Association of Body Mass Index and Body Mass Index Change with Mortality in Incident Peritoneal Dialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Ethics Statement
2.3. Data Collection
2.4. Outcomes
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics at Baseline
| Variable * | Underweight | Normal Weight | Overweight | Obesity | p Value |
|---|---|---|---|---|---|
| Number of patients | 191 (15.1) | 707 (56.0) | 202 (16.0) | 163 (12.9) | |
| BMI (kg/m2) | 17.27 ± 0.99 | 20.74 ± 1.20 | 23.94 ± 0.58 | 27.31 ± 1.92 | <0.001 |
| Age (years) | 43.52 ± 16.23 | 46.82 ± 14.94 | 52.43 ± 13.81 | 51.01 ± 13.13 | <0.001 |
| Male | 71 (37.2) | 436 (61.7) | 129 (63.9) | 104 (63.8) | <0.001 |
| Cause of ESRD | <0.001 | ||||
| Glomerulonephritis | 148 (77.5) | 457 (64.6) | 91 (45.0) | 74 (45.4) | |
| Diabetic nephropathy | 16 (8.4) | 137 (19.4) | 68 (33.7) | 55 (33.7) | |
| Hypertension | 6 (3.1) | 49 (6.9) | 23 (11.4) | 20 (12.3) | |
| Other | 21 (11.0) | 64 (9.1) | 20 (9.9) | 14 (8.6) | |
| Diabetes | 18 (9.4) | 150 (21.2) | 77 (38.1) | 60 (36.8) | <0.001 |
| History of CVD | 84 (44) | 348 (49.2) | 97 (48.0) | 80 (49.1) | 0.634 |
| MAP (mmHg) | 101.3 ± 13.6 | 102.4 ± 14.2 | 103.1 ± 13.3 | 104.0 ± 13.5 | 0.299 |
| Hemoglobin (g/L) | 110.8 ± 23.4 | 108.6 ± 22.8 | 109.1 ± 21.6 | 104.0 ± 13.9 | 0.086 |
| Albumin (g/L) | 37.91 ± 5.66 | 37.89 ± 4.93 | 38.22 ± 4.87 | 37.87 ± 5.34 | 0.866 |
| TC (mmol/L) | 5.19 (4.40–5.94) | 5.00 (4.30–5.80) | 4.95 (4.20–5.98) | 5.20 (4.39–6.30) | 0.076 |
| TG (mmol/L) | 1.24 (0.94–1.75) | 1.36 (0.94–1.82) | 1.63 (1.53–2.35) | 1.98 (1.46–3.14) | <0.001 |
| hs-CRP (mg/L) | 0.86 (0.38–4.88) | 1.45 (0.55–4.40) | 3.12 (1.45–8.99) | 3.16 (1.40–10.39) | <0.001 |
| rGFR (mL/min/1.73m2) | 2.54 (1.20–3.60) | 2.83 (1.51–4.48) | 3.45 (2.06–5.28) | 3.26 (1.74–5.14) | <0.001 |
| Kt/Vurea | 2.50 ± 0.57 | 2.36 ± 0.63 | 2.34 ± 0.64 | 2.20 ± 0.61 | 0.001 |
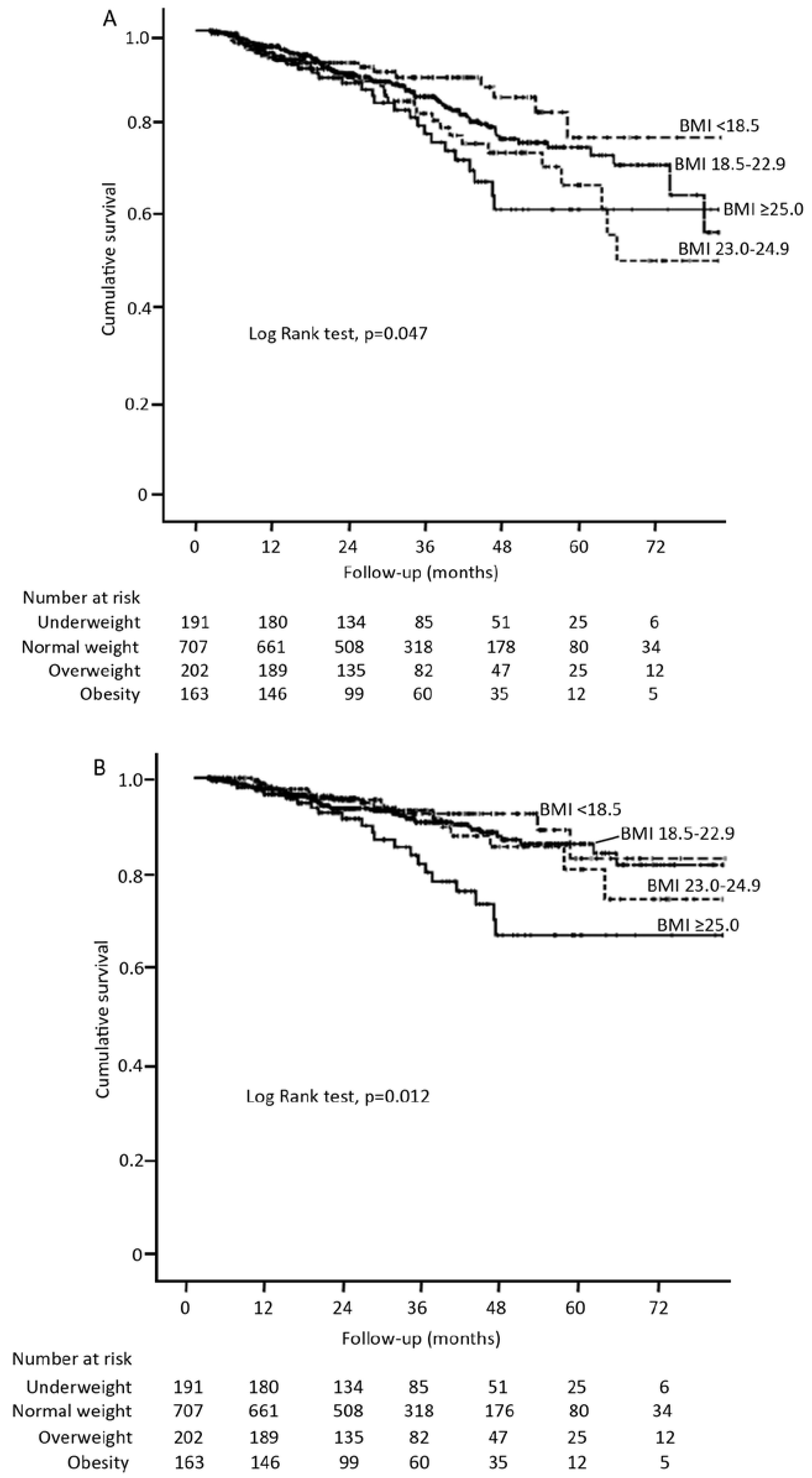
3.2. BMI and Patient Survival
| Variable | Univariate | Multivariate * | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| All-cause mortality | ||||
| Underweight | 0.75 (0.46–1.22) | 0.246 | 0.61 (0.30–1.25) | 0.178 |
| Normal weight | 1.00 (ref.) | 1.00 (ref.) | ||
| Overweight | 1.33 (0.90–1.97) | 0.153 | 0.99 (0.61–1.59) | 0.949 |
| Obesity | 1.57 (1.03–2.39) | 0.038 | 1.54 (0.94–2.52) | 0.085 |
| Cardiovascular mortality | ||||
| Underweight | 0.81 (0.44–1.52) | 0.516 | 0.79 (0.35–1.81) | 0.582 |
| Normal weight | 1.00 (ref.) | 1.00 (ref.) | ||
| Overweight | 1.03 (0.57–1.79) | 0.973 | 0.78 (0.41–1.49) | 0.437 |
| Obesity | 2.07 (1.26–3.40) | 0.004 | 2.01 (1.14–3.54) | 0.016 |
3.3. BMI Change and Mortality
| Variable * | Q1 (<−0.80%) | Q2 (−0.80%–2.69%) | Q3 (2.70%–7.40%) | Q4 (>7.40%) | p Value |
|---|---|---|---|---|---|
| Patients (n) | 220 | 220 | 220 | 220 | |
| Baseline BMI (kg/m2) | 21.73 ± 3.1 | 21.87 ± 2.99 | 21.56 ± 3.01 | 20.87 ± 2.98 | 0.003 |
| Baseline BMI group | 0.084 | ||||
| Underweight | 32 (14.6) | 27 (12.3) | 31 (14.1) | 46 (20.9) | |
| Normal weight | 121 (55.0) | 126 (57.3) | 125 (56.8) | 132 (60.0) | |
| Overweight | 36 (16.4) | 38 (17.3) | 40 (18.2) | 22 (10.0) | |
| Obesity | 31 (14.1) | 29 (13.2) | 24 (10.9) | 20 (9.1) | |
| Age (years) | 48.1 ± 15.8 | 48.1 ± 14.7 | 46.9 ± 14.3 | 48.3 ± 14.5 | 0.757 |
| Male | 122 (55.5) | 129 (58.6) | 139 (63.2) | 111 (50.5) | 0.052 |
| Diabetes | 55 (25.0) | 56 (25.5) | 41 (18.6) | 56 (25.5) | 0.253 |
| History of CVD | 102 (46.4) | 106 (48.2) | 100 (45.5) | 116 (52.7) | 0.429 |
| MAP (mmHg) | 103.1 ± 14.2 | 101.4 ± 13.2 | 101.9 ± 13.0 | 102.5 ± 12.5 | 0.519 |
| Hemoglobin (g/L) | 111.1 ± 21.1 | 110.1 ± 22.2 | 112.1 ± 21.5 | 110.5 ± 21.8 | 0.791 |
| Albumin (g/L) | 38.2 ± 4.7 | 38.3 ± 4.7 | 39.0 ± 5.0 | 38.2 ± 5.1 | 0.244 |
| TC (mmol/L) | 5.20 (4.40–5.90) | 5.00 (4.30–5.98) | 5.07 (4.30–5.88) | 5.20 (4.30–6.20) | 0.785 |
| TG (mmol/L) | 1.54 (1.06–2.23) | 1.44 (1.05–2.03) | 1.36 (0.95–2.05) | 1.42 (1.05–1.98) | 0.372 |
| hs-CRP (mg/L) | 1.73 (0.58–6.34) | 1.90 (0.58–6.14) | 1.96 (0.53–5.20) | 1.78 (0.72–5.51) | 0.591 |
| rGFR (mL/min/1.73m2) | 3.08 (1.47–5.04) | 3.04 (1.65–4.40) | 2.92 (1.66–4.49) | 2.95 (1.21–4.75) | 0.704 |
| Kt/Vurea | 2.40 ± 0.67 | 2.33 ± 0.66 | 2.35 ± 0.65 | 2.38 ± 0.56 | 0.654 |
| Variable | Univariate | Multivariate * | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| All-cause mortality | ||||
| Q1 | 2.01 (1.18–3.41) | 0.010 | 2.21 (1.23–3.95) | 0.008 |
| Q2 | 1.00 (ref.) | 1.00 (ref.) | ||
| Q3 | 0.96 (0.52–1.78) | 0.903 | 1.09 (0.54–2.19) | 0.818 |
| Q4 | 1.10 (0.61–2.01) | 0.747 | 1.19 (0.63–2.26) | 0.600 |
| Cardiovascular mortality | ||||
| Q1 | 2.24 (1.13–4.44) | 0.021 | 2.31 (1.11–4.84) | 0.026 |
| Q2 | 1.00 (ref.) | 1.00 (ref.) | ||
| Q3 | 1.17 (0.54–2.53) | 0.693 | 1.23 (0.58–3.23) | 0.479 |
| Q4 | 1.21 (0.56–2.61) | 0.630 | 1.22 (0.53–2.77) | 0.643 |


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Thun, M.J.; Petrelli, J.M.; Rodriguez, C.; Heath, C.W., Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N. Engl. J. Med. 1999, 341, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Cai, J.; Pamuk, E.R.; Williamson, D.F.; Thun, M.J.; Wood, J.L. The effect of age on the association between body-mass index and mortality. N. Engl. J. Med. 1998, 338, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Byers, T. Body weight and mortality. N. Engl. J. Med. 1995, 333, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; McCulloch, C.E.; Iribarren, C.; Darbinian, J.; Go, A.S. Body mass index and risk for end-stage renal disease. Ann. Intern. Med. 2006, 144, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Hsu, C.Y. Excess weight as a risk factor for kidney failure. Curr. Opin. Nephrol. Hypertens. 2007, 16, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Praga, M.; Morales, E. Obesity, proteinuria and progression of renal failure. Curr. Opin. Nephrol. Hypertens. 2006, 15, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Chazot, C.; Gassia, J.P.; di Benedetto, A.; Cesare, S.; Ponce, P.; Marcelli, D. Is there any survival advantage of obesity in southern European haemodialysis patients? Nephrol. Dial. Transplant. 2009, 24, 2871–2876. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Abbott, K.C.; Salahudeen, A.K.; Kilpatrick, R.D.; Horwich, T.B. Survival advantages of obesity in dialysis patients. Am. J. Clin. Nutr. 2005, 81, 543–554. [Google Scholar] [PubMed]
- Port, F.K.; Ashby, V.B.; Dhingra, R.K.; Roys, E.C.; Wolfe, R.A. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J. Am. Soc. Nephrol. 2002, 13, 1061–1066. [Google Scholar] [PubMed]
- Schmidt, D.S.; Salahudeen, A.K. Obesity—Survival paradox—Still a controversy? Semin. Dial. 2007, 20, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Herzig, K.A.; Purdie, D.M.; Chang, W.; Brown, A.M.; Rigby, R.J.; Campbell, S.B.; Nicol, D.L.; Hawley, C.M. Is obesity a favorable prognostic factor in peritoneal dialysis patients? Perit. Dial. Int. 2000, 20, 715–721. [Google Scholar] [PubMed]
- Snyder, J.J.; Foley, R.N.; Gilbertson, D.T.; Vonesh, E.F.; Collins, A.J. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int. 2003, 64, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.P.; Collins, J.F.; Johnson, D.W. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J. Am. Soc. Nephrol. 2003, 14, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Unal, A.; Sipahioglu, M.H.; Kocyigit, I.; Elmali, F.; Tokgoz, B.; Oymak, O. Does body mass index affect survival and technique failure in patients undergoing peritoneal dialysis? Pak. J. Med. Sci. 2014, 30, 41–44. [Google Scholar] [PubMed]
- De Mutsert, R.; Grootendorst, D.C.; Boeschoten, E.W.; Dekker, F.W.; Krediet, R.T. Is obesity associated with a survival advantage in patients starting peritoneal dialysis? Contrib. Nephrol. 2009, 163, 124–131. [Google Scholar] [PubMed]
- Fernandes, N.M.; Bastos, M.G.; Franco, M.R.; Chaoubah, A.; Lima Mda, G.; Divino-Filho, J.C.; Qureshi, A.R. Body size and longitudinal body weight changes do not increase mortality in incident peritoneal dialysis patients of the Brazilian peritoneal dialysis multicenter study. Clinics 2013, 68, 51–58. [Google Scholar] [CrossRef]
- Johansen, K.L.; Young, B.; Kaysen, G.A.; Chertow, G.M. Association of body size with outcomes among patients beginning dialysis. Am. J. Clin. Nutr. 2004, 80, 324–332. [Google Scholar] [PubMed]
- Kiran, V.R.; Zhu, T.Y.; Yip, T.; Lui, S.L.; Lo, W.K. Body mass index and mortality risk in Asian peritoneal dialysis patients in Hong Kong—Impact of diabetes and cardiovascular disease status. Perit. Dial. Int. 2014, 34, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, S.H.; Kim, H.W.; Kim, Y.O.; Jin, D.C.; Song, H.C.; Choi, E.J.; Kim, Y.L.; Kim, Y.S.; Kang, S.W.; et al. The association between body mass index and mortality on peritoneal dialysis: A prospective cohort study. Perit. Dial. Int. 2014, 34, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a who consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Xu, Q.; Xiong, L.; Fan, L.; Xu, F.; Yang, Y.; Li, H.; Peng, X.; Cao, S.; Zheng, Z.; Yang, X.; et al. Association of pulmonary hypertension with mortality in incident peritoneal dialysis patients. Perit. Dial. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Psaty, B.M.; Kuller, L.H.; Bild, D.; Burke, G.L.; Kittner, S.J.; Mittelmark, M.; Price, T.R.; Rautaharju, P.M.; Robbins, J. Methods of assessing prevalent cardiovascular disease in the cardiovascular health study. Ann. Epidemiol. 1995, 5, 270–277. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Sarnak, M.J.; Katz, R.; Fried, L.F.; Seliger, S.L.; Newman, A.B.; Siscovick, D.S.; Stehman-Breen, C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N. Engl. J. Med. 2005, 352, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Wang, M.; Woo, J.; Lam, C.W.; Li, P.K.; Lui, S.F.; Sanderson, J.E. Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: A prospective study. J. Am. Soc. Nephrol. 2003, 14, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Abbott, K.C.; Glanton, C.W.; Trespalacios, F.C.; Oliver, D.K.; Ortiz, M.I.; Agodoa, L.Y.; Cruess, D.F.; Kimmel, P.L. Body mass index, dialysis modality, and survival: Analysis of the United States renal data system dialysis morbidity and mortality wave II study. Kidney Int. 2004, 65, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Stack, A.G.; Murthy, B.V.; Molony, D.A. Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney Int. 2004, 65, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.; Bernardini, J.; Piraino, B. Neither size nor weight predicts survival in peritoneal dialysis patients. Perit. Dial. Int. 1996, 16, 357–361. [Google Scholar] [PubMed]
- Chen, Y.; Copeland, W.K.; Vedanthan, R.; Grant, E.; Lee, J.E.; Gu, D.; Gupta, P.C.; Ramadas, K.; Inoue, M.; Tsugane, S.; et al. Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: Pooled analysis of prospective data from the Asia cohort consortium. BMJ 2013, 347. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, G.; Offer, A.; Zhou, M.; Smith, M.; Peto, R.; Ge, H.; Yang, L.; Whitlock, G. Body mass index and mortality in China: A 15-year prospective study of 220,000 men. Int. J. Epidemiol. 2012, 41, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; Sull, J.W.; Park, J.; Lee, S.Y.; Ohrr, H.; Guallar, E.; Samet, J.M. Body-mass index and mortality in Korean men and women. N. Engl. J. Med. 2006, 355, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Billington, C.J.; Epstein, L.H.; Goodwin, N.J.; Hill, J.O.; Pi-Sunyer, F.X.; Rolls, B.J.; Stern, J.; Wadden, T.A.; Weinsier, R.L.; Wilson, G.T.; et al. Overweight, obesity, and health risk. Arc. Intern. Med. 2000, 160, 898–904. [Google Scholar]
- Asia Pacific Cohort Studies Collaboration. Body mass index and cardiovascular disease in the Asia-Pacific region: An overview of 33 cohorts involving 310,000 participants. Int. J. Epidemiol. 2004, 33, 751–758. [Google Scholar]
- Memish, Z.A.; el Bcheraoui, C.; Tuffaha, M.; Robinson, M.; Daoud, F.; Jaber, S.; Mikhitarian, S.; Al Saeedi, M.; AlMazroa, M.A.; Mokdad, A.H.; et al. Obesity and associated factors—Kingdom of Saudi Arabia, 2013. Prev. Chronic Dis. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Nikolic, D.; Katsiki, N.; Montalto, G.; Isenovic, E.R.; Mikhailidis, D.P.; Rizzo, M. Lipoprotein subfractions in metabolic syndrome and obesity: Clinical significance and therapeutic approaches. Nutrients 2013, 5, 928–948. [Google Scholar] [CrossRef] [PubMed]
- Stompor, T.; Sulowicz, W.; Dembinska-Kiec, A.; Janda, K.; Wojcik, K.; Zdzienicka, A. An association between body mass index and markers of inflammation: Is obesity the proinflammatory state in patients on peritoneal dialysis? Perit. Dial. Int. 2003, 23, 79–83. [Google Scholar] [PubMed]
- Kalantar-Zadeh, K.; Kilpatrick, R.D.; Kuwae, N.; Wu, D.Y. Reverse epidemiology: A spurious hypothesis or a hardcore reality? Blood Purif. 2005, 23, 57–63. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, L.; Cao, S.; Xu, F.; Zhou, Q.; Fan, L.; Xu, Q.; Yu, X.; Mao, H. Association of Body Mass Index and Body Mass Index Change with Mortality in Incident Peritoneal Dialysis Patients. Nutrients 2015, 7, 8444-8455. https://doi.org/10.3390/nu7105405
Xiong L, Cao S, Xu F, Zhou Q, Fan L, Xu Q, Yu X, Mao H. Association of Body Mass Index and Body Mass Index Change with Mortality in Incident Peritoneal Dialysis Patients. Nutrients. 2015; 7(10):8444-8455. https://doi.org/10.3390/nu7105405
Chicago/Turabian StyleXiong, Liping, Shirong Cao, Fenghua Xu, Qian Zhou, Li Fan, Qingdong Xu, Xueqing Yu, and Haiping Mao. 2015. "Association of Body Mass Index and Body Mass Index Change with Mortality in Incident Peritoneal Dialysis Patients" Nutrients 7, no. 10: 8444-8455. https://doi.org/10.3390/nu7105405




