In Vitro Bioaccessibility, Human Gut Microbiota Metabolites and Hepatoprotective Potential of Chebulic Ellagitannins: A Case of Padma Hepaten® Formulation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. MC-RP-HPLC-UV Conditions
2.2.1. HPLC Apparatus
2.2.2. HPLC Conditions for Separation of Padma Hepaten Phenolics
2.2.3. Preparation of Standard Solutions of Phenolics for Quantification
2.2.4. Sample Preparation for Quantification
2.3. Simulated Gastrointestinal Digestion in Dynamic Conditions
2.3.1. Preparation of Digestive Fluids
2.3.2. Simulated Gastrointestinal Digestion
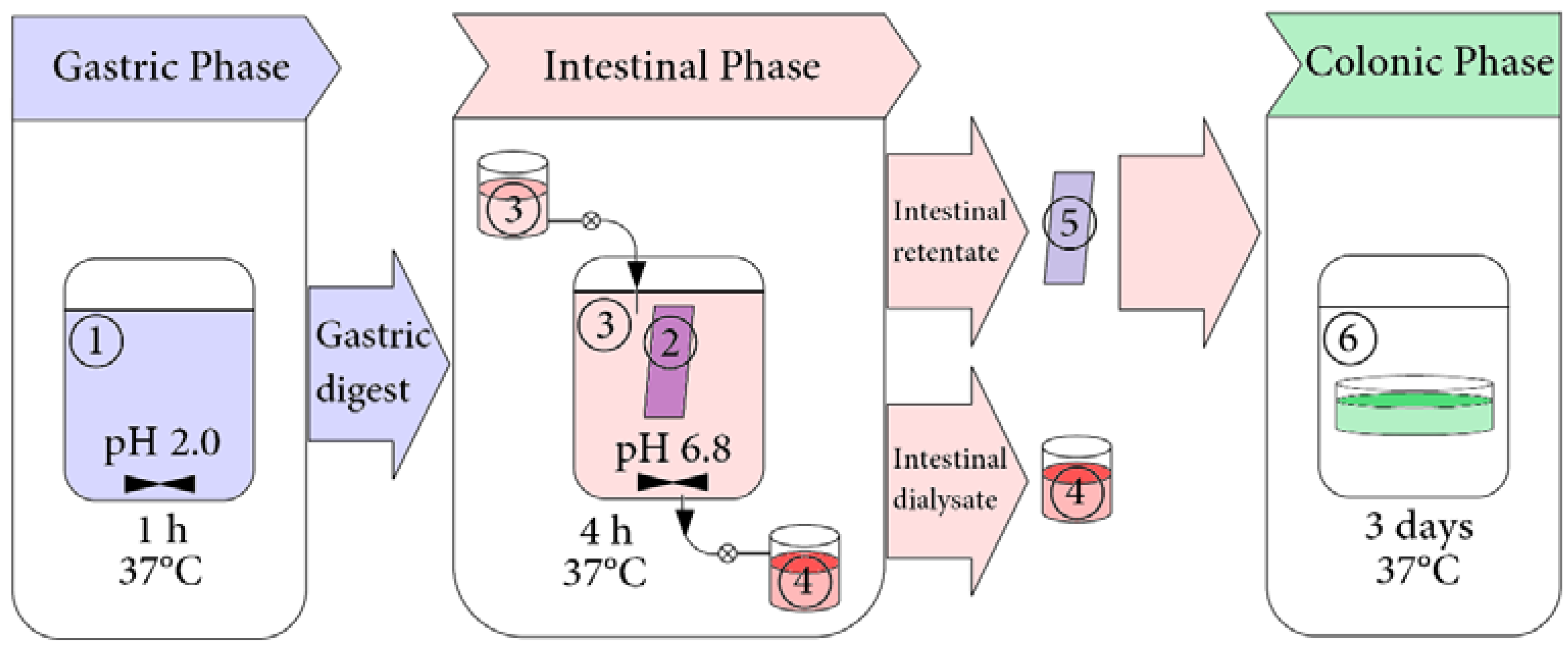
2.3.3. Collection of Samples for HPLC Analysis
2.4. Ex vivo Conditions of Microbial Metabolites Formation with Human Gut Microbiote
2.4.1. Sample Preparation
2.4.2. Incubation of the Samples
2.4.3. Collection of Samples for HPLC Analysis
2.4.4. HPLC Conditions for Separation of Human Gut Microbiota Metabolites
2.4.5. Preparation of Standard Solutions of Ellagic Acid, Chebulinic Acid, Chebulagic Acid, and Urolithins for Quantification
2.5. Ex vivo Conditions of t-BHP-Induced Oxidative Hepatotoxicity in Cultured Rat Hepatocytes
2.6. Statistical Analysis
3. Results
3.1. HPLC Fingerprint of Padma Hepaten
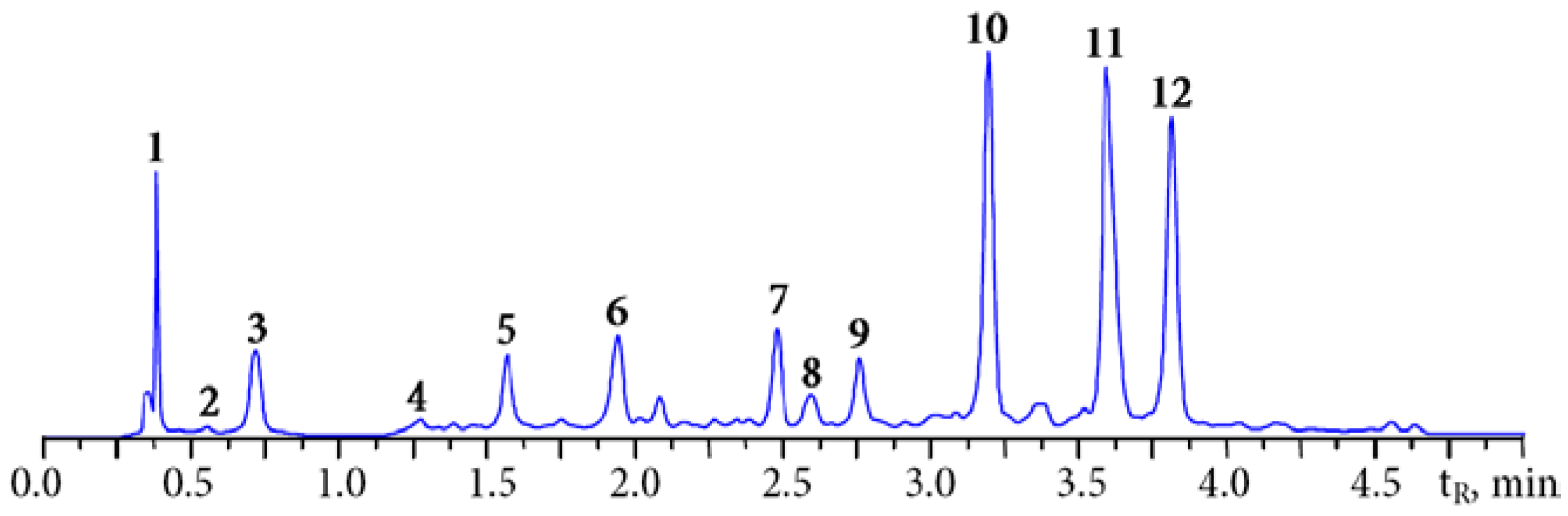
| Compound | Content, mg/g |
|---|---|
| Chebulic acid and chebulic ellagitannins | 185.42 |
| Chebulic acid | 6.73 ± 0.18 |
| Chebulanin | 13.89 ± 0.33 |
| Chebulagic acid | 84.94 ± 1.95 |
| Chebulinic acid | 79.86 ± 1.83 |
| Non-chebulic ellagitannins | 43.73 |
| α-Punicalagin | 12.27 ± 0.31 |
| β-Punicalagin | 15.13 ± 0.36 |
| Corilagin | 16.33 ± 0.34 |
| Gallic acid and simple gallate esters | 43.05 |
| Gallic acid | 18.36 ± 0.42 |
| Glucogallin | 8.45 ± 0.18 |
| 1,6-Di-O-galloyl-β-d-glucose | 7.17 ± 0.14 |
| 3,4,6-Tri-O-galloyl-β-d-glucose | 9.07 ± 0.19 |
| Other compounds | 16.14 |
| Ellagic acid | 16.14 ± 0.51 |
| Total | 288.36 |
3.2. Release of Phenolic Compounds from Padma Hepaten During In vitro Dynamic Gastrointestinal Digestion
3.2.1. Gastric Phase of Digestion
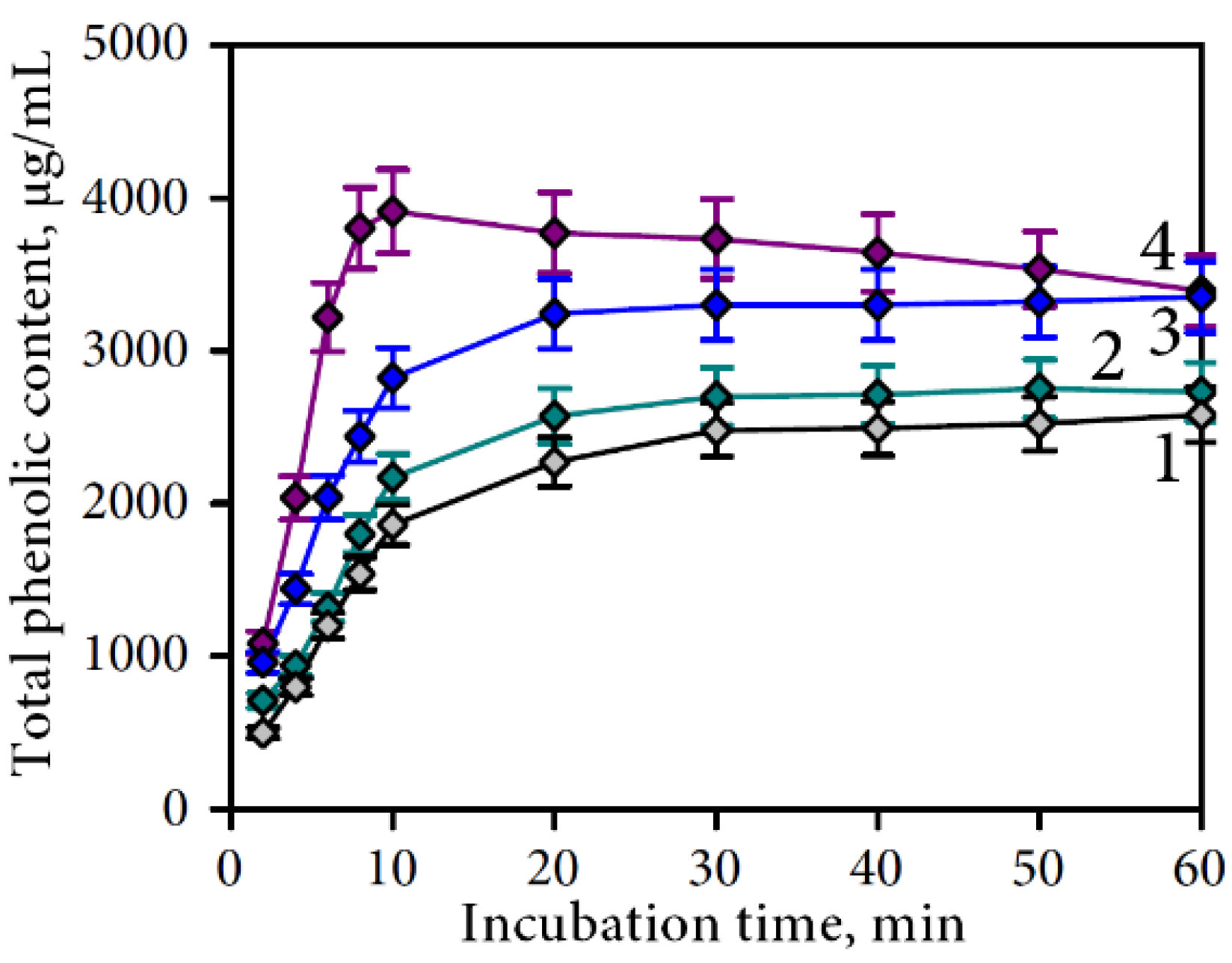
| Compound | Undigested Sample | Gastric Media | Intestine Media Retentate | Intestine Media Dialysate | Plant Residue after Intestine Phase | Total Recovery, % | Degradation Percentage, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amount, mg | Recovery, % | Amount, mg | Recovery, % | Amount, mg | Recovery, % | Amount, mg | Recovery, % | Amount, mg | Recovery, % | |||
| Chebulic acid and chebulic ellagitannins | ||||||||||||
| Chebulic acid | 2.69 ± 0.07 | 100 | 2.15 ± 0.10 | 79.93 | 0.78 ± 0.04 | 28.99 | 1.21 ± 0.05 | 44.98 | 0.09 ± 0.00 | 3.35 | 77.23 | 22.77 |
| Chebulanin | 5.56 ± 0.15 | 100 | 4.40 ± 0.22 | 79.14 | 1.11 ± 0.05 | 19.96 | 3.07 ± 0.12 | 55.21 | 0.30 ± 0.01 | 5.40 | 80.63 | 19.37 |
| Chebulagic acid | 33.97 ± 0.88 | 100 | 28.18 ± 1.35 | 82.96 | 18.67 ± 0.86 | 54.96 | 7.14 ± 0.36 | 21.02 | 0.19 ± 0.00 | 0.56 | 76.55 | 23.45 |
| Chebulinic acid | 31.95 ± 0.87 | 100 | 21.55 ± 1.01 | 67.45 | 13.36 ± 0.65 | 41.82 | 5.80 ± 0.26 | 18.15 | 0.91 ± 0.04 | 2.85 | 62.81 | 37.19 |
| Subtotal | 74.17 | 100 | 56.28 | 75.88 | 33.92 | 45.73 | 17.22 | 23.22 | 1.49 | 2.01 | 70.96 | 29.04 |
| Non-chebulic ellagitannins | ||||||||||||
| α-Punicalagin | 4.91 ± 0.14 | 100 | 4.25 ± 0.18 | 86.56 | 3.47 ± 0.15 | 70.67 | 0.56 ± 0.02 | 11.40 | 0.04 ± 0.00 | 0.81 | 82.87 | 17.13 |
| β-Punicalagin | 6.05 ± 0.18 | 100 | 4.37 ± 0.19 | 72.23 | 3.20 ± 0.16 | 52.89 | 1.05 ± 0.05 | 17.36 | n.d. | 0.00 | 70.25 | 29.75 |
| Corilagin | 6.53 ± 0.19 | 100 | 5.80 ± 0.26 | 88.83 | 3.01 ± 0.16 | 46.09 | 2.40 ± 0.11 | 36.75 | 0.30 ± 0.01 | 4.59 | 87.50 | 12.50 |
| Subtotal | 17.49 | 100 | 14.42 | 82.45 | 9.68 | 55.35 | 4.01 | 22.93 | 0.34 | 1.94 | 80.29 | 19.71 |
| Gallic acid and simple gallate esters | ||||||||||||
| Gallic acid | 6.19 ± 0.14 | 100 | 5.88 ± 0.26 | 95.00 | 1.14 ± 0.06 | 18.42 | 4.72 ± 0.22 | 76.25 | 0.23 ± 0.00 | 3.72 | 82.89 | 17.11 |
| Glucogallin | 3.38 ± 0.08 | 100 | 3.28 ± 0.16 | 97.04 | 1.12 ± 0.05 | 33.14 | 2.14 ± 0.08 | 63.31 | n.d. | 0.00 | 96.39 | 3.61 |
| 1,6-Di-O-galloyl-β-d-glucose | 2.87 ± 0.06 | 100 | 2.59 ± 0.13 | 90.24 | 0.56 ± 0.03 | 19.51 | 2.00 ± 0.08 | 69.69 | n.d. | 0.00 | 89.36 | 10.64 |
| 3,4,6-Tri-O-galloyl-β-d-glucose | 3.63 ± 0.10 | 100 | 3.05 ± 0.13 | 84.02 | 0.74 ± 0.04 | 20.39 | 2.18 ± 0.09 | 60.06 | 0.13 ± 0.00 | 3.58 | 83.99 | 16.01 |
| Subtotal | 16.07 | 100 | 14.80 | 92.10 | 3.56 | 22.15 | 11.04 | 68.70 | 0.36 | 2.24 | 86.85 | 13.15 |
| Other compounds | ||||||||||||
| Ellagic acid | 6.45 ± 0.22 | 100 | 3.83 ± 0.17 | 59.38 | 1.83 ± 0.10 | 28.37 | 1.68 ± 0.07 | 26.05 | 0.66 ± 0.03 | 10.23 | 64.67 | 35.33 |
| Subtotal | 6.45 | 100 | 3.83 | 59.38 | 1.83 | 28.37 | 1.68 | 26.05 | 0.66 | 10.23 | 64.67 | 35.33 |
| Total | 114.18 | 100 | 89.33 | 78.24 | 48.99 | 42.91 | 33.95 | 29.73 | 2.85 | 2.48 | 74.39 | 25.61 |
3.2.2. Intestine Phase of Digestion

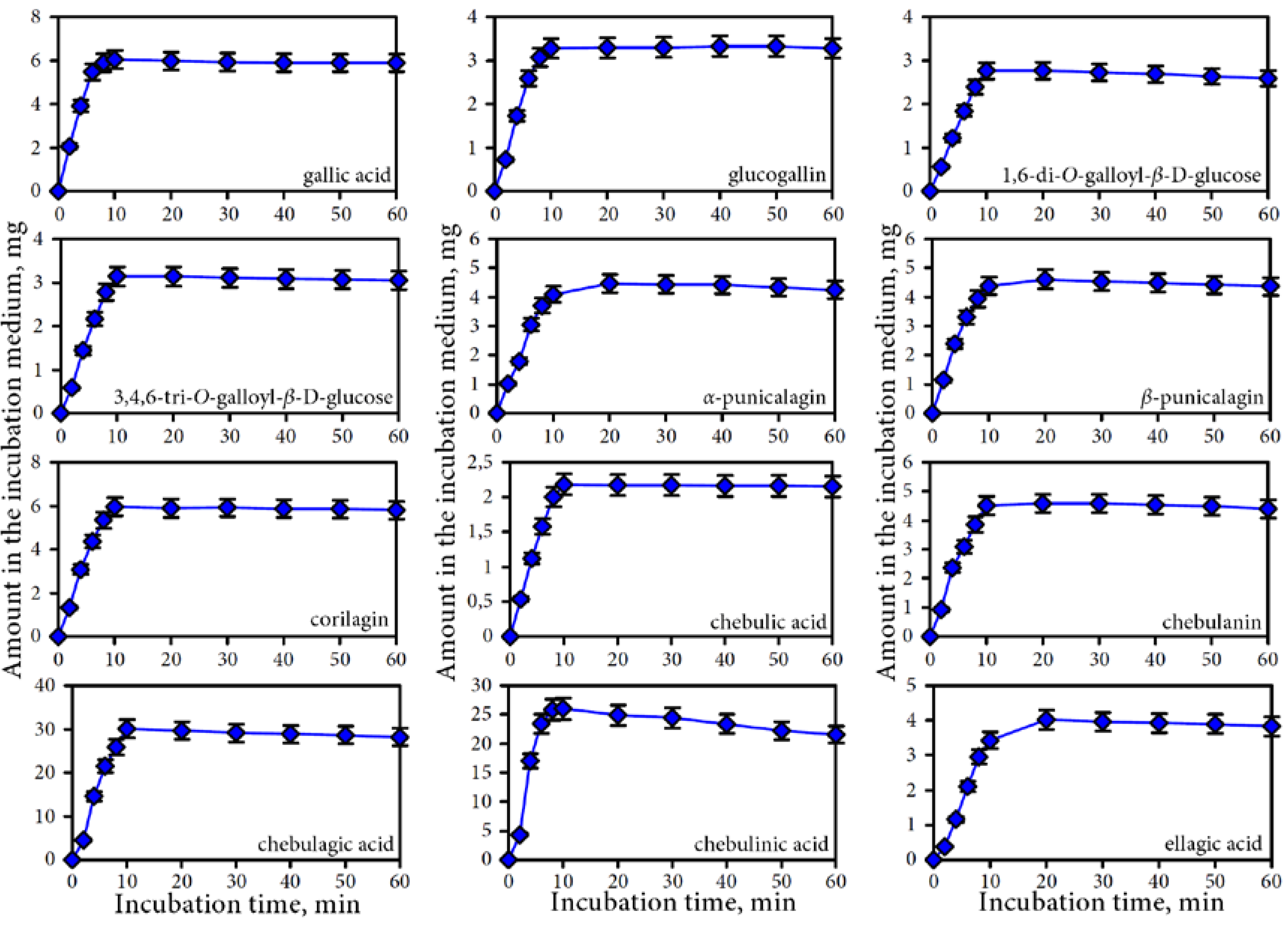
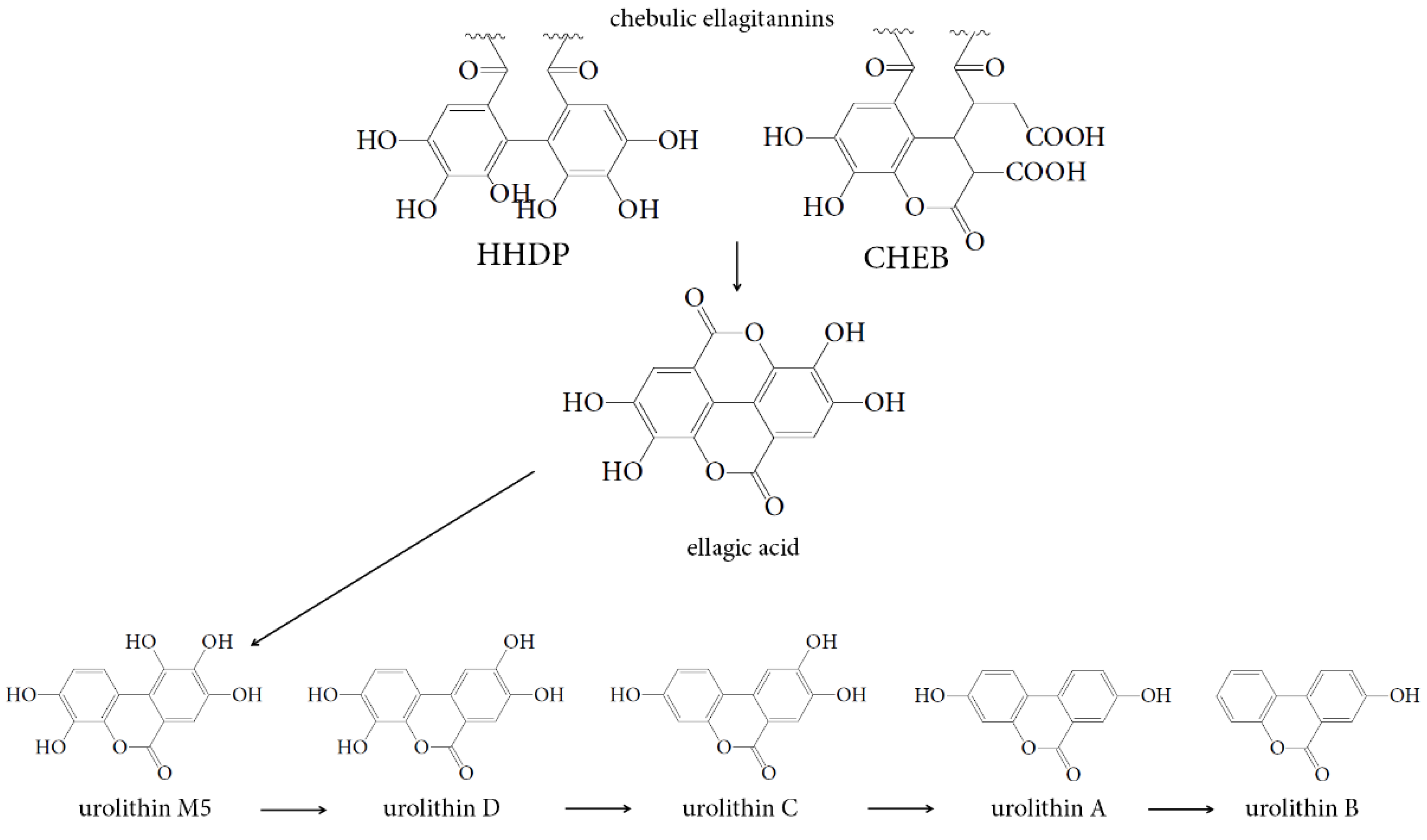
3.3. Human Gut Microbiota Metabolites of Intestine Media Retentate of Padma Hepaten
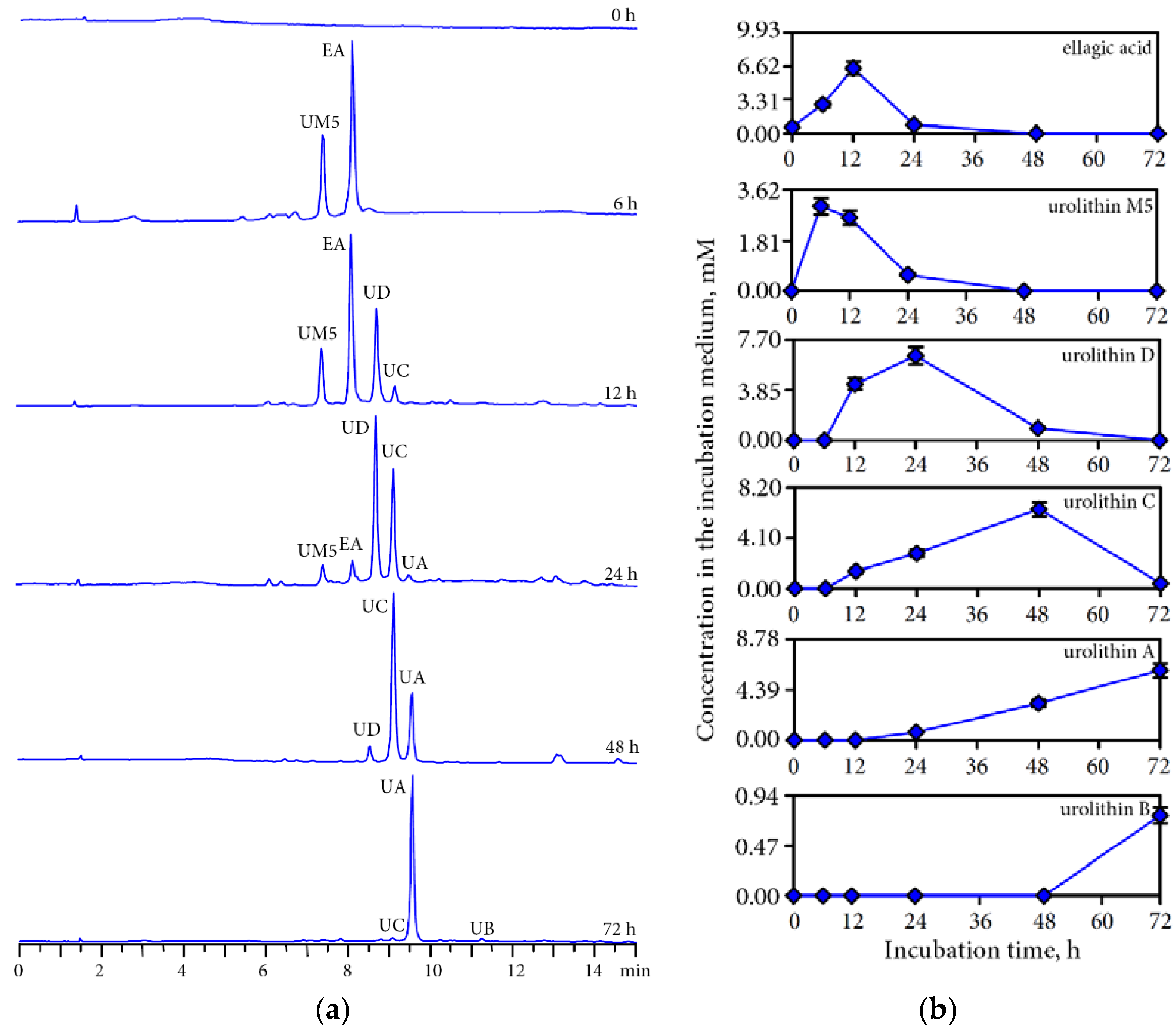

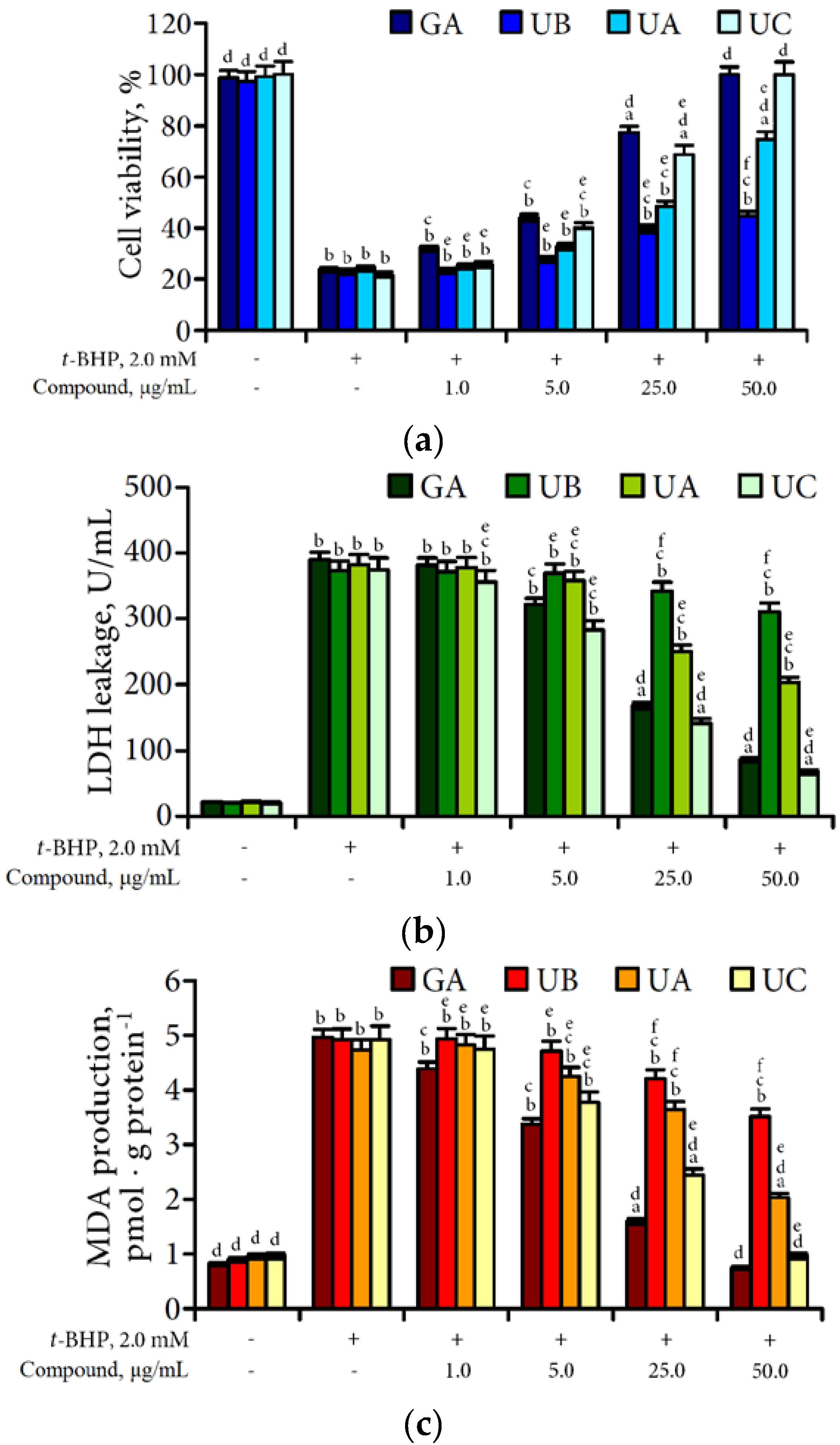
3.4. Hepatoprotective Potential of Urolithins Against t-BHP-Induced Oxidative Injury of Hepatocytes
4. Discussion
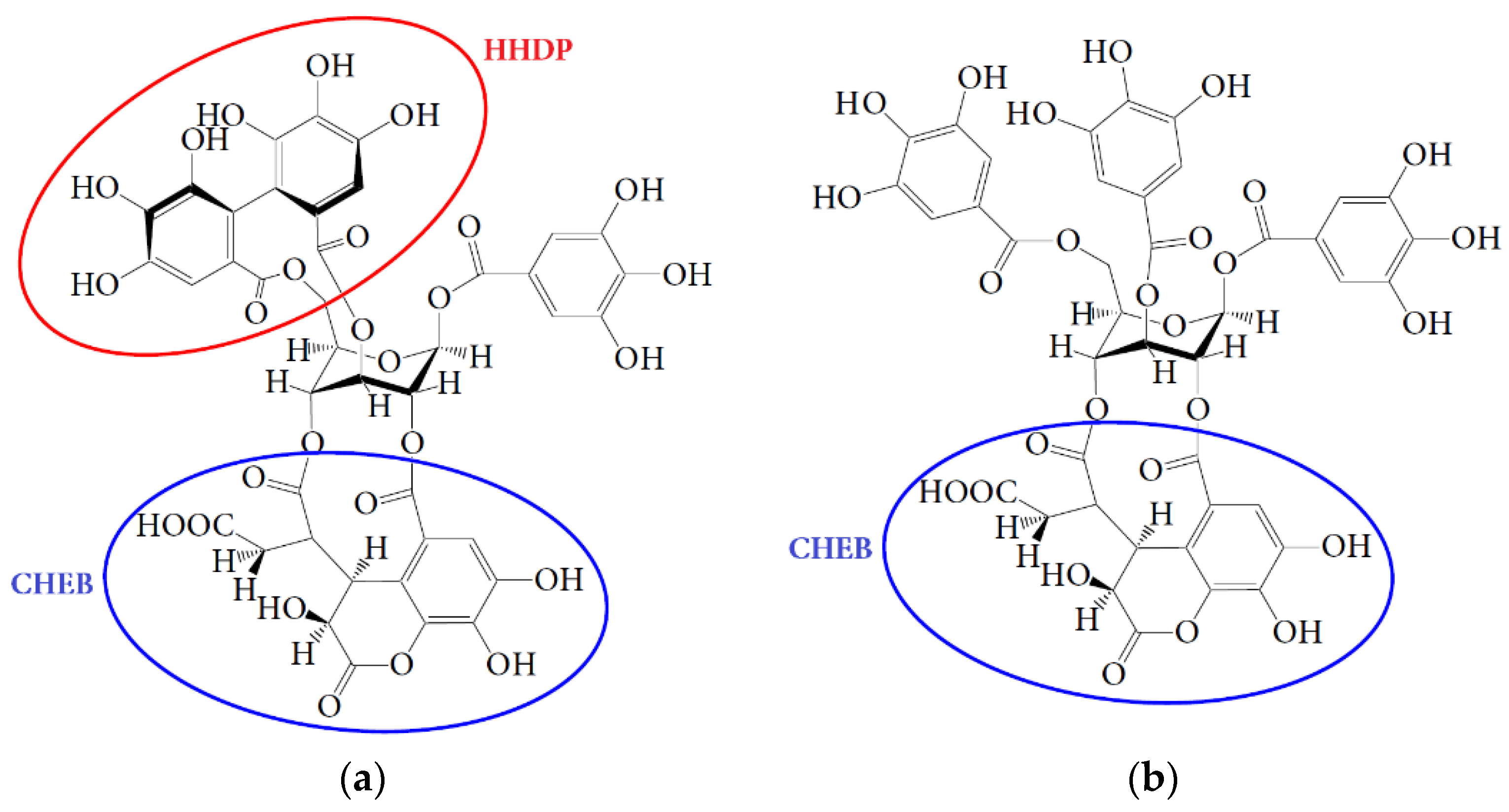
4.1. Bioaccessibility of Compounds of Padma Hepaten
4.2. Gut Microbiota Metabolites of Compounds of Padma Hepaten
4.3. Hepatoprotective Effect of Urolithins Against t-BHP-Induced Oxidative Injury of Hepatocytes
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heaney, R.P. Factors influencing the measurement of bioavailability, taking calcium as a model. J. Nutr. 2001, 131, 1344–1348. [Google Scholar]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Comp. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Chen, J.; Gaikwad, V.; Holmes, M.; Murray, B.; Povey, M.; Wang, Y.; Zhang, Y. Development of a simple model device for in vitro gastric digestion investigation. Food Funct. 2011, 2, 174–182. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, Y. Review of in vitro digestion models for rapid screening of emulsion-based systems. Food Funct. 2010, 1, 32–59. [Google Scholar] [CrossRef] [PubMed]
- Courraud, J.; Berger, J.; Cristol, J.P.; Avallone, S. Stability and bioaccessibility of different forms of carotenoids and vitamin A during in vitro digestion. Food Chem. 2013, 136, 871–877. [Google Scholar] [CrossRef]
- Garret, D.A.; Faila, M.L.; Sarama, R.J. Estimation of carotenoids bioavailability from fresh stir-fried vegetables using an in vitro digestion/Caco-2 cell culture model. J. Nutr. Biochem. 2000, 49, 1035–1041. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Samardžić, J.; Janković, T.; Šavikin, K.; Mojsin, M.; Topalović, V.; Stevanović, M. Antioxidant and antiproliferative activity of chokeberry juice phenolics during in vitro simulated digestion in the presence of food matrix. Food Chem. 2015, 175, 516–522. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potentional uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Kern, S.M.; Bennett, R.N.; Needs, P.W.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.T. Characterization of metabolites of hydroxycinnamates in the in vitro model of human small intestinal epithelium Caco-2 cells. J. Agric. Food Chem. 2003, 51, 7884–7891. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Husheem, M.; Härkönen, P.; Pihlaya, K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J. Ethnopharmacol. 2002, 81, 327–336. [Google Scholar] [CrossRef]
- Cho, H.; Jung, H.; Lee, H.; Yi, H.C.; Kwak, H.-K.; Hwang, K.I. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT-29 colon cancer cells. Food Funct. 2015, 6, 1675–1683. [Google Scholar] [CrossRef]
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Baliga, M.S.; Meera, S.; Mathai, B.; Rai, M.P.; Pawar, V.; Palatty, P.L. Scientific validation of the ethnomedicinal properties of the Ayurvedic drug Triphala: A review. Chin. J. Integr. Med. 2012, 18, 946–954. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Rani, A.; Kumari, R. Therapeutic potential of Triphala against human diseases. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 5–13. [Google Scholar]
- Balasubramani, S.P.; Venkatasubramanian, P.; Kukkupuni, S.K.; Patwardhan, B. Plant-based Rasayana drugs from Ayurveda. Chin. J. Integr. Med. 2011, 17, 88–94. [Google Scholar] [CrossRef]
- Pfundstein, B.; el Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Thilagavathi, G. Terminalia chebula—Review on pharmacological and biochemical studies. Int. J. PharmTech Res. 2014, 6, 97–116. [Google Scholar]
- Saraswathi Motamarri, N.; Karthikeyan, M.; Kannan, M.; Rajasekar, S. Terminalia belerica Roxb.—A phytopharmacological review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 96–99. [Google Scholar]
- Espín, J.C.; González-Barrio, R.; Cerdá, B.; López-Bote, C.; Rey, A.I.; Tomás-Barberán, F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. [Google Scholar] [CrossRef]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Zhang, Y.; Suchard, M.; Li, Z.; Heber, D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 h. J. Nutr. 2006, 136, 2481–2485. [Google Scholar] [PubMed]
- Dell’agli, M.; Galli, G.V.; Bulgari, M.; Basilico, N.; Romeo, S.; Bhattacharya, D.; Taramelli, D.; Bosisio, M. Ellagitannins of the fruit rind of the pomegranate (Punica granatum) antagonize in vitro the host inflammatory response mechanism involved in the onset of malaria. Malar. J. 2010, 9, 208. [Google Scholar] [CrossRef]
- Gimenez-Bastida, J.A.; Gonzalez-Sarrias, A.; Larrosa, M.; Tomás-Barberán, F.; Espín, J.C.; Garcia-Conesa, M.T. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-alpha-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol. Nutr. Food Res. 2012, 56, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Bastida, J.A.; Larrosa, M.; Gonzalez-Sarrias, A.; Tomás-Barberán, F.; Espín, J.C.; Garcia-Conesa, M.T. Intestinal ellagitannin metabolites ameliorate cytokine-induced inflammation and associated molecular markers in human colon fibroblasts. J. Agric. Food Chem. 2012, 60, 8866–8876. [Google Scholar] [CrossRef]
- Bialonska, D.; Kasimsetty, S.G.; Khan, S.I.; Ferreira, D. Urolithins, intestinal microbial metabolites of pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J. Agric. Food Chem. 2009, 57, 10181–10186. [Google Scholar] [CrossRef]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55, S35–S43. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Larrosa, M.; Tomas-Barberan, F.A.; Dolara, P.; Espin, J.C. NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br. J. Nutr. 2010, 104, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.K.; Granica, S.; Stolarczyk, M.; Melzig, M.F. Epigenetic modulation of mechanisms involved in inflammation: Influence of selected polyphenolic substances on histone acetylation state. Food Chem. 2012, 131, 1015–1020. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Granica, S.; Zwierzyńska, M.; Stefańska, J.; Schopohl, P.; Melzig, M.F.; Kiss, A.K. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant material. J. Ethnopharmacol. 2014, 155, 801–809. [Google Scholar] [CrossRef]
- PADMA: Tibetische Arzneimittel Seit 1969: PADMA Hepaten. Available online: http://www.padma.at/produkte/padma-hepaten (accessed on 15 May 2011).
- Ginsburg, I.; Koren, E.; Horani, A.; Mahamid, M.; Doron, S.; Muhanna, N.; Amer, J.; Safadi, R. Amelioration of hepatic fibrosis via Padma Hepaten is associated with altered natural killer T lymphocytes. Clin. Exp. Immun. 2009, 157, 155–164. [Google Scholar] [CrossRef]
- United States Pharmacopeia. Simulated Gastric Fluid and Simulated Intestinal Fluid. In The United States Pharmacopeia 23, the National Formulary 18; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 1995; p. 2053. [Google Scholar]
- Marambe, H.K.; Shand, P.J.; Wanasundara, J.P.D. Release of angiotensin I-converting enzyme inhibitory peptides from flaxseed (Linum usitatissimum L.) protein under simulated gastrointestinal digestion. J. Agric. Food Chem. 2011, 59, 9596–9604. [Google Scholar] [CrossRef] [PubMed]
- Oomen, A.G.; Rompelberg, C.J.M.; Bruil, M.A.; Dobbe, C.J.G.; Pereboom, D.P.K.H.; Sips, A.J.A.M. Development of an in vitro digestion model for estimating the bioaccessibility of soil contaminants. Arch. Environ. Contam. Toxicol. 2003, 44, 281–287. [Google Scholar] [CrossRef]
- Savoie, L.; Gauthier, S.F. Dialysis cell for the in vitro measurement of the protein digestibility. J. Food Sci. 1986, 51, 494–498. [Google Scholar] [CrossRef]
- Lee, H.S.; Won, N.H.; Kim, K.H.; Lee, H.; Jun, W.; Lee, K.W. Antioxidant effects of aqueous extract of Terminalia chebula in vivo and in vitro. Biol. Pharm. Bull. 2005, 28, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.H.; Wang, C.J.; Kao, E.S.; Chu, H.Y. Hibiscus protocatechuic acid protects against oxidative damage induced by tert-butylhydroperoxide in rat primary hepatocytes. Chem. Biol. Interact. 1996, 101, 137–148. [Google Scholar] [CrossRef]
- Yagi, K. Lipid peroxides and human diseases. Chem. Phys. Lipids 1987, 45, 337–351. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Schwabl, H.; Vennos, C.; Loepfe, C. New gallates of mucic acid from Phyllanthus emblica. Chem. Nat. Compd. 2015, 51, 612–617. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Schwabl, H.; Vennos, C. Application of MC-RP-HPLC-UV for the rapid and sensitive analysis of phenolic compounds in Terminalia species. Pharm. Chem. J. 2015, in press. [Google Scholar]
- Anand, K.K.; Singh, B.; Saxena, A.K.; Chandan, B.K.; Gupta, V.N.; Bhardwaj, V. 3,4,5-Trihydroxy benzoic acid (gallic acid), the hepatoprotective principle in the fruits of Terminalia belerica-bioassay guided activity. Pharmacol. Res. 1997, 36, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Shahrzad, S.; Bitsch, I. Determination of gallic acid and its metabolites in human plasma and urine by high-performance liquid chromatography. J. Chrom. B 1998, 705, 87–95. [Google Scholar] [CrossRef]
- Shahrzad, S.; Aoyagi, K.; Winter, A.; Koyama, A.; Bitsch, I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J. Nutr. 2001, 131, 1207–1210. [Google Scholar] [PubMed]
- Mertens-Talcott, S.U.; Jilma-Stohlawetz, P.; Rios, J.; Hingorani, L.; Derendorf, H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J. Agric. Food Chem. 2006, 54, 8956–8961. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Xing, D.M.; Xiang, L.; Zhao, Y.N.; Wang, W.; Zhang, L.J.; Du, L.J. Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J. Chrom. B 2003, 796, 189–194. [Google Scholar] [CrossRef]
- Haidara, K.; Morel, I.; Abaléa, V.; Barré, M.G.; Denizeau, F. Mechanism of tert-butylhydroperoxide induced apoptosis in rat hepatocytes: Involvement of mitochondria and endoplasmic reticulum. Biochim. Biophys. Acta 2002, 1542, 173–185. [Google Scholar] [CrossRef]
- Pfundstein, B.; Haubner, R.; Würtele, G.; Gehres, N.; Ulrich, C.M.; Owen, R.W. Pilot walnut intervention study of urolithin bioavailability in human volunteers. J. Agric. Food Chem. 2014, 62, 10264–10273. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. In Vitro Bioaccessibility, Human Gut Microbiota Metabolites and Hepatoprotective Potential of Chebulic Ellagitannins: A Case of Padma Hepaten® Formulation. Nutrients 2015, 7, 8456-8477. https://doi.org/10.3390/nu7105406
Olennikov DN, Kashchenko NI, Chirikova NK. In Vitro Bioaccessibility, Human Gut Microbiota Metabolites and Hepatoprotective Potential of Chebulic Ellagitannins: A Case of Padma Hepaten® Formulation. Nutrients. 2015; 7(10):8456-8477. https://doi.org/10.3390/nu7105406
Chicago/Turabian StyleOlennikov, Daniil N., Nina I. Kashchenko, and Nadezhda K. Chirikova. 2015. "In Vitro Bioaccessibility, Human Gut Microbiota Metabolites and Hepatoprotective Potential of Chebulic Ellagitannins: A Case of Padma Hepaten® Formulation" Nutrients 7, no. 10: 8456-8477. https://doi.org/10.3390/nu7105406