Effects of Fructans from Mexican Agave in Newborns Fed with Infant Formula: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects and Study Design
2.2. DNA Purification from Feces
2.3. Gut Bacteria
| Target Organisms (Amplicon Size) | Primer/Probe | Sequence (5′ to 3′) | Tm, °C | Source |
|---|---|---|---|---|
| Bifidobacterium spp. (231 bp) | Forward primer | GGGATGCTGGTGTGGAAGAGA | 60 | Haarman & Knol, 2005 [18] |
| Reverse primer | TGCTCGCGTCCACTATCCAGT | 60 | ||
| Probe | VIC-TCAAACCACCACGCGCCA-NFQ-MGB | 70 | ||
| Enterobacteriaceae (96 bp) | Forward primer | CATGCC GCGTGTATGAAGAA | 59 | Huijsdens et al., 2002 [19] |
| Reverse primer | CGGGTAACGTCAATGAGCAAA | 59 | ||
| Probe | 6-FAM-TATTAACTTTACTCCCTTCCTCCCCGCTGAA-TAMRA | 68 | ||
| Lactobacillus spp. (92 bp) | Forward primer | TGG ATG CCT TGG CAC TAG GA | 58 | Haarman & Knol, 2006 [20] |
| Reverse primer | AAA TCT CCG GAT CAA AGC TTA CTTAT | 58 | ||
| Probe | VIC-TATTAGTTCCGTCCTTCATC-NFQ-MGB | 68 | ||
| Clostridium Cluster XI (139 pb) | Forward primer | ACGCTACTT GAGGAGGA | 58 | Nakamura et al., 2009 [21] |
| Reverse primer | GAGCCG TAG CCT TTC ACT | 58 | ||
| Probe | 6-FAM-GTGCCAGCAGCCGCGGTAATACG-TAMRA | 63 | ||
| Bacteroides fragilis (99 bp) | Forward primer | CTACAGGCTTAACACATGCAAGTC | 54 | This study |
| Reverse primer | GCAGGTTGGATACGTGTTACTCA | 54 | ||
| Probe | 6-FAM-TCGCCAGCAAAGAAA-NFQ-MGB | 64 | ||
| Veillonella spp. (128 bp) | Forward primer | ATCAACCTGCCCTTCAGAGG | 54 | This study |
| Reverse primer | AATCCCCTCCTTCAGTGATAGCTTA | 54 | ||
| Probe | 6-FAM-TAGCAGTCGTTTCCAACTGT-NFQ-MGB | 68 | ||
| Total Count (467 bp) | Forward primer | TCCTACGGGAGGCAGCAGT | 59 | Nadkarni et al., 2002 [22] |
| Reverse primer | GGACTACCAGGGTATCTAATCCTGTT | 58 | ||
| Probe | 6-FAM-CGTATTACCGCGGCTGCTGGCAC-BHQ1 | 70 |
2.4. Saliva IgA
2.5. C-Reactive Protein and Serum Ferritin, Triglycerides, Cholesterol, and Lipoproteins
2.6. Bone Metabolism
2.7. Statistical Analyses
3. Results
3.1. Feeding Frequency
3.2. Changes in Gut Microbiota
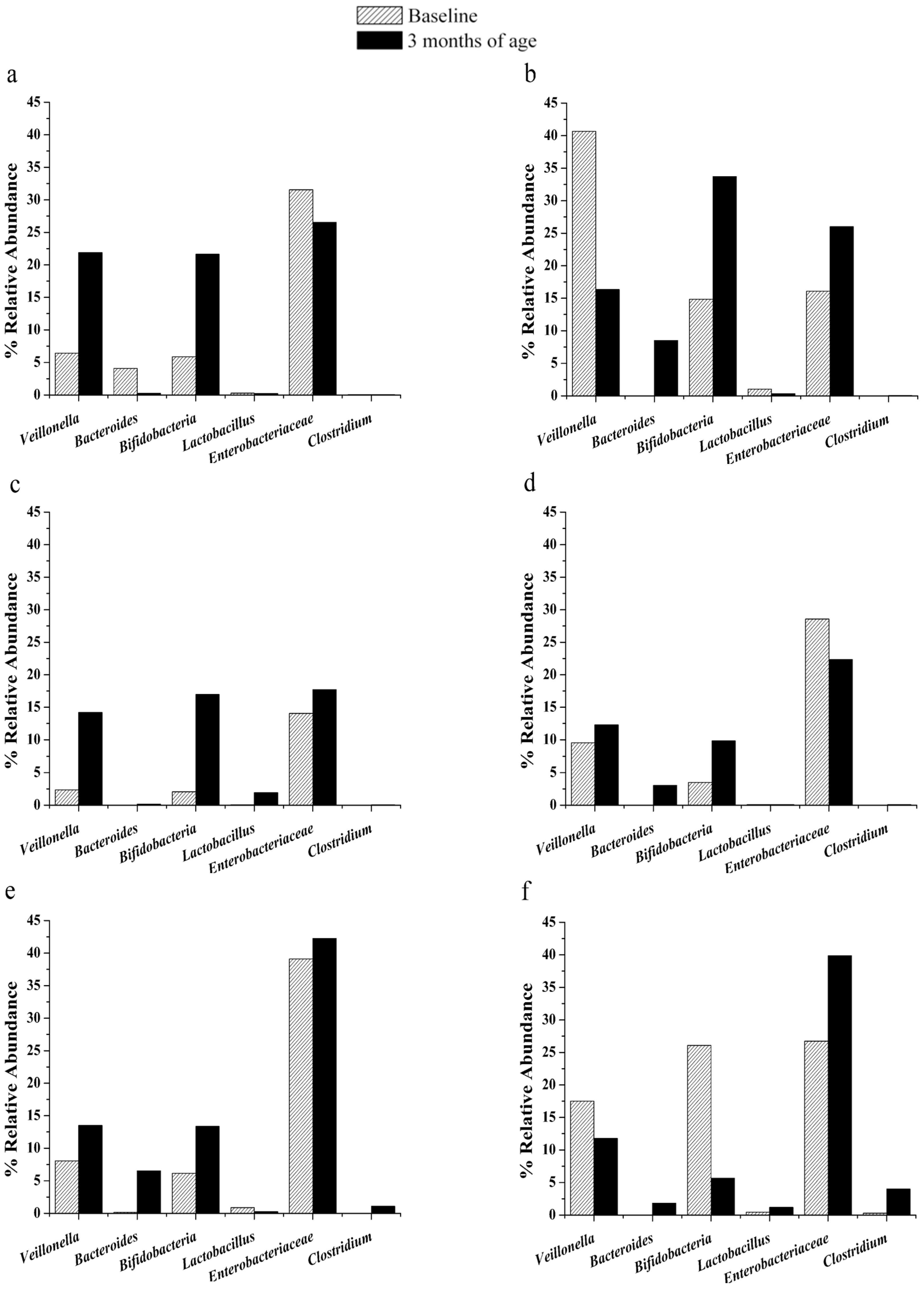

3.3. Immune Response
| Variable | Probiotics + Metlin + Metlos (n = 93) | Probiotics + Metlin (n = 93) | Probiotics + Metlos (n = 89) | Probiotics (n = 89) | Only Formula (n = 89) | Breast Milk (n = 147) |
|---|---|---|---|---|---|---|
| IgA in Saliva Basal (μg/mL) (X ± SD) | 11.8 ± 8.5 | 14.1 ± 10.1 | 12.1 ± 8.3 | 11.6 ± 7.7 | 14.0 ± 9.9 | 13.9 ± 9.3 |
| IgA in Saliva Final (μg/mL) (X ± SD) | 26.6 ± 11.8 | 24.8 ± 13.3 | 23.1 ± 11.4 | 21.3 ± 8.9 | 21.7 ± 9.7 | 24.4 ± 11.4 |
| Differences IgA in Saliva (μg/mL) (X ± SD) | 17.7 ± 8.1 ** | 13.9 ± 6.5 | 12.6 ± 7.5 | 11.1 ± 10.5 | 9.6 ± 6.1 | 12.3 ± 8.3 |
3.4. C-Reactive Protein, Serum Ferritin, Cholesterol, Triglycerides, and Lipoproteins
| Group | n | C-Protein Initial (mg/dL) Median Mean 95%CI | C-Protein Final (mg/dL) Median Mean 95%CI | Ferritin Initial (ng/mL) Median Mean 95%CI | Ferritin Final (ng/mL) Median Mean 95%CI |
|---|---|---|---|---|---|
| Probiotics + Metlin + Metlos | 27 | 0.290 | 328.0 | 146.0 | |
| 0.329 | 382.74 | 155.26 | |||
| 0.248 to 0.410 | 318.1 to 447.2 | 113.3 to 197.2 | |||
| Probiotics + Metlin | 32 | 0.290 | 348.5 | 147.5 | |
| 0.296 | 379.46 | 161.0 | |||
| 0.282 to 0.310 | 333.4 to 425.4 | 121.0 to 200.9 | |||
| Probiotics + Metlos | 25 | 0.290 | 264 | 129.0 | |
| 0.295 | 281.96 | 131.05 | |||
| 0.287 to 0.302 | 244.5 to 319.3 | 97.4 to 167.7 | |||
| Probiotics | 31 | 0.290 | 0.290 | 331.0 | 125 |
| 0.297 | 0.402 | 337.28 | 135.78 | ||
| 0.285 to 0.308 | 0.173 to 0.631 | 283.1 to 391.4 | 98.31 to 173.2 | ||
| Only formula | 20 | 0.290 | 321 | 137.5 | |
| 0.291 | 330.65 | 153.43 | |||
| 0.288 to 0.294 | 262.3 to 398.9 | 111.3 to 195.5 | |||
| Breast Milk | 56 | 0.290 | 360.0 | 134.0 | |
| 348.11 | 154.76 | ||||
| 0.374 to 0.484 | 306.1 to 390.0 | 126.9 to 182.6 |
| Parameter | Group Probiotics + Metlin + Metlos (n = 93) | Group Probiotics + Metlin (n = 93) | Group Probiotics + Metlos (n = 89) | Group Probiotics (n = 89) | Group only Formula (n = 89) | Group Breast Milk (n = 147) |
|---|---|---|---|---|---|---|
| Triglycerides (mg/Dl) | 121 ± 8 * | 124 ± 23 | 126 ± 40 | 125 ± 18 | 142 ± 30 | 123 ± 11 |
| Total Cholesterol (mg/Dl) | 123 ± 6 | 125 ± 13 | 124 ± 17 | 124 ± 8 | 132 ± 11 | 120 ± 19 |
| HDL-Cholesterol | 48 ± 7 * | 41 ± 7 | 43 ± 11 | 42 ± 10 | 32 ± 6 | 49 ± 11 |
| LDL-Cholesterol | 38 ± 7 * | 38 ± 8 | 40 ± 6 | 41 ± 16 | 65 ± 14 | 36 ± 11 |
| VLDL-Cholesterol | 25 ± 11 | 27 ± 14 | 27 ± 8 | 26 ± 17 | 28 ± 4 | 23 ± 11 |
3.5. Bone Metabolism
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roberfroid, M.B. Prebiotics: Preferential substrates for specific germs? Am. J. Clin. Nutr. 2001, 73, 406S–409S. [Google Scholar] [PubMed]
- Palacio, M.I.; Etcheverría, A.I.; Manrique, G.D. Fermentation by Lactobacillus paracasei of galactooligosaccharides and low-molecular weight carbohydrates extracted from squash (Curcubita. maxima) and lupin (Lupinus. albus) seeds. J. Microbiol. Biotech. Food Sci. 2014, 3, 329–332. [Google Scholar]
- Bornet, F.R.; Brouns, F.; Tashiro, Y.; Duvillier, V. Nutritional aspects of short-chain fructooligosaccharides: Natural occurrence, chemistry, physiology and health implications. Dig. Liver Dis. 2002, 34, S111–S120. [Google Scholar] [CrossRef]
- Roberfroid, M.B.; van Loo, J.A.E.; Gibson, G.R. The bifidogenic nature of chicory inulin and its hydrolysis product. J. Nutr. 1998, 128, 11–19. [Google Scholar] [PubMed]
- Grimoud, J.; Durand, H.; Courtin, C.; Monsan, P.; Ouarné, F.; Theodorou, V.; Roques, C. In vitro screening of probiotic lactic acid bacteria and prebiotic glucooligosaccharides to select effective synbiotics. Anaerobe 2010, 16, 493–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vrese, M.; Schrezenmeir, J. Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng. Biotechnol. 2008, 111, 1–66. [Google Scholar] [PubMed]
- Lasekan, J.; Baggs, G.; Acosta, S.; Mackey, A. Soy protein-based infant formulas with supplemental fructooligosaccharides: Gastrointestinal tolerance and hydration status in newborn infants. Nutrients 2015, 7, 3022–3037. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B. Innovations in infant milk feeding: From the past to the future. Nestle Nutr. Workshop Ser. Pediatr. Program. 2010, 66, 1–17. [Google Scholar] [PubMed]
- Risk assessment on use of Lactobacillus rhamnosus (LGG) as an ingredient in infant formula and baby foods (II). Available online: http://www.vkm.no/dav/63bb45d3eb.pdf (accessed on 22 October 2015).
- European Food Safety Authority. Scientific opinion on the substantiation of a health claim related Immunofortis and strengthening of the baby’s immune system pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1430. [Google Scholar]
- Garcia Mendoza, A. Distribution of Agave (Agavaceae) in Mexico. Available online: http://www.agavaceae.com/botanik/pflanzen/botanzeige_scan_es.asp?gnr=110&cat=&scan=110-4 (accessed on 22 October 2015).
- Praznik, W.; Löppert, R.; Cruz Rubio, J.M.; Zangger, K.; Huber, A. Structure of fructo-oligosaccharides from leaves and stem of Agave tequilana Weber, var. azul. Carbohydr. Res. 2013, 15, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Tuohy, K.M.; Gibson, G.R.; Klinder, A.; Costabile, A. In vitro evaluation of the fermentation properties and potential prebiotic activity of Agave fructans. J. Appl. Microbiol. 2010, 108, 2114–2121. [Google Scholar] [PubMed]
- Cieslik, E.; Topolska, K.; Praznik, W.; Cruz Rubio, J.M. Effect of Agave fructans on selected parameters of calcium metabolism and bone condition in rats. J. Aging Res. Clin. Prac. 2012, 1, 103–108. [Google Scholar]
- Gracia, M.I.; Tinoco, M.M.; Rivera, H.M.; Sanchez, B.F.; Tapia, P.G.; Altamirano, L.M.; Romero, R.L.; García, O.L. Acute toxicity and genotoxic evaluation of Metlin® and Metlos® (Organic Agave Fructans). Food Nutr. Sci. 2013, 4, 106–112. [Google Scholar] [CrossRef]
- López-Velázquez, G.; Díaz-García, L.; Anzo, A.; Parra-Ortiz, M.; Llamosas-Gallardo, B.; Ortiz-Hernández, A.A.; Mancilla-Ramírez, J.; Cruz-Rubio, J.M.; Gutiérrez-Castrellón, P. Safety of a dual potential prebiotic system from Mexican agave "Metlin® and Metlos®", incorporated to an infant formula for term newborn babies: A randomized controlled trial. Rev. Investig. Clin. 2013, 65, 483–490. [Google Scholar]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Haarman, M.; Knol, J. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium. species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 2005, 71, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Gronlund, M.M.; Lehtonen, O.P.; Eerola, E.; Kero, P. Fecal microflora in healthy infants born by different methods of delivery: Permanent changes in intestinal flora after cesarean delivery. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Haarman, M.; Knol, J. Quantitative Real-Time PCR analysis of fecal Lactobacillus species in Infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 2006, 72, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Gaskins, H.R.; Collier, C.T.; Nava, G.M.; Rai, D.; Petschow, B.; Russell, W.M.; Harris, C.; Mackie, R.I.; Wampler, J.L.; et al. Molecular ecological analysis of fecal bacterial populations from term infants fed formula supplemented with selected blends of prebiotics. Appl. Environ. Microbiol. 2009, 75, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, M.A.; Martin, F.E.; Jacques, N.A.; Hunter, N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primer set. Microbiology 2002, 148, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Fredrickson, D.S. Estimation of concentration of low density lipoprotein cholesterol in plasma without use of ultracentrifuge. Clin. Chem. 1972, 18, 449–502. [Google Scholar] [PubMed]
- Harmsen, H.J.M.; Wildeboer-Veloo, A.C.M.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Huebner, J.; Wehling, R.L.; Hutkins, R.W. Functional activity of commercial prebiotics. Int. Dairy J. 2007, 17, 770–775. [Google Scholar] [CrossRef]
- Goldsmith, F.; O'Sullivan, A.; Smilowitz, J.T.; Freeman, S.L. Lactation and Intestinal Microbiota: How Early Diet Shapes the Infant Gut. J. Mammary Gland Biol. Neoplasia. 2015. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Rastall, R.A.; Gibson, G.R. Developing a quantitative approach for determining the in vitro prebiotic potential of dietary oligosaccharides. FEMS Microbiol. Lett. 2004, 236, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Costalos, C.; Kapiki, A.; Apostolou, M.; Papathoma, E. The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Hum. Dev. 2008, 84, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Hachelaf, W.; Suau, A.; Boudraa, G.; Bouziane-Nedjadi, K.; Rigottier-Gois, L.; Touhami, M.; Desjeux, J.; Pochart, P. Effects on faecal microbiota of dietary and acidic oligosaccharides in children during partial formula feeding. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Stark, P.L.; Lee, A. The bacterial colonization of the large bowel of pre-term low birth weight neonates. J. Hyg. 1982, 89, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Minoli, I.; Mosca, M.; Fanaro, S.; Jelinek, J.; Stahl, B.; Boehm, G. Dosage related bifidogenic effects of galacto and fructooligosaccharides in formula-fed term infants. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Niers, L.E.; Hoekstra, M.O.; Timmerman, H.M.; van Uden, N.O.; de Graaf, P.M.; Smits, H.H.; Kimpen, J.L.; Rijkers, G.T. Selection of probiotic bacteria for prevention of allergic diseases: Immunomodulation of neonatal dendritic cells. Clin. Exp. Immunol. 2007, 149, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Kirjavainen, P.; Eerola, E.; Kero, P.; Salminen, S.; Isolauri, E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 2001, 107, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Isolauri, E.; He, F.; Hashimoto, H.; Benno, Y.; Salminen, S. Differences in Bifidobacterium flora composition in allergic and healthy infants. J. Allergy Clin. Immunol. 2001, 108, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, P.A.; Alliet, P.; Raes, M.; Alles, M.S.; Kroes, H.; Boehm, G.; Knippels, L.M.; Knol, J.; Vandenplas, Y. Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J. Nutr. 2008, 138, 1141–1147. [Google Scholar] [PubMed]
- Jin, H.X.; Wang, R.S.; Chen, S.J.; Wang, A.P.; Liu, X.Y. Early and late Iron supplementation for low birth weight infants: A meta-analysis. Ital. J. Pediatr. 2015, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Cani, P.D. Gut microbiota and metabolic disorders: How prebiotic can work? Br. J. Nutr. 2013, 109, S81–S85. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Jackson, K.G. Inulin and oligofructose: Effects on lipid metabolism from human studies. Br. J. Nutr. 2002, 87, S261–S264. [Google Scholar] [CrossRef] [PubMed]
- Rauchenzauner, M.; Schmid, A.; Heinz-Erian, P.; Kapelari, K.; Falkensammer, G.; Griesmacher, A.; Finkenstedt, G.; Högler, W. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J. Clin. Endocrinol. Metab. 2007, 92, 443–449. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Velázquez, G.; Parra-Ortiz, M.; Mora, I.D.l.M.-D.l.; García-Torres, I.; Enríquez-Flores, S.; Alcántara-Ortigoza, M.A.; Angel, A.G.-d.; Velázquez-Aragón, J.; Ortiz-Hernández, R.; Cruz-Rubio, J.M.; et al. Effects of Fructans from Mexican Agave in Newborns Fed with Infant Formula: A Randomized Controlled Trial. Nutrients 2015, 7, 8939-8951. https://doi.org/10.3390/nu7115442
López-Velázquez G, Parra-Ortiz M, Mora IDlM-Dl, García-Torres I, Enríquez-Flores S, Alcántara-Ortigoza MA, Angel AG-d, Velázquez-Aragón J, Ortiz-Hernández R, Cruz-Rubio JM, et al. Effects of Fructans from Mexican Agave in Newborns Fed with Infant Formula: A Randomized Controlled Trial. Nutrients. 2015; 7(11):8939-8951. https://doi.org/10.3390/nu7115442
Chicago/Turabian StyleLópez-Velázquez, Gabriel, Minerva Parra-Ortiz, Ignacio De la Mora-De la Mora, Itzhel García-Torres, Sergio Enríquez-Flores, Miguel Angel Alcántara-Ortigoza, Ariadna González-del Angel, José Velázquez-Aragón, Rosario Ortiz-Hernández, José Manuel Cruz-Rubio, and et al. 2015. "Effects of Fructans from Mexican Agave in Newborns Fed with Infant Formula: A Randomized Controlled Trial" Nutrients 7, no. 11: 8939-8951. https://doi.org/10.3390/nu7115442







