In Vitro Anti-Osteoporosis Properties of Diverse Korean Drynariae rhizoma Phenolic Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Drynariae Rhizoma Extracts
2.2. Extraction and Purification of Phenolic Acids and Flavonoids
2.3. Reagents, Standards, and Solvents
2.4. HPLC-DAD Analysis
2.5. Superoxide Anion (O2−) Radical Scavenging Activity
2.6. Hydroxyl Radical (OH−) Scavenging Activity
2.7. Free Radical Scavenging Activity on DPPH Assay
2.8. Cell Culture
2.9. MTT Assay
2.10. 3H-TdR Assay
2.11. Alkaline Phosphatase (ALP) Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield, Total Phenolic and Flavonoid Contents
| Phenolic Compound | RT (min) | DR-DW (mg/100 g Extract) | DR-EtOH (mg/100 g Extract) |
|---|---|---|---|
| Phenolic acid | |||
| benzoic acid | |||
| phloroglucinol | 7.27 | - | 129.29 ± 0.69 |
| 4-hydroxy benzhydrazide derivative | 7.57 | 72.73 ± 0.05 | 72.63 ± 0.19 |
| gallic acid | 8.45 | 20.70 ± 0.42 a | 15.59 ± 0.29 b |
| vanillic acid | 21.91 | 32.18 ± 0.31 b | 42.94 ± 0.37 a |
| protocatechuic acid ethyl ester | 34.92 | 17.17 ± 19.84 b | 32.37 ± 0.18 a |
| 2-amino-3,4-dimethyl-benzoic acid | 33.37 | 182.55 ± 0.63 | 206.44 ± 0.48 a |
| p-anisic acid | 34.62 | 9.41 ± 0.12 a | 2.94 ± 0.09 b |
| alizarin | 44.00 | 7.86 ± 0.06 a | 0.05 ± 0.07 b |
| 4-(3,5-dimethyl-pyrazol-1-yl)-benzoic acid | 39.85 | 59.85 ± 0.06 b | 127.23 ± 0.17 a |
| cinnamic acid | |||
| chlorogenic acid | 23.17 | 71.26 ± 0.36 b | 212.76 ± 1.76 a |
| caffeic acid | 23.16 | 6.43 ± 0.25 a | 5.91 ± 0.9 b |
| syringic acid | 24.10 | 4.09 ± 0.13 b | 27.37 ± 0.21 a |
| p-coumaric acid | 30.76 | 22.77 ± 0.01 a | 16.46 ± 0.05 b |
| chlorogenic derivative | 31.23 | 268.66 ± 0.18 a | 71.16 ± 0.85 b |
| trans-ferulic acid | 32.51 | 547.54 ± 0.62 b | 620.02 ± 0.03 a |
| coumarin | 35.66 | - | - |
| Total of phenolic acid | 1323.22 ± 19.85 | 1583.16 ± 0.46 | |
| Flavonoids | |||
| Flavanols | |||
| (+)-catechin hydrate | 21.24 | 255.58 ± 0.59 | 258.41 ± 0.15 |
| catechin gallate | 33.77 | 13.38 ± 0.12 | 13.33 ± 0.19 |
| gallocatechin | 17.68 | - | - |
| (−)-epigallocatechin | 18.58 | 7,152.94 ± 2.91 b | 8,954.97 ± 1.46 a |
| epicatechin | 25.60 | - | - |
| epigallocatechin gallate | 27.27 | 42.18 ± 0.12 b | 70.18 ± 0.11 a |
| quercetin hydrate | 35.45 | 1.25 ± 0.07 | 1.40 ± 0.14 |
| myricetin | 37.59 | - | - |
| morin hydrate | 38.96 | 58.41 ± 14.23 a | 40.37 ± 0.19 b |
| quercetin dihydrate | 40.50 | 56.99 ± 0.02 b | 78.40 ± 0.35 |
| morin derivative | 42.19 | 10.23 ± 0.24 | 10.13 ± 0.10 |
| kaempferol | 42.43 | 7.26 ± 0.19 | 7.19 ± 0.09 |
| 3-hydroxyflavone | 45.80 | 210.63 ± 0.23 a | - b |
| Flavones | |||
| rutin hydrate | 33.77 | 9.84 ± 0.20 | 9.74 ± 0.34 |
| luteolin | 40.67 | 7.08 ± 0.12 b | 18.48 ± 0.39 a |
| Flavanones | |||
| naringin | 34.77 | 78.84 ± 0.20 a | 58.79 ± 0.13 b |
| Anthraquinones | |||
| rhein | 44.47 | - | - |
| emodin | 45.83 | 4.48 ± 0.03 b | 22.07 ± 0.04 a |
| Total of flavonoids | 7909.11 ± 10.84 b | 9543.45 ± 1.07 a |
3.2. Antioxidant Activity of D. rhizoma
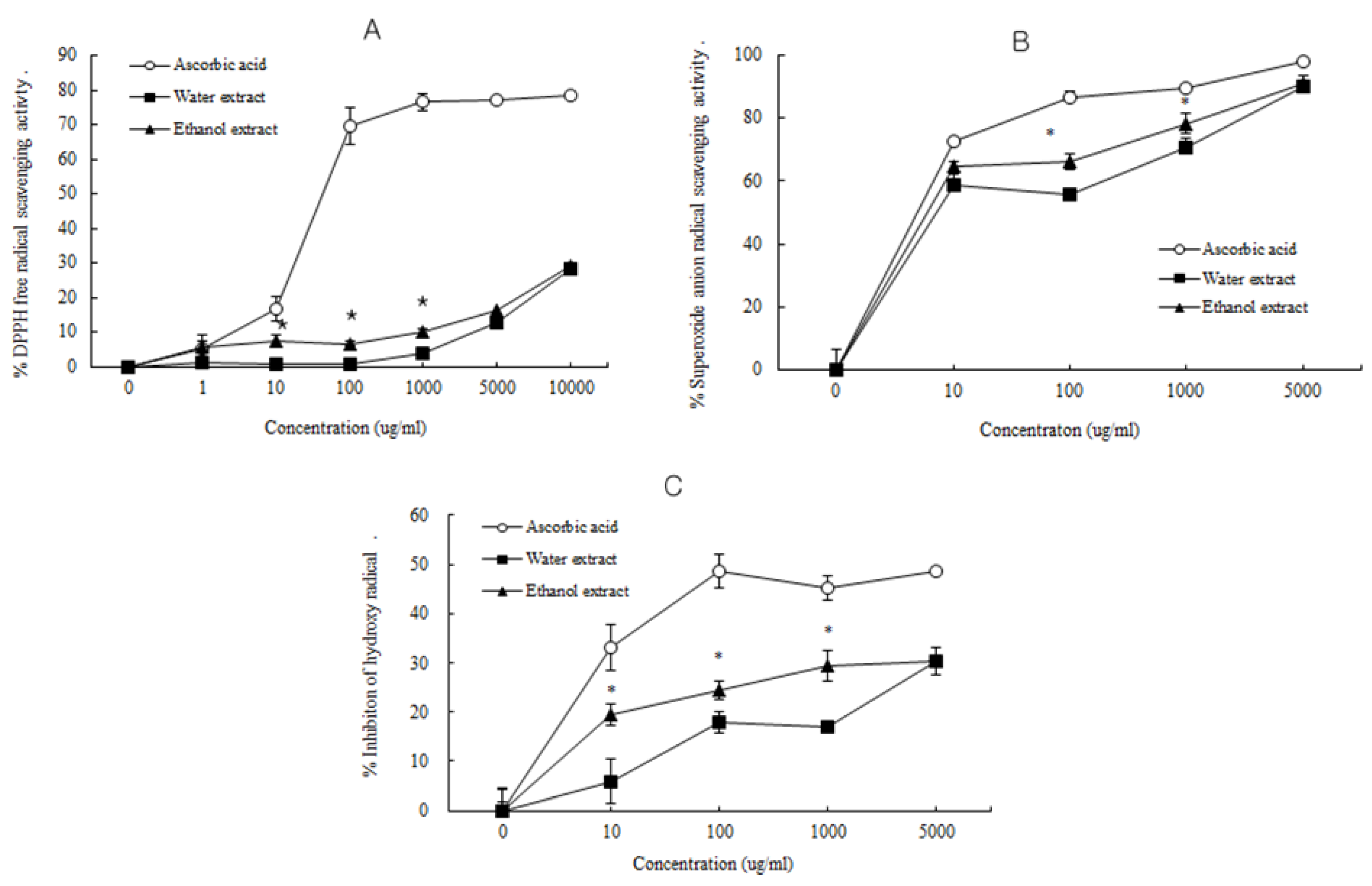
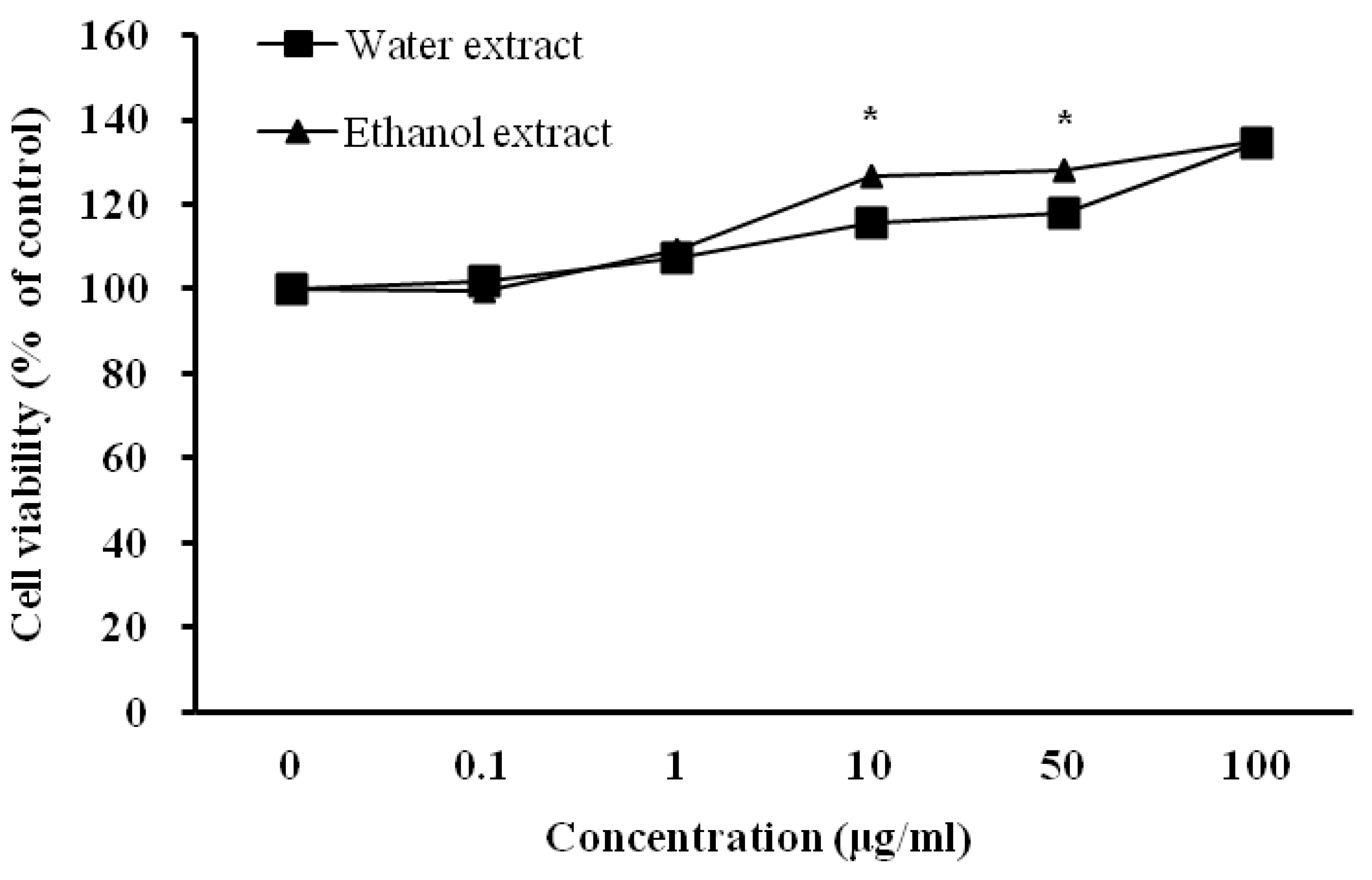
3.3. Influence of D. rhizoma on the Proliferation of Mouse Osteoblastic Cells


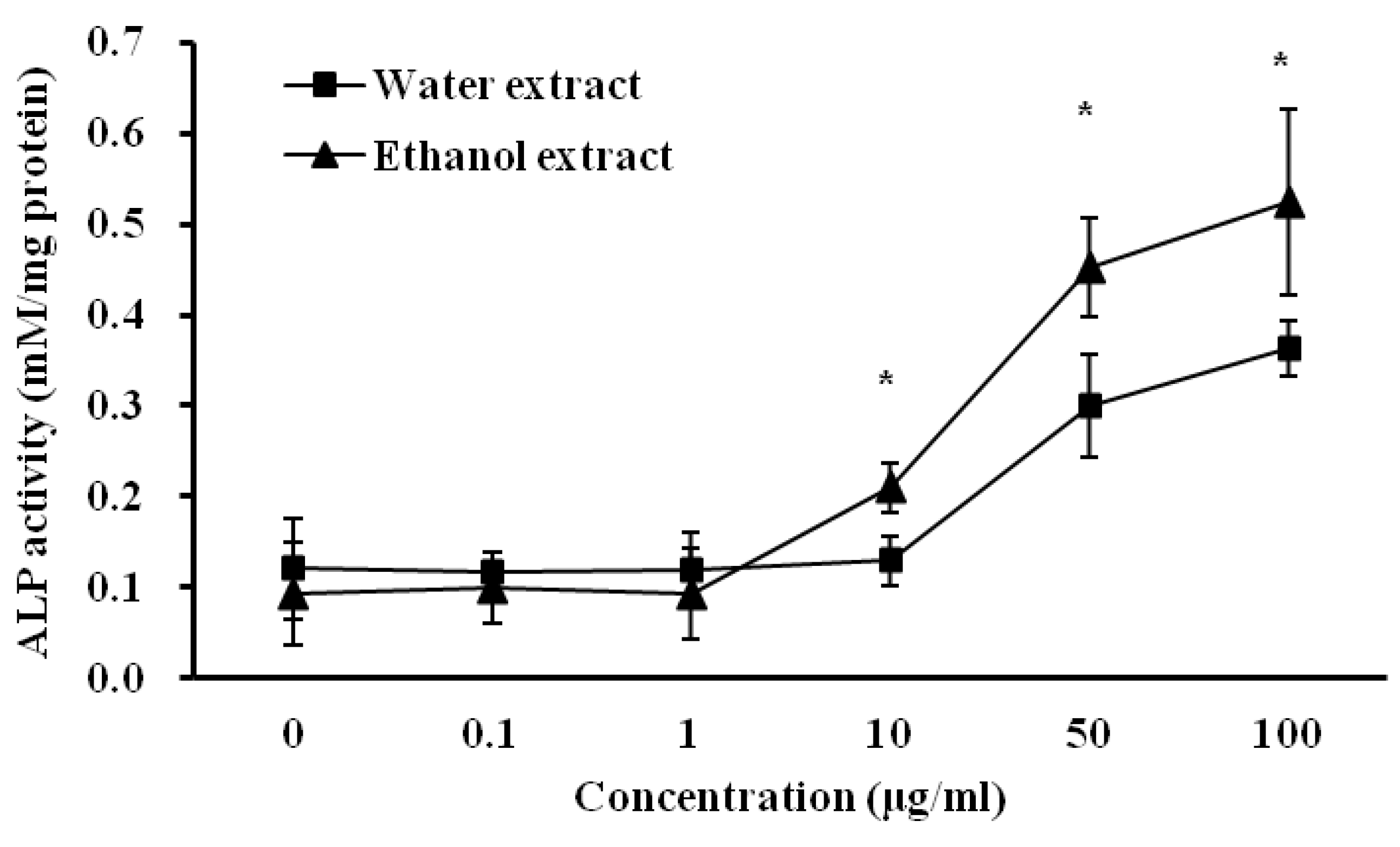
3.4. Influence of D. rhizoma on the Differentiation of Mouse Osteoblastic Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lim, L.S.; Hoeksema, L.J.; Sherin, K. Screening for osteoporosis in the adult U.S. population: ACPM position statement on preventive practice. Am. J. Prev. Med. 2009, 36, 366–375. [Google Scholar] [CrossRef]
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Chen, Q.M. Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints. Ann. N. Y. Acad. Sci. 2000, 908, 111–125. [Google Scholar]
- Altindag, O.; Erel, O.; Soran, N.; Celik, H.; Selek, S. Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol. Int. 2008, 11, 317–321. [Google Scholar]
- Garrett, I.R.; Boyce, B.F.; Oreffo, R.O.; Bonewald, L.; Poser, J.; Mundy, G.R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J. Clin. Investig. 1990, 85, 632–639. [Google Scholar] [CrossRef]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, M.A.; Ruiz-Ramos, M.; Correa-Munoz, E.; Mendoza-Nunez, V.M. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet. Disord. 2007, 8, 124. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar]
- Jeong, J.C.; Lee, J.W.; Yoon, C.H.; Kim, H.M.; Kim, C.H. Drynariae rhizoma promotes osteoblast differentiation and mineralization in MC3T3-E1 cells through regulation of bone morphogenetic protein-2, alkaline phosphatase, type I collagen and collagenase-1. Toxicol. In Vitro 2004, 18, 829–834. [Google Scholar] [CrossRef]
- Jeong, J.C.; Lee, J.W.; Yoon, C.H.; Lee, Y.C.; Chung, K.H.; Kim, M.G.; Kim, C.H. Stimulative effects of Drynariae rhizoma extracts on the proliferation and differentiation of osteoblastic MC3T3-E1 cells. J. Ethnopharmacol. 2005, 96, 489–495. [Google Scholar]
- Ettinger, B.; Genant, H.K.; Cann, C.E. Postmenopausal bone loss is prevented by treatment with low-dosage estrogen with calcium. Ann. Intern. Med. 1987, 106, 40–45. [Google Scholar] [CrossRef]
- Gambacciani, M.; Spinetti, A.; Piaggesi, L.; Cappagli, B.; Taponeco, F.; Manetti, P.; Weiss, C.; Teti, G.C.; la Commare, P.; Facchini, V. Ipriflavone prevents the bone mass reduction in premenopausal women treated with gonadotropin hormone-releasing hormone agonists. Bone Miner. 1994, 26, 19–26. [Google Scholar] [CrossRef]
- Rubin, M.R.; Bilezikian, J.P. New anabolic therapies in osteoporosis. Endocrinol. Metab. Clin. North Am. 2003, 32, 285–307. [Google Scholar] [CrossRef]
- Genant, H.K.; Baylink, D.J.; Gallagher, J.C. Estrogens in the prevention of osteoporosis in postmenopausal women. Am. J. Obstet. Gynecol. 1989, 161, 1842–1846. [Google Scholar] [CrossRef]
- Hovik, P.; Sundsbak, H.P.; Gaasemyr, M.; Sandvik, L. Comparison of continuous and sequential oestrogen-progestogen treatment in women with climacteric symptoms. Maturitas 1989, 11, 75–82. [Google Scholar] [CrossRef]
- Phillipson, J.D.; Anderson, L.A. Ethnopharmacology and Western medicine. J. Ethnopharmacol. 1989, 25, 61–72. [Google Scholar] [CrossRef]
- Xiu, R.J. Microcirculation and traditional Chinese medicine. JAMA 1988, 260, 1755–1757. [Google Scholar] [CrossRef]
- Jeong, J.C.; Kang, S.K.; Youn, C.H.; Jeong, C.W.; Kim, H.M.; Lee, Y.C.; Chang, Y.C.; Kim, C.H. Inhibition of Drynariae rhizoma extracts on bone resorption mediated by processing of cathepsin K in cultured mouse osteoclasts. Int. Immunopharmacol. 2003, 3, 1685–1697. [Google Scholar] [CrossRef]
- Jeong, J.C.; Lee, B.T.; Yoon, C.H.; Kim, H.M.; Kim, C.H. Effects of Drynariae rhizoma on the proliferation of human bone cells and the immunomodulatory activity. Pharmacol. Res. 2005, 51, 125–136. [Google Scholar]
- Liu, X.; Zhang, S.; Lu, X.; Zheng, S.; Li, F.; Xiong, Z. Metabonomic study on the anti-osteoporosis effect of Rhizoma Drynariae and its action mechanism using ultra-performance liquid chromatography-tandem mass spectrometry. J. Ethnopharmacol. 2012, 139, 311–317. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Khorsandi, K.; Hemmaty, S. Study of the effect of surfactants on extraction and determination of polyphenolic compounds and antioxidant capacity of fruits extracts. PLoS One 2013, 8, e57353. [Google Scholar] [CrossRef]
- Ramirez-Rodriques, M.M.; Plaza, M.L.; Azeredo, A.; Balaban, M.O.; Marshall, M.R. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J. Food Sci. 2011, 76, C428–C435. [Google Scholar]
- Prenesti, E.; Berto, S.; Daniele, P.G.; Toso, S. Antioxidant power quantification of decoction and cold infusions of Hibiscus sabdariffa flowers. Food Chem. 2007, 100, 433–438. [Google Scholar] [CrossRef]
- Liu, F.; Ooi, V.E.; Chang, S.T. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997, 60, 763–771. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Cassiede, P.; Dennis, J.E.; Ma, F.; Caplan, A.I. Osteochondrogenic potential of marrow mesenchymal progenitor cells exposed to TGF-β1 or PDGF-BB as assayed in vivo and in vitro. J. Bone Miner. Res. 1996, 11, 1264–1273. [Google Scholar] [CrossRef]
- Okada, Y.; Ishimaru, A.; Suzuki, R.; Okuyama, T. A new phloroglucinol derivative from the brown alga Eisenia bicyclis: Potential for the effective treatment of diabetic complications. J. Nat. Prod. 2004, 67, 103–105. [Google Scholar] [CrossRef]
- Chotimarkorn, C.; Ushio, H. The effect of trans-ferulic acid and gamma-oryzanol on ethanol-induced liver injury in C57BL mouse. Phytomedicine 2008, 15, 951–958. [Google Scholar] [CrossRef]
- Yasukawa, K.; Akihisa, T.; Kimura, Y.; Tamura, T.; Takido, M. Inhibitory effect of cycloartenol ferulate, a component of rice bran, on tumor promotion in two-stage carcinogenesis in mouse skin. Biol. Pharm. Bull. 1998, 21, 1072–1076. [Google Scholar] [CrossRef]
- Plumb, G.W.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Williamson, G. Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Redox Rep. 2002, 7, 41–46. [Google Scholar] [CrossRef]
- Jacobo-Velazquez, D.A.; Cisneros-Zevallos, L. Correlations of antioxidant activity against phenolic content revisited: A new approach in data analysis for food and medicinal plants. J. Food Sci. 2009, 74, R107–113. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Wang, L.J.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Ozgocmen, S.; Kaya, H.; Fadillioglu, E.; Aydogan, R.; Yilmaz, Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol. Cell. Biochem. 2007, 295, 45–52. [Google Scholar] [CrossRef]
- Sheweita, S.A.; Khoshhal, K.I. Calcium metabolism and oxidative stress in bone fractures: Role of antioxidants. Curr. Drug Metab. 2007, 8, 519–525. [Google Scholar] [CrossRef]
- Hung, T.Y.; Chen, T.L.; Liao, M.H.; Ho, W.P.; Liu, D.Z.; Chuang, W.C.; Chen, R.M. Drynaria fortunei J. Sm. promotes osteoblast maturation by inducing differentiation-related gene expression and protecting against oxidative stress-induced apoptotic insults. J. Ethnopharmacol. 2010, 131, 70–77. [Google Scholar]
- Liu, H.C.; Chen, R.M.; Jian, W.C.; Lin, Y.L. Cytotoxic and antioxidant effects of the water extract of the traditional Chinese herb gusuibu (Drynaria fortunei) on rat osteoblasts. J. Formos. Med. Assoc. 2001, 100, 383–388. [Google Scholar]
- Lee, J.S.; Hong, E.K. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int. Immunopharmacol. 2011, 11, 1226–1233. [Google Scholar] [CrossRef]
- Peterson, W.J. A method to assess the proliferative activity of small numbers of murine peripheral blood mononuclear cells. J. Immunol. Methods 1987, 96, 171–177. [Google Scholar] [CrossRef]
- Carusi, D. Phytoestrogens as hormone replacement therapy: An evidence-based approach. Prim. Care Update Ob Gyns 2000, 7, 253–259. [Google Scholar] [CrossRef]
- Fanti, P.; Monier-Faugere, M.C.; Geng, Z.; Schmidt, J.; Morris, P.E.; Cohen, D.; Malluche, H.H. The phytoestrogen genistein reduces bone loss in short-term ovariectomized rats. Osteoporos. Int. 1998, 8, 274–281. [Google Scholar] [CrossRef]
- Von Rosenberg, S.; Wehr, U.; Bachmann, H. Effect of vitamin D-containing plant extracts on osteoporotic bone. J. Steroid Biochem. Mol. Biol. 2007, 103, 596–600. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kang, S.-N.; Lee, J.S.; Park, J.-H.; Cho, J.-H.; Park, J.-H.; Cho, K.-K.; Lee, O.-H.; Kim, I.-S. In Vitro Anti-Osteoporosis Properties of Diverse Korean Drynariae rhizoma Phenolic Extracts. Nutrients 2014, 6, 1737-1751. https://doi.org/10.3390/nu6041737
Kang S-N, Lee JS, Park J-H, Cho J-H, Park J-H, Cho K-K, Lee O-H, Kim I-S. In Vitro Anti-Osteoporosis Properties of Diverse Korean Drynariae rhizoma Phenolic Extracts. Nutrients. 2014; 6(4):1737-1751. https://doi.org/10.3390/nu6041737
Chicago/Turabian StyleKang, Suk-Nam, Jong Seok Lee, Joung-Hyun Park, Jae-Hyeon Cho, Jae-Hong Park, Kwang-Keun Cho, Ok-Hwan Lee, and Il-Suk Kim. 2014. "In Vitro Anti-Osteoporosis Properties of Diverse Korean Drynariae rhizoma Phenolic Extracts" Nutrients 6, no. 4: 1737-1751. https://doi.org/10.3390/nu6041737





