Dietary Intervention Restored Menses in Female Athletes with Exercise-Associated Menstrual Dysfunction with Limited Impact on Bone and Muscle Health
Abstract
:1. Introduction
2. Experimental Section
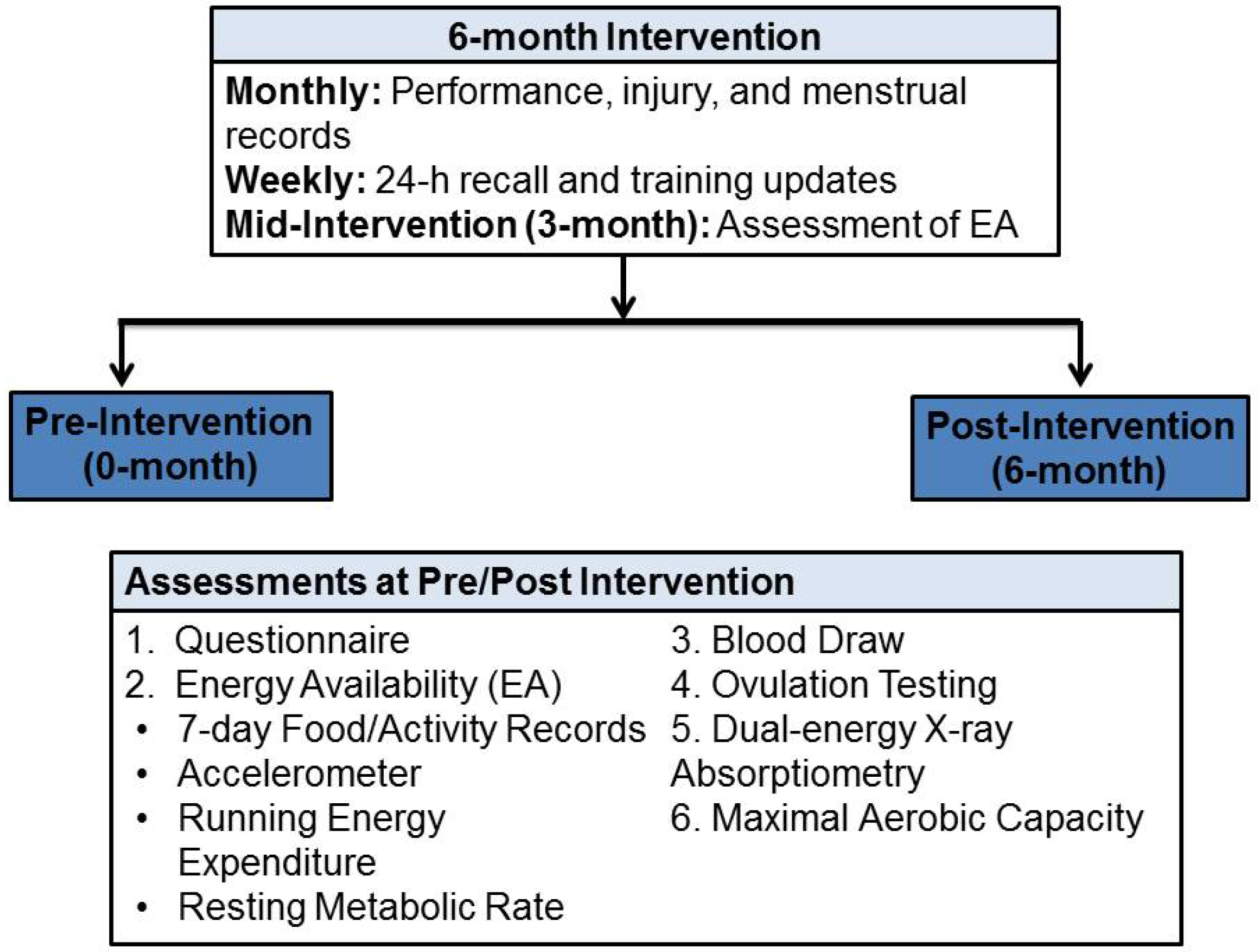
2.1. Study Protocol and Participants
2.2. Maximal Aerobic Capacity Test (VO2max)
2.3. Blood Biochemistry
2.4. Energy Intake and Expenditure Measurement and Analysis
2.4.1. Measurements
2.4.2. Analysis
2.5. Bone Density and Body Composition
2.6. Muscle Strength and Power
2.7. Submaximal Exercise Protocol and Skeletal Muscle Analysis
2.7.1. Submaximal Exercise and Post-Exercise Muscle Biopsy
2.7.2. Muscle Biopsy Preparation and Antibodies and Positive Controls
2.7.3. Western Blotting
2.8. Intervention Assessments
2.9. Statistical Analysis
3. Results
3.1. Physical Characteristic
3.2. Energy Availability, Energy Balance, and Energy and Nutrient Intakes
| Values expressed as Mean ± SD | Eumenorrheic Controls (n = 9) | Women with ExMD (n = 8) | |
|---|---|---|---|
| Description | 0-months | 0-months | 6-months |
| Age (year) | 23.1 ± 4.3 | 22.6 ± 3.3 | - |
| Age at Menarche (year) | 12.7 ± 1.3 | 13.5 ± 2.0 | - |
| Weight (kg) | 66.8 ± 9.3 | 62.4 ± 7.8 | 64.0 ± 8.0 |
| Lean Body Mass (kg) d | 48.5 ± 4.7 | 46.2 ± 4.4 | 46.1 ± 4.7 |
| Fat Free Mass (kg) d | 51.0 ± 5.0 | 48.5 ± 4.6 | 48.4 ± 4.8 |
| Body Mass Index (BMI) (kg/m2) | 23.2 ± 2.8 | 22.3 ± 2.5 | 22.9 ± 2.5 |
| Body Fat (%) d | 23.2 ± 4.4 | 22.0 ± 4.7 | 24.1 ± 3.9 |
| Exercise (h/week) e | 7.4 ± 3.6 | 7.4 ± 3.2 | 7.1 ± 3.4 |
| VO2max (mL/kg/min) f | 50.6 ± 5.2 | 49.0 ± 5.8 | 49.3 ± 6.0 |
| VO2max (L/min) f | 3.3 ± 0.4 | 3.0 ± 0.3 | 3.1 ± 0.4 |
| Values expressed as Mean ± SD | Eumenorrheic Controls (n = 9) | Women with ExMD (n = 8) | |
|---|---|---|---|
| Description | 0-months | 0-months | 6-months |
| Bone Mineral Content (g) d | |||
| Whole Body | 2492 ± 332 | 2326 ± 314 | 2331 ± 280 |
| Total Hip | 39 ± 6 | 33 ± 8 | 34 ± 8 |
| Total Spine | 66 ± 13 | 61 ± 14 | 61 ± 13 |
| Bone Mineral Density (g·cm2) d | |||
| Whole Body | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 |
| Total Hip | 1.1 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| Total Spine | 1.1 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| Energy Intake (kcal/day) | 2430 ± 524 | 2312 ± 324 | 2694 ± 541 |
| Resting Metabolic Rate (kcal/day) | 1491 ± 117 | 1514 ± 142 | 1522 ± 134 |
| Energy Balance (kcal/day) | −171 ± 459 | −510 ± 361 | −44 ± 707 |
| Energy Balance (kcal/kg FFM/day) | −3.0 ± 9.7 | −10.3 ± 6.9 | −0.7 ± 15.1 |
| Energy Availability (kcal/day) | 1945 ± 452 | 1760 ± 429 | 2177 ± 645 |
| Energy Availability (kcal/kg FFM/day) e | 38.3 ± 10.3 | 36.7 ± 10.2 | 45.4 ± 14.7 |
| Values Expressed as Mean ± SD | Eumenorrheic Controls (n = 9) | Women with ExMD (n = 8) | |
|---|---|---|---|
| Description | 0-months | 0-months | 6-months |
| Carbohydrate | |||
| % of Total Energy | 50 ± 5 | 53 ± 7 | 51 ± 6 |
| g/day | 304 ± 69 | 308 ± 56 | 340 ± 42 |
| g/kg/day | 4.6 ± 1.0 | 5.0 ± 1.2 | 5.4 ± 0.4 |
| Protein | |||
| % of Total Energy | 15 ± 3 | 15 ± 4 | 17 ± 2 |
| g/day | 89 ± 21 | 87 ± 17 | 114 ± 27 |
| g/kg/day | 1.3 ± 0.3 | 1.4 ± 0.2 | 1.8 ± 0.5 |
| Fat | |||
| % of Total Energy | 34 ± 5 | 30 ± 3 | 30 ± 5 |
| g/day | 93 ± 27 | 76 ± 16 | 93 ± 33 |
| Alcohol | |||
| % of Total Energy | 2 ± 3 | 3 ± 4 | 3 ± 4 |
| g/day | 9 ± 10 | 11 ± 14 | 11 ± 14 |
| Fiber (g/day) | 29 ± 11 | 28 ± 9 | 26 ± 9 |
| Vitamins and Minerals | |||
| Folate (μg/day) | 449 ± 207 | 532 ± 468 | 403 ± 242 |
| Vitamin B12 (μg/day) | 8 ± 5 | 14 ± 25 | 6 ± 2 |
| Vitamin D (IU/day) | 385 ± 314 | 379 ± 321 | 383 ± 316 |
| Calcium (mg/day) | 1211 ± 385 | 1320 ± 571 | 1725 ± 555 |
| Iron (mg/day) | 24 ± 9 | 29 ± 15 | 22 ± 5 |
| Magnesium (mg/day) | 365 ± 194 | 288 ± 115 | 330 ± 69 |
| Phosphorous (mg/day) | 1098 ± 471 | 911 ± 384 | 1034 ± 386 |
| Zinc (mg/day) | 14 ± 6 | 13 ± 8 | 16 ± 5 |
| Values expressed as Mean ± SD | Eumenorrheic Controls (n = 10) | Women with ExMD (n = 8) | NormalValues c | |
|---|---|---|---|---|
| Description | 0-months | 0-months | 6-months | |
| Bone Markers d | ||||
| P1NP (ng/mL) | 42.0 ± 30.8 | 52.9 ± 36.4 | 58.5 ± 19.2 | 27.7–127.6 e |
| Osteocalcin (nmol/L) | 4.7 ± 1.4 | 4.5 ± 1.3 | 4.4 ± 1.7 | 1.8–7.8 e |
| CTX (ng/mL) | 0.754 ± 0.248 | 0.661 ± 0.324 | 0.660 ± 0.214 | 0.000-0.700 e |
| Hormones | ||||
| T (nmol/L) | 1.70 ± 0.16 | 1.61 ± 0.32 | 1.62 ± 0.24 | 1.20–2.74 |
| Estradiol (pmol/L) | 158.1 ± 115.4 | 232.6 ± 260.7 | 399.9 ± 557.8 | 45.9–609.4 |
| Progesterone (nmol/mL) | 1.8 ± 1.1 | 3.2 ± 3.1 | 2.5 ± 1.4 | 0.6–4.8 |
| LH (IU/L) | 5.9 ± 3.3 | 5.6 ± 4.1 | 15.3 ± 16.3 | 2.4–12.6 |
| FSH (IU/L) | 5.5 ± 1.2 | 4.1 ± 2.2 d | 5.1 ± 2.0 | 4–13 |
| Vitamins | ||||
| Folate (ng/mL) | 14.9 ± 3.1 | 16.2 ± 2.8 | 15.0 ± 2.0 | >9.1 |
| B12 (pg/ml) | 561 ± 116 | 704 (381) | 689 ± 290 | 211–946 |
| 25-OH Vit D (/mL) | 105.4 ± 30.1 | 106.7 ± 24.6 | 115.1 ± 19.2 | 14.7–162.0 |
| Iron Panel | ||||
| Serum Iron (/dL) | 115 ± 58 | 104 ± 61 | 74 ± 33 | 37–145 |
| TIBC (/dL) | 313 ± 57 | 364 ± 62 | 346 ± 43 | 250–450 |
| % Saturation | 37 ± 18 | 29 ± 16 | 22 ± 11 | 15–50 |
| Ferritin (ng/mL) | 36.5 ± 26.0 | 27.5 ± 16.6 | 31.2 ± 15.8 | 13–150 |
| Ovulation f | n = 10 | n = 0 | n = 7 | - |
3.3. Menstrual Status
3.4. Bone Health
| Months since Last Menses b | n Size | Total Hip z-score (mean) | Total Spine (L1–L4) z-score (mean) | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| 0–3 c | 1 | 2.4 | 2.2 | 1.4 | 1.1 |
| >3–6 | 4 | 1.2 | 1.0 | −0.1 | −0.1 |
| >6–12 | 1 | 0.8 | 0.7 | 0.0 | 0.2 |
| >12 | 2 | −0.2 | 0.1 | −1.6 | −1.4 |
| All | 8 | 0.5 | 0.5 | −0.2 | −0.1 |
| Range | −0.6 to 3.2 | −0.4 to 3.0 | −2.9 to 1.4 | −2.3 to 1.1 | |
3.5. Muscle Power and Torque and Markers of Protein Synthesis and Degradation
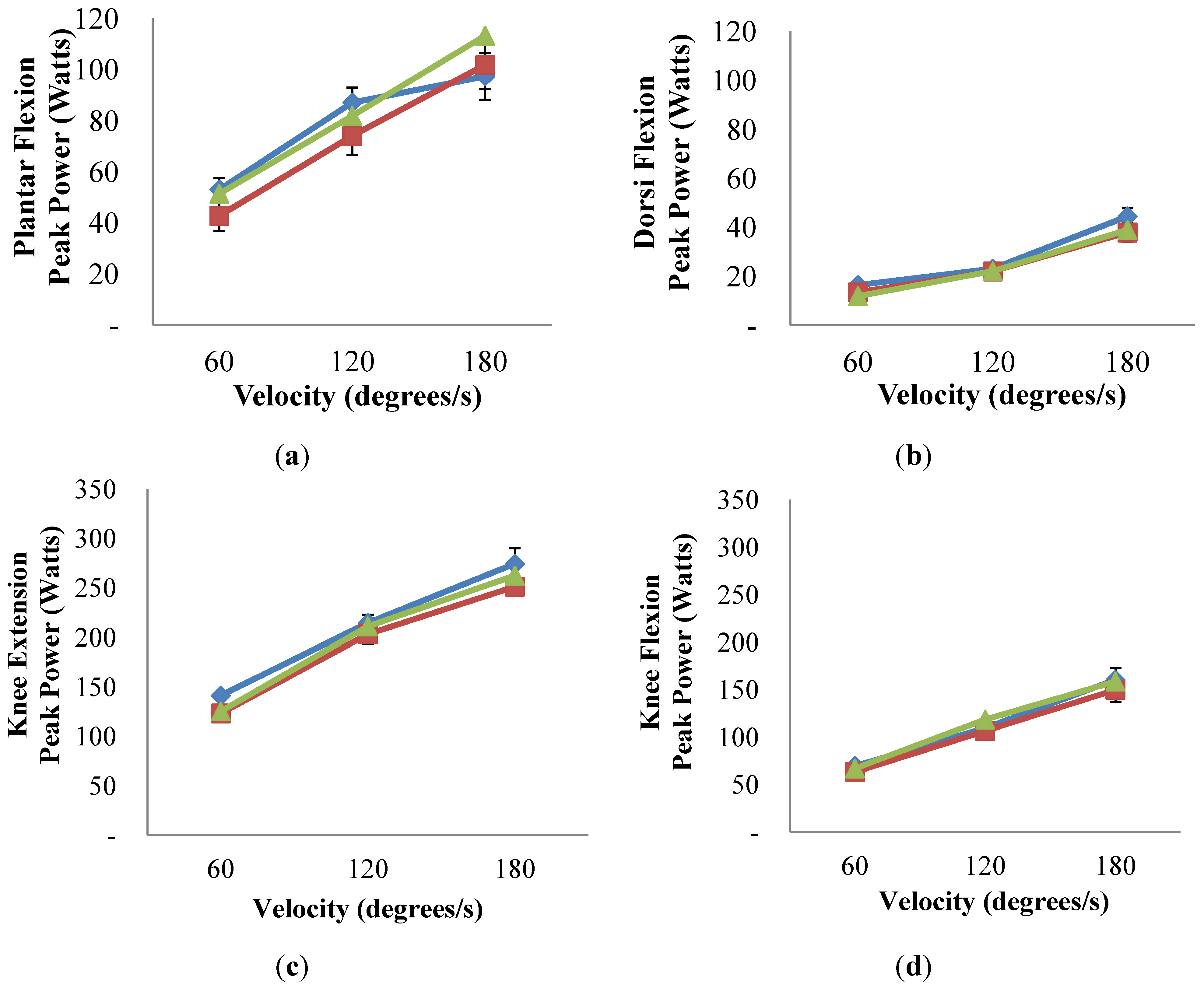
 ) only and compared to ExMD at 0-month (
) only and compared to ExMD at 0-month (  ) and at 6-months (
) and at 6-months (  ). No differences were detected (p-value > 0.05 for all comparisons). Multiple linear regression was used to compare Eumen and ExMD groups and 0-month vs. 6-months in ExMD group, blocking on participant and controlling for baseline age and weight and false discovery rate (FDR) of 5%.
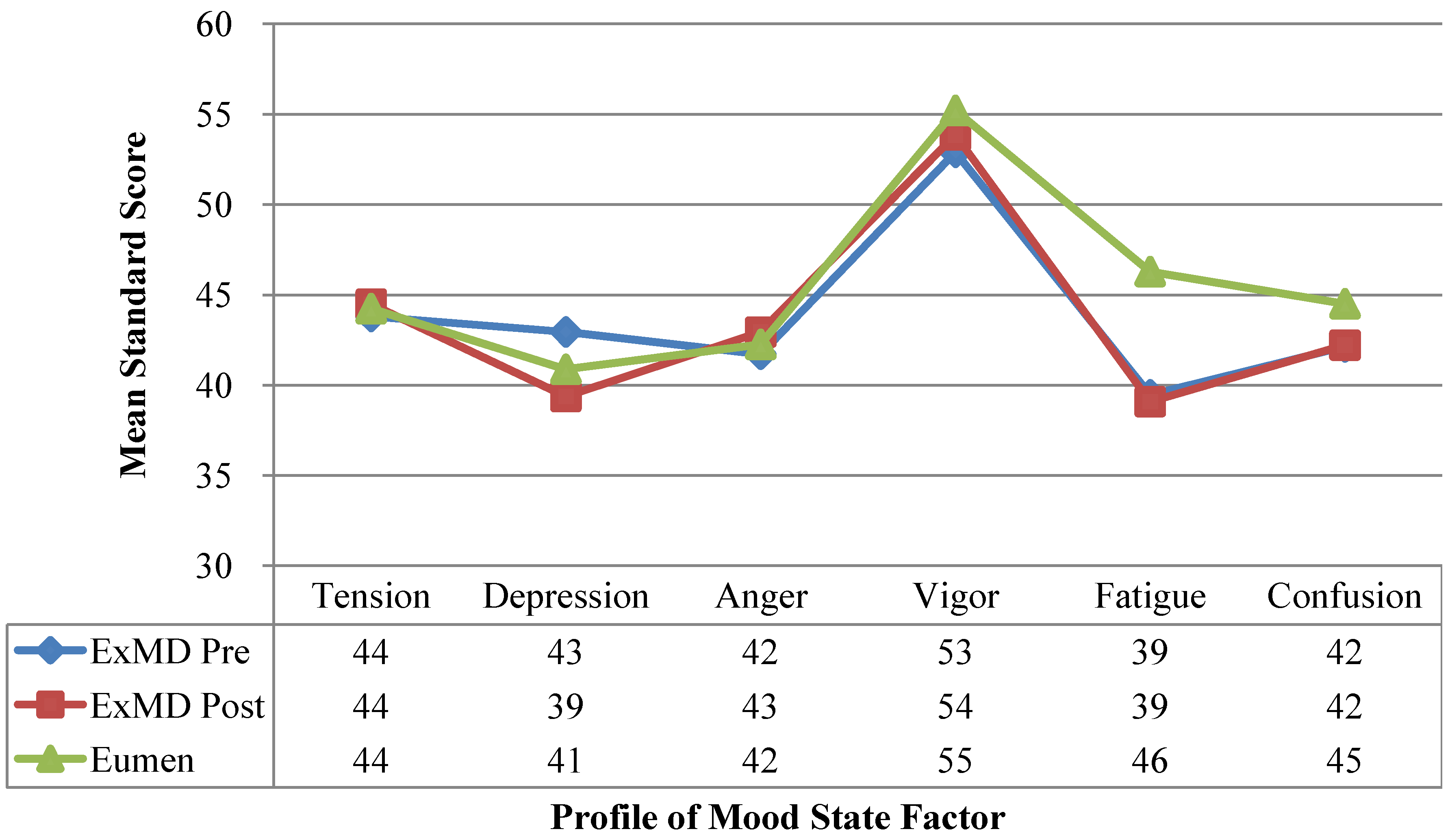
). No differences were detected (p-value > 0.05 for all comparisons). Multiple linear regression was used to compare Eumen and ExMD groups and 0-month vs. 6-months in ExMD group, blocking on participant and controlling for baseline age and weight and false discovery rate (FDR) of 5%.3.6. Profile of Mood States
4. Discussion
4.1. Energy and Nutrient Intakes
4.2. Menstrual Status and Blood Hormone
4.3. Bone Health
4.4. Blood Bone Markers
4.5. Muscle Torque and Power and Markers of Protein Synthesis and Degradation
4.6. Profile of Mood State
4.7. Strengths and Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rodriguez, N.R.; di Marco, N.M.; Langley, S. American college of sports medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar] [CrossRef]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P.; American College of Sports Medicine. American college of sports medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007, 39, 1867–1882. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The ioc consensus statement: Beyond the female athlete triad--relative energy deficiency in sport (red-s). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.C.; Williams, N.I.; de Souza, M.J. Prevalence of individual and combined components of the female athlete triad. Med. Sci. Sports Exerc. 2013, 45, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.P.; Perlroth, N.E. The effects of intense exercise on the female reproductive system. J. Endocrinol. 2001, 170, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Nattiv, A.; Kennedy, G.; Barrack, M.T.; Abdelkerim, A.; Goolsby, M.A.; Arends, J.C.; Seeger, L.L. Correlation of mri grading of bone stress injuries with clinical risk factors and return to play: A 5-year prospective study in collegiate track and field athletes. Am. J. Sports Med. 2013, 41, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Hoch, A.Z.; Lal, S.; Jurva, J.W.; Gutterman, D.D. The female athlete triad and cardiovascular dysfunction. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 385–400. [Google Scholar]
- Enns, D.L.; Tiidus, P.M. The influence of estrogen on skeletal muscle: Sex matters. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Enns, D.L.; Iqbal, S.; Tiidus, P.M. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol. (Oxf.) 2008, 194, 81–93. [Google Scholar] [CrossRef]
- Carter, A.; Dobridge, J.; Hackney, A.C. Influence of estrogen on markers of muscle tissue damage following eccentric exercise. Fiziol. Cheloveka 2001, 27, 133–137. [Google Scholar] [PubMed]
- Kanaley, J.A.; Ji, L.L. Antioxidant enzyme activity during prolonged exercie in amenorrheic and eumenorrheic athletes. Metab. Clin. Exp. 1991, 40, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Sipaviciene, S.; Daniuseviciute, L.; Kliziene, I.; Kamandulis, S.; Skurvydas, A. Effects of estrogen fluctuation during the menstrual cycle on the response to stretch-shortening exercise in females. BioMed. Res. Int. 2013, 2013, 243572. [Google Scholar]
- Markofski, M.M.; Braun, W.A. Influence of menstrual cycle on indices of contraction-induced muscle damage. J. Strength Cond. Res. 2014, in press. [Google Scholar]
- Miller, B.F.; Hansen, M.; Olesen, J.L.; Flyvbjerg, A.; Schwarz, P.; Babraj, J.A.; Smith, K.; Rennie, M.J.; Kjaer, M. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E163–E168. [Google Scholar] [CrossRef] [PubMed]
- Manore, M.M.; Kam, L.C.; Loucks, A.B.; International Association of Athletics Federations. The female athlete triad: Components, nutrition issues, and health consequences. J. Sports Sci. 2007, 25 (Suppl. 1), S61–S71. [Google Scholar]
- American Society of Health-System Pharmacists, Inc. Estrogen and Progestin (Oral Contraceptives). Available online: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a601050.html (accessed on 31 January 2014).
- Arends, J.C.; Cheung, M.Y.; Barrack, M.T.; Nattiv, A. Restoration of menses with nonpharmacologic therapy in college athletes with menstrual disturbances: A 5-year retrospective study. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 98–108. [Google Scholar] [PubMed]
- Kopp-Woodroffe, S.A.; Manore, M.M.; Dueck, C.A.; Skinner, J.S.; Matt, K.S. Energy and nutrient status of amenorrheic athletes participating in a diet and exercise training intervention program. Int. J. Sport Nutr. 1999, 9, 70–88. [Google Scholar] [PubMed]
- Garner, D.M.; Olmstead, M.P.; Polivy, J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int. J. Eat. Disord. 1983, 2, 15–34. [Google Scholar] [CrossRef]
- Sundgot-Borgen, J. Nutrient intake of female elite athletes suffering from eating disorders. Int. J. Sport Nutr. 1993, 3, 431–442. [Google Scholar] [PubMed]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Revised Manual for the Profile of Mood States; Educational and Industrial Testing Services: San Diego, CA, USA, 1992. [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil. Steril. 2008, 90, S219–S225. [Google Scholar] [PubMed]
- Guebels, C.P.; Kam, L.C.; Maddalozzo, G.F.; Manore, M.M. Active women before/after an intervention designed to restore menstrual function: Resting metabolic rate and comparison of four methods to quantify energy expenditure and energy availability. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Woolf, K.; LoBuono, D.L.; Manore, M.M. B-vitamins and physical activity: Is need increased? In Nutrition and the Female Athlete, 2nd ed.; Beals, K.A., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 139–182. [Google Scholar]
- Willis, K.S.; Smith, D.T.; Broughton, K.S.; Larson-Meyer, D.E. Vitamin d status and biomarkers of inflammation in runners. Open Access J. Sports Med. 2012, 3, 35–42. [Google Scholar] [PubMed]
- Seibel, M.J. Biochemical markers of bone turnover: Part I: Biochemistry and variability. Clin. Biochem. Rev. 2005, 26, 97–122. [Google Scholar] [PubMed]
- Tomten, S.E.; Hostmark, A.T. Energy balance in weight stable athletes with and without menstrual disorders. Scand. J. Med. Sci. Sports 2006, 16, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar]
- Writing Group for the ISCD Position Development Conference. Diagnosis of osteoporosis in men, premenopausal women, and children. J. Clin. Densitom. 2004, 7, 17–26. [Google Scholar]
- Drouin, J.M.; Valovich-mcLeod, T.C.; Shultz, S.J.; Gansneder, B.M.; Perrin, D.H. Reliability and validity of the biodex system 3 pro isokinetic dynamometer velocity, torque and position measurements. Eur. J. Appl. Physiol. 2004, 91, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bassey, E.J.; Short, A.H. A new method for measuring power output in a single leg extension: Feasibility, reliability and validity. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, R.J.; Williams, N.I.; Olmsted, M.P.; Scheid, J.L.; Riddle, E.S.; de Souza, M.J. A case report of recovery of menstrual function following a nutritional intervention in two exercising women with amenorrhea of varying duration. J. Int. Soc. Sports Nutr. 2013, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Verdun, M.; Heath, E.M. Low energy availability, not stress of exercise, alters l h pulsatility in exercising women. J. Appl. Physiol. 1998, 84, 37–46. [Google Scholar] [PubMed]
- Gremion, G.; Rizzoli, R.; Slosman, D.; Theintz, G.; Bonjour, J.P. Oligo-amenorrheic long-distance runners may lose more bone in spine than in femur. Med. Sci. Sports Exerc. 2001, 33, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rencken, M.L.; Chesnut, C.H., III; Drinkwater, B.L. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA 1996, 276, 238–240. [Google Scholar]
- De Souza, M.J.; Maguire, M.S.; Maresh, C.M.; Kraemer, W.J.; Rubin, K.R.; Loucks, A.B. Adrenal activation and the prolactin response to exercise in eumenorrheic and amenorrheic runners. J. Appl. Physiol. 1991, 70, 2378–2387. [Google Scholar] [PubMed]
- Wilmore, J.H.; Wambsgans, K.C.; Brenner, M.; Broeder, C.E.; Paijmans, I.; Volpe, J.A.; Wilmore, K.M. Is there energy conservation in amenorrheic compared with eumenorrheic distance runners? J. Appl. Physiol. 1992, 72, 15–22. [Google Scholar]
- Glass, A.R.; Deuster, P.A.; Kyle, S.B.; Yahiro, J.A.; Vigersky, R.A.; Schoomaker, E.B. Amenorrhea in olympic marathon runners. Fertil. Steril. 1987, 48, 740–745. [Google Scholar] [PubMed]
- Zanker, C.L.; Swaine, I.L. Relation between bone turnover, oestradiol, and energy balance in women distance runners. Br. J. Sports Med. 1998, 32, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Zanker, C.L.; Swaine, I.L. Bone turnover in amenorrhoeic and eumenorrhoeic women distance runners. Scand. J. Med. Sci. Sports 1998, 8, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B. Energy balance and body composition in sports and exercise. J. Sports Sci. 2004, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Snead, D.B.; Stubbs, C.C.; Weltman, J.Y.; Evans, W.S.; Veldhuis, J.D.; Rogol, A.D.; Teates, C.D.; Weltman, A. Dietary patterns, eating behaviors, and bone mineral density in women runners. Am. J. Clin. Nutr. 1992, 56, 705–711. [Google Scholar] [PubMed]
- Snead, D.B.; Weltman, A.; Weltman, J.Y.; Evans, W.S.; Veldhuis, J.D.; Varma, M.M.; Teates, C.D.; Dowling, E.A.; Rogol, A.D. Reproductive hormones and bone mineral density in women runners. J. Appl. Physiol. 1992, 72, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Stacey, E.; Korkia, P.; Hukkanen, M.V.; Polak, J.M.; Rutherford, O.M. Decreased nitric oxide levels and bone turnover in amenorrheic athletes with spinal osteopenia. J. Clin. Endocrinol. Metab. 1998, 83, 3056–3061. [Google Scholar] [PubMed]
- Fredericson, M.; Kent, K. Normalization of bone density in a previously amenorrheic runner with osteoporosis. Med. Sci. Sports Exerc. 2005, 37, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, B.L.; Nilson, K.; Ott, S.; Chesnut, C.H., III. Bone mineral density after resumption of menses in amenorrheic athletes. JAMA 1986, 256, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Zanker, C.L.; Cooke, C.B.; Truscott, J.G.; Oldroyd, B.; Jacobs, H.S. Annual changes of bone density over 12 years in an amenorrheic athlete. Med. Sci. Sports Exerc. 2004, 36, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Goltzman, D.; Langsetmo, L.; Joseph, L.; Jackson, S.; Kreiger, N.; Tenenhouse, A.; Davison, K.S.; Josse, R.G.; Prior, J.C.; et al. Peak bone mass from longitudinal data: Implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J. Bone Miner. Res. 2010, 25, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; West, S.L.; Jamal, S.A.; Hawker, G.A.; Gundberg, C.M.; Williams, N.I. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone 2008, 43, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.H.; Mitchell, A.; Harries, M.G.; Reeve, J. Nutritional and exercise-related determinants of bone density in elite female runners. Osteoporos. Int. 2004, 15, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, U.; Stalnacke, B.; Ahlenius, G.; Henriksson-Larsen, K.; Lorentzon, R. Low bone mass density at multiple skeletal sites, including the appendicular skeleton in amenorrheic runners. Calcif. Tissue Int. 1999, 64, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Seibel, M.J.; Robins, S.P.; Bilezikian, J.P. Dynamics of bone and cartilage metabolism, 2nd ed.; Academic Press: San Diego, CA, USA, 2006; pp. 916–919. [Google Scholar]
- Janse de Jonge, X.A.; Boot, C.R.; Thom, J.M.; Ruell, P.A.; Thompson, M.W. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J. Physiol. 2001, 530, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, C.M.; McKenzie, D.C.; Prior, J.C.; Taunton, J.E. Effects of menstrual cycle phase on athletic performance. Med. Sci. Sports Exerc. 1995, 27, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Williams, N.I.; Olmsted-Kramer, L.C.; Leidy, H.J.; Putukian, M. Neuromuscular performance and knee laxity do not change across the menstrual cycle in female athletes. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 817–822. [Google Scholar] [CrossRef] [PubMed]
- DiBrezzo, R.; Fort, I.L.; Brown, B. Relationships among strength, endurance, weight and body fat during three phases of the menstrual cycle. J. Sports Med. Phy. Fit. 1991, 31, 89–94. [Google Scholar]
- Abt, J.P.; Sell, T.C.; Laudner, K.G.; McCrory, J.L.; Loucks, T.L.; Berga, S.L.; Lephart, S.M. Neuromuscular and biomechanical characteristics do not vary across the menstrual cycle. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.K.; Sanderson, A.G.; Birch, K.; Bruce, S.A.; Woledge, R.C. Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. J. Physiol. 1996, 496, 551–557. [Google Scholar] [PubMed]
- Hallas, J.; Bjerrum, L.; Stovring, H.; Andersen, M. Use of a prescribed ephedrine/caffeine combination and the risk of serious cardiovascular events: A registry-based case-crossover study. Am. J. Epidemiol. 2008, 168, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Bar-Eli, M. Essential Readings in Sport and Exercise Psychology; Human Kinetics: Champaign, IL, USA, 2007. [Google Scholar]
- Terry, P.C.; Lane, A.M. Normative values for the profile of mood states for use with athletic samples. J. Appl. Sport Psychol. 2000, 12, 93–109. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cialdella-Kam, L.; Guebels, C.P.; Maddalozzo, G.F.; Manore, M.M. Dietary Intervention Restored Menses in Female Athletes with Exercise-Associated Menstrual Dysfunction with Limited Impact on Bone and Muscle Health. Nutrients 2014, 6, 3018-3039. https://doi.org/10.3390/nu6083018
Cialdella-Kam L, Guebels CP, Maddalozzo GF, Manore MM. Dietary Intervention Restored Menses in Female Athletes with Exercise-Associated Menstrual Dysfunction with Limited Impact on Bone and Muscle Health. Nutrients. 2014; 6(8):3018-3039. https://doi.org/10.3390/nu6083018
Chicago/Turabian StyleCialdella-Kam, Lynn, Charlotte P. Guebels, Gianni F. Maddalozzo, and Melinda M. Manore. 2014. "Dietary Intervention Restored Menses in Female Athletes with Exercise-Associated Menstrual Dysfunction with Limited Impact on Bone and Muscle Health" Nutrients 6, no. 8: 3018-3039. https://doi.org/10.3390/nu6083018




