Supplementation of Adult Rats with Moderate Amounts of β-Carotene Modulates the Redox Status in Plasma without Exerting Pro-Oxidant Effects in the Brain: A Safer Alternative to Food Fortification with Vitamin A?
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals
2.2. Treatment
2.3. Biochemical Analyses
2.4. Statistical Analyses
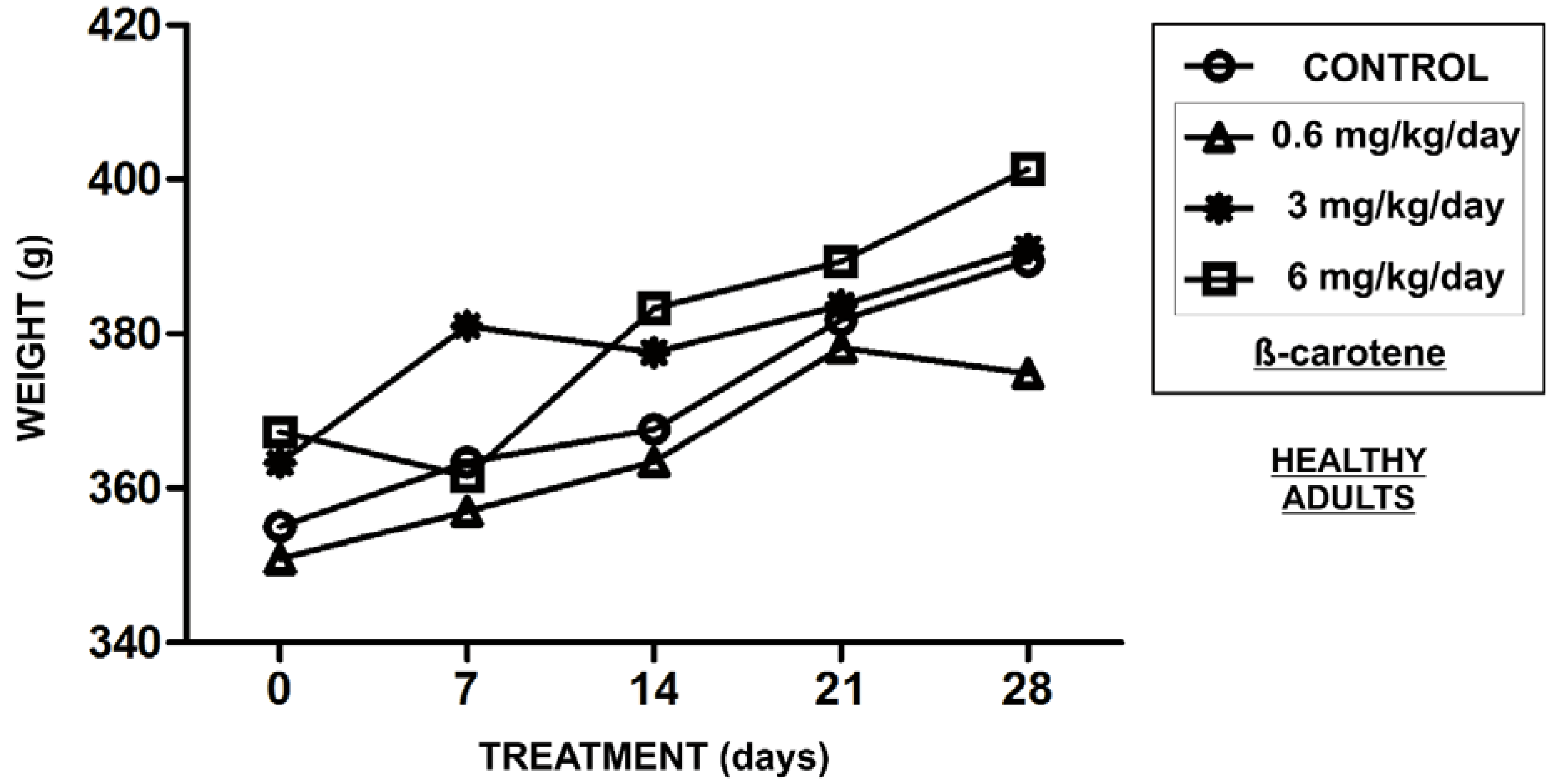
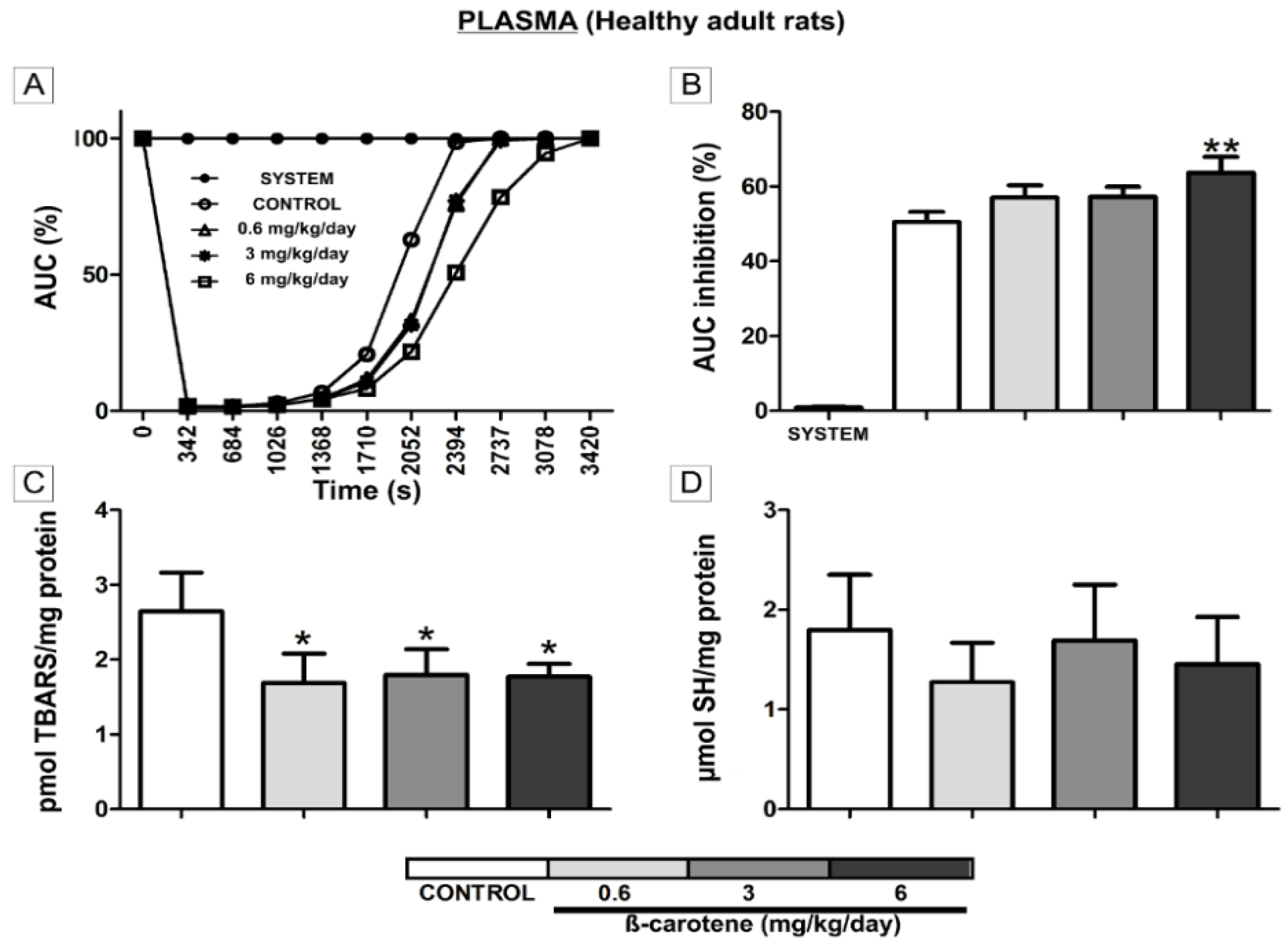
3. Results
| Redox Parameters | β-CAROTENE (mg/kg/day) | |||
|---|---|---|---|---|
| 0 (Control) | 0.6 | 3 | 6 | |
| Number of supplemented rats | 6 | 7 | 7 | 7 |
| Hippocampus | ||||
| TBARS level (nmol MDA/mg protein) | 3.67 ± 0.66 | 3.29 ± 0.34 | 3.24 ± 0.53 | 2.96 ± 0.31 |
| Total thiol content (mmol SH/mg protein) | 17.7 ± 1.4 | 18.1 ± 0.8 | 17.8 ± 1.1 | 17.2 ± 1.3 |
| CAT activity (U CAT/mg protein) | 1.19 ± 0.21 | 1.04 ± 0.18 | 1.28 ± 0.22 | 1.09 ± 0.25 |
| SOD activity (U SOS/mg protein) | 32.1 ± 1.6 | 31.9 ± 0.8 | 33.4 ± 0.8 | 32.6 ± 1.7 |
| Striatum | ||||
| TBARS level (nmol MDA/mg protein) | 3.09 ± 0.73 | 2.63 ± 0.23 | 2.71 ± 0.56 | 2.67 ± 0.69 |
| Total thiol content (mmol SH/mg protein) | 17.7 ± 1.3 | 18.9 ± 1.9 | 20.4 ± 3.0 | 19.9 ± 2.5 |
| CAT activity (U CAT/mg protein) | 1.47 ± 0.6 | 1.16 ± 0.51 | 1.22 ± 0.48 | 0.96 ± 0.39 |
| SOD activity (U SOD/mg protein) | 32.7 ± 4.8 | 33.6 ± 2.9 | 36.1 ±2.2 | 33.6 ± 2.8 |
| Cerebral Cortex | ||||
| TBARS level (nmol MDA/mg protein) | 1.08 ± 0.18 | 1.3 ± 0.36 | 1.17 ± 0.35 | 0.99 ± 0.37 |
| Total thiol content (mmol SH/mg protein) | 22.4 ± 3.4 | 24.9 ± 3.1 | 21.9 ± 4.1 | 18.9 ± 4.9 |
| CAT activity (U CAT/mg protein) | 1.53 ± 0.37 | 1.86 ± 0.54 | 2.41 ± 0.39 * | 1.79 ± 0.29 |
| SOD activity (U SOD/mg protein) | 37.9 ± 2.7 | 34.5 ± 4.4 | 37.4 ± 3.1 | 33.5 ± 5.1 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allen, L.H.; Haskell, M. Estimating the potential for vitamin A toxicity in women and young children. J. Nutr. 2002, 132, 2907S–2919S. [Google Scholar] [PubMed]
- Malaspina, A.; Michael-Titus, A.T. Is the modulation of retinoid and retinoid-associated signaling a future therapeutic strategy in neurological trauma and neurodegeneration? J. Neurochem. 2008, 104, 584–595. [Google Scholar] [PubMed]
- Snodgrass, S.R. Vitamin neurotoxicity. Mol. Neurobiol. 1992, 6, 41–73. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; Silvestrin, R.B.; Mello, E.; Souza, T.; Moreira, J.C. Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: Effects of sub acute vitamin A supplementation at therapeutic doses. Neurotoxicology 2007, 28, 1191–1199. [Google Scholar]
- De Oliveira, M.R.; de Bittencourt Pasquali, M.A.; Silvestrin, R.B.; Mello, E.; Souza, T.; Moreira, J.C. Vitamin A supplementation induces a prooxidative state in the striatum and impairs locomotory and exploratory activity of adult rats. Brain Res. 2007, 1169, 112–119. [Google Scholar]
- De Oliveira, M.R.; Moreira, J.C. Acute and chronic vitamin A supplementation at therapeutic doses induces oxidative stress in submitochondrial particles isolated from cerebral cortex and cerebellum of adult rats. Toxicol. Lett. 2007, 173, 145–150. [Google Scholar]
- Schnorr, C.E.; da Silva Morrone, M.; Simões-Pires, A.; da Rocha, R.F.; Behr, G.A.; Moreira, J.C. Vitamin A supplementation in rats under pregnancy and nursing induces behavioral changes and oxidative stress upon striatum and hippocampus of dams and their offspring. Brain Res. 2011, 1369, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurham, D.; Yin, S.A.; Biesalski, H.K. β-Carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- US National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- US National Research Council. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press (US): Washington, DC, USA, 2001. [Google Scholar]
- Tang, G.; Hu, Y.; Yin, S.A.; Wang, Y.; Dallal, G.E.; Grusak, M.A.; Russell, R.M. β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children. Am. J. Clin. Nutr. 2012, 96, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [PubMed]
- Ellman, G.L. Tissue Sulfydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Lissi, E.; Salim-Hanna, M.; Pascual, C.; del Castillo, M.D. Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Radic. Biol. Med. 1995, 18, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Dresch, M.T.; Rossato, S.B.; Kappel, V.D.; Biegelmeyer, R.; Hoff, M.L.; Mayorga, P.; Zuanazzi, J.A.; Henriques, A.T.; Moreira, J.C. Optimization and validation of an alternative method to evaluate total reactive antioxidant potential. Anal. Biochem. 2009, 385, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- The ATBC Study Group. The effect of vitamin E and β-carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of β-carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef]
- Lee, I.-M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Hennekens, C.H. β-Carotene supplementation and incidence of cancer and cardiovascular disease: The Women’s Health Study. J. Natl. Cancer Inst. 1999, 91, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.R.; Baron, J.A.; Karagas, M.R.; Stukel, T.A.; Nierenberg, D.W.; Stevens, M.M.; Mandel, J.S.; Haile, R.W. Mortality associated with low plasma concentration of β carotene and the effect of oral supplementation. JAMA 1996, 275, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.M. The enigma of β-carotene in carcinogenesis: What can be learned from animal studies. J. Nutr. 2004, 134, 262S–268S. [Google Scholar] [PubMed]
- Great Britain: Committee on Medical Aspects of Food Policy; Great Britain: Department of Health; Acheson, D. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom; HMSO: London, UK, 1991; pp. 1–210. [Google Scholar]
- Australian National Health and Medical Research Council; New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; NHRMC Press (AU): Camberra, Australia, 2006. [Google Scholar]
- US National Research Council. Nutrient Requirements of Laboratory Animals, 4th ed.; The National Academies Press (US): Washington, DC, USA, 1995. [Google Scholar]
- Rice-Evans, C.A.; Sampson, J.; Bramley, P.M.; Holloway, D.E. Why do we expect carotenoids to be antioxidants in vivo? Free Radic. Res. 1997, 26, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning). Free Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Briviba, K.; Schnäbele, K.; Rechkemmer, G.; Bub, A. Supplementation of a diet low in carotenoids with tomato or carrot juice does not affect lipid peroxidation in plasma and feces of healthy men. J. Nutr. 2004, 134, 1081–1083. [Google Scholar] [PubMed]
- Cao, G.; Booth, S.L.; Sadowski, J.A.; Prior, R.L. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am. J. Clin. Nutr. 1998, 68, 1081–1087. [Google Scholar] [PubMed]
- Cao, G.; Rusell, R.M.; Lischner, N.; Prior, R.L. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J. Nutr. 1998, 128, 2383–2390. [Google Scholar] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A.; Shapiro, S.S. Effect of β-carotene and canthaxanthin on immune responses of the rat. J. Nutr. 1986, 116, 2254–2262. [Google Scholar] [PubMed]
- Schunemann, H.J.; Grant, B.J.B.; Freudenheim, J.L.; Muti, P.; Browne, R.W.; Drake, J.A.; Klocke, R.A.; Trevisan, M. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am. J. Respir. Crit. Care Med. 2001, 163, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Issing, W.J. Micronutrients as intermediate biomarkers in chemotherapy and enhancement for cancer treatments. In Primary and Secondary Preventive Nutrition Part II; Bendich, A., Deckelbaum, R.J., Eds.; Humana Press: Totowa, NJ, USA, 2001; Volume 4, pp. 55–74. [Google Scholar]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chun, O.K.; Song, W.O. Plasma and dietary antioxidant status as cardiovascular disease risk factors: A review of human studies. Nutrients 2013, 5, 2969–3004. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 3, CD007176. [Google Scholar] [PubMed]
- Shete, V.; Quadro, L. Mammalian metabolism of β-carotene: Gaps in knowledge. Nutrients 2013, 5, 4849–4868. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.; Nagarajan, P.; Raj, T.A.; Jahan, P.; Ganapathy, H.S.; Mahesh Kumar, M.J. Effect of dietary β carotene on cerebral aneurysm and subarachnoid haemorrhage in the brain apo E−/− mice. J. Thromb. Thrombolysis 2011, 32, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Jama, J.W.; Launer, L.J.; Witteman, J.C.; den Breeijen, J.H.; Breteler, M.M.; Grobbee, D.E.; Hofman, A. Dietary antioxidants and cognitive function in a population-based sample of older persons. The Rotterdam Study. Am. J. Epidemiol. 1996, 144, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, N.T.; Faure, H.; Gourlet, V.; Favier, A.; Berr, C. Plasma carotenoid levels and cognitive performance in an elderly population: Results of the EVA Study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Von Arnim, C.A.; Herbolsheimer, F.; Nikolaus, T.; Peter, R.; Biesalski, H.K.; Ludolph, A.C.; Riepe, M.; Nagel, G. Dietary antioxidants and dementia in a population-based case-control study among older people in south Germany. J. Alzheimers Dis. 2012, 31, 717–724. [Google Scholar] [PubMed]
- Wengreen, H.J.; Munger, R.G.; Corcoran, C.D.; Zandi, P.; Hayden, K.M.; Fotuhi, M.; Skoog, I.; Norton, M.C.; Tschanz, J.; Breitner, J.C.; et al. Antioxidant intake and cognitive function of elderly men and women: The Cache County Study. J. Nutr. Health Aging 2007, 11, 230–237. [Google Scholar] [PubMed]
- Hu, P.; Bretsky, P.; Crimmins, E.M.; Guralnik, J.M.; Reuben, D.B.; Seeman, T.E. Association between serum β-carotene levels and decline of cognitive function in high-functioning older persons with or without apolipoprotein E 4 alleles: MacArthur studies of successful aging. J. Gerontol. 2006, 61, 616–620. [Google Scholar] [CrossRef]
- Engelhart, M.J.; Geerlings, M.I.; Ruitenberg, A.; van Swieten, J.C.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002, 287, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F.; Kang, J.H.; Glynn, R.J.; Cook, N.R.; Gaziano, J.M. A randomized trial of β carotene supplementation and cognitive function in men: The physicians’ health study II. Arch. Intern. Med. 2007, 167, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnorr, C.E.; Morrone, M.D.S.; Simões-Pires, A.; Bittencourt, L.D.S.; Zeidán-Chuliá, F.; Moreira, J.C.F. Supplementation of Adult Rats with Moderate Amounts of β-Carotene Modulates the Redox Status in Plasma without Exerting Pro-Oxidant Effects in the Brain: A Safer Alternative to Food Fortification with Vitamin A? Nutrients 2014, 6, 5572-5582. https://doi.org/10.3390/nu6125572
Schnorr CE, Morrone MDS, Simões-Pires A, Bittencourt LDS, Zeidán-Chuliá F, Moreira JCF. Supplementation of Adult Rats with Moderate Amounts of β-Carotene Modulates the Redox Status in Plasma without Exerting Pro-Oxidant Effects in the Brain: A Safer Alternative to Food Fortification with Vitamin A? Nutrients. 2014; 6(12):5572-5582. https://doi.org/10.3390/nu6125572
Chicago/Turabian StyleSchnorr, Carlos Eduardo, Maurilio Da Silva Morrone, André Simões-Pires, Leonardo Da Silva Bittencourt, Fares Zeidán-Chuliá, and José Cláudio Fonseca Moreira. 2014. "Supplementation of Adult Rats with Moderate Amounts of β-Carotene Modulates the Redox Status in Plasma without Exerting Pro-Oxidant Effects in the Brain: A Safer Alternative to Food Fortification with Vitamin A?" Nutrients 6, no. 12: 5572-5582. https://doi.org/10.3390/nu6125572





