint, M.J.M.; van Ederen, A.M.; van Veen, L.; Hulskamp-Koch, C. Serum amyloid A and transferrin in chicken. A preliminary investigation of using acute phase variables to assess diseases in chickens. Vet. Q. 1999, 21, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Huff, G.R.; Huff, W.E.; Balog, J.M.; Rath, N.C. Effects of ovotransferrin on chicken macrophages and heterophil-granulocytes. Dev. Comp. Immunol. 2002, 26, 805–815. [Google Scholar] [CrossRef]
- Xie, H.; Huff, G.R.; Huff, W.E.; Balog, J.M.; Holt, P.; Rath, N.C. Identification of ovotransferrin as an acute phase protein in chickens. Poult. Sci. 2002, 81, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Rath, N.C.; Xie, H.; Huff, W.E.; Huff, G.R. Avian acute phase protein ovotransferrin modulates phagocyte function. In New Immunology Research Development; Muller, G.V., Ed.; Nova Science Publishers: New York, NY, USA, 2008; pp. 95–108. [Google Scholar]
- Rath, N.C.; Anthony, N.B.; Kannan, L.; Huff, W.E.; Huff, G.R.; Chapman, H.D.; Erf, G.F.; Wakenell, P. Serum ovotransferrin as a biomarker of inflammatory diseases in chickens. Poult. Sci. 2009, 88, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Giardi, M.F.; Massucci, M.T.; Botti, D.; Antonini, G. Ovotransferrin expression and release by chicken cell lines infected with Marek’s disease virus. Biochem. Cell Biol. 2007, 85, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Structure and activity study of egg protein ovotransferrin derived peptides (IRW and IQW) on endothelial inflammatory response and oxidative stress. J. Agric. Food Chem. 2013, 61, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Phillips, M.; Mine, Y. Advances in the value of eggs and egg components for human health. J. Agric. Food Chem. 2005, 53, 8421–8431. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Kiyono, T. Novel anticancer activity of the autocleaved ovotransferrin against human colon and breast cancer cells. J. Agric. Food Chem. 2009, 57, 11383–11390. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Cheng, J.T.; Enomoto, T.; Nakano, Y. One peptide derived from hen ovotransferrin as pro-drug to inhibit angiotensin converting enzyme. J. Food Drug Anal. 2006, 14, 31–35. [Google Scholar] [CrossRef]
- Wu, J.; Acero-Lopez, A. Ovotransferrin: Structure, bioactivities and preparation. Food Res. Int. 2012, 46, 480–487. [Google Scholar] [CrossRef]
- Shimo-Oka, T.; Hagiwara, Y.; Ozawa, E. Class specificity of transferrin as a muscle trophic factor. J. Cell. Physiol. 1986, 126, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.F.; Connor, J.R. Functional roles of transferrin in the brain. Biochim. Biophys. Acta 2012, 1820, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.H.; Shin, H.Y.; Kim, J.W.; Park, S.H.; Son, J.H.; Kim, D.G. Primary culture of central neurocytoma: A case report. J. Korean Med. Sci. 2010, 25, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Castagnola, P.; Cancedda, F.D.; Dozin, B.; Quarto, R. Developmental control of chondrogenesis and osteogenesis. Int. J. Dev. Biol. 2000, 44, 707–714. [Google Scholar] [PubMed]
- Gentili, C.; Doliana, R.; Bet, P.; Campanile, G.; Colombatti, A.; Cancedda, F.D.; Cancedda, R. Ovotransferrin and ovotransferrin receptor expression during chondrogenesis and endochondral bone formation in developing chick embryo. J. Cell Biol. 1994, 124, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bruinink, A.; Sidler, C.; Birchler, F. Neurotrophic effects of transferrin on embryonic chick brain and neural retinal cell cultures, Int. J. Dev. Neurosci. 1996, 14, 785–795. [Google Scholar] [CrossRef]
- Leboffe, L.; Giansanti, F.; Antonini, G. Antifungal and antiparasitic activities of lactoferrin. Anti-Infect. Agents Med. Chem. 2009, 8, 114–127. [Google Scholar] [CrossRef]
- Massucci, M.T.; Giansanti, F.; di Nino, G.; Turacchio, M.; Giardi, M.F.; Botti, D.; Ippoliti, R.; de Giulio, B.; Siciliano, R.A.; Donnarumma, G.; et al. Proteolytic activity of bovine lactoferrin. Biometals 2004, 17, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Hendrixson, D.R.; Qiu, J.; Shewry, S.C.; Fink, D.L.; Petty, S.; Baker, E.N.; Plaut, A.G.; St Geme, J.W., 3rd. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol. Microbiol. 2003, 47, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Tatsumoto, S.; Ono, H.; van Immerseel, F.; Raspoet, R.; Miyata, T. A novel antibiotic-delivery system by using ovotransferrin as targeting Molecule. Eur. J. Pharm. Sci. 2015, 66, 59–69. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giansanti, F.; Leboffe, L.; Angelucci, F.; Antonini, G. The Nutraceutical Properties of Ovotransferrin and Its Potential Utilization as a Functional Food. Nutrients 2015, 7, 9105-9115. https://doi.org/10.3390/nu7115453
Giansanti F, Leboffe L, Angelucci F, Antonini G. The Nutraceutical Properties of Ovotransferrin and Its Potential Utilization as a Functional Food. Nutrients. 2015; 7(11):9105-9115. https://doi.org/10.3390/nu7115453
Chicago/Turabian StyleGiansanti, Francesco, Loris Leboffe, Francesco Angelucci, and Giovanni Antonini. 2015. "The Nutraceutical Properties of Ovotransferrin and Its Potential Utilization as a Functional Food" Nutrients 7, no. 11: 9105-9115. https://doi.org/10.3390/nu7115453