Antioxidant Effectiveness of Vegetable Powders on the Lipid and Protein Oxidative Stability of Cooked Turkey Meat Patties: Implications for Health
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Turkey Patties
2.2. Compositional Analysis of Turkey Patties
2.3. Determination of Oxidative Stability of Patties
2.4. Statistical Analysis
3. Results
3.1. Composition of Patties
| Patty Type | Cooked weight (g) | Fat (g 100 g−1) | Protein (g 100 g−1) | CHO (g 100 g−1) | Fibre (g 100 g−1) | GE (kJ 100 g−1) |
|---|---|---|---|---|---|---|
| Plain | 142 | 28.3 | 67.3 | 0.62 | 0.53 | 2543 |
| Carrot | 149 | 23.6 | 62.6 | 4.78 | 2.59 | 2363 |
| Swede | 146 | 25.6 | 59.7 | 4.07 | 3.67 | 2443 |
| Broccoli | 150 | 24.7 | 63.7 | 1.20 | 4.08 | 2305 |
| Celery | 148 | 24.8 | 61.8 | 3.78 | 3.63 | 2423 |
| Beetroot | 148 | 24.1 | 63.3 | 4.89 | 2.43 | 2327 |
| Spinach | 150 | 24.6 | 64.9 | 0.53 | 2.68 | 2437 |
| Yellow pea | 151 | 24.7 | 64.6 | 6.18 | 2.46 | 2412 |
| Onion | 149 | 25.4 | 60.5 | 2.90 | 2.22 | 2527 |
| Red pepper | 147 | 25.2 | 61.3 | 2.05 | 2.32 | 2532 |
| Green pea | 148 | 24.6 | 62.5 | 5.69 | 2.29 | 2279 |
| Tomato | 145 | 21.9 | 65.5 | 2.69 | 1.44 | 2264 |
| Patty Type | Total Phenols (mg GA 100 g−1) | Free Phenols (mg GA 100 g−1) | Vitamin C (µg 100 g−1) | α-Tocopherol (µg 100 g−1) | γ-Tocopherol (µg 100 g−1) | HORAC Value (µM GA mL−1) |
|---|---|---|---|---|---|---|
| Plain | 629 ± 10 | 225 ± 34 | 305 ± 18 | 97 ± 6 | 86 ± 2 | 11.2 ± 0.7 |
| Carrot | 1065 ± 31 | 300 ± 12 | 3712 ± 52 | 184 ± 16 | 99 ± 1 | 9.3 ± 0.5 |
| Swede | 883 ± 15 | 256 ± 39 | 2901 ± 142 | 114 ± 10 | 110 ± 2 | 9.0 ± 0.2 |
| Broccoli | 806 ± 22 | 251 ± 15 | 19,673 ± 560 | 708 ± 8 | 108 ± 4 | 11.5 ± 0.7 |
| Celery | 685 ± 36 | 258 ± 12 | 6742 ± 374 | 166 ± 8 | 92 ± 1 | 9.6 ± 0.6 |
| Beetroot | 940 ± 20 | 295 ± 12 | 6913 ± 472 | 136 ± 2 | 93 ± 4 | 9.7 ± 0.2 |
| Spinach | 1137 ± 30 | 347 ± 8 | 613 ± 31 | 838 ± 65 | 212 ± 23 | 13.9 ± 1.2 |
| Yellow pea | 917 ± 8 | 232 ± 8 | 2637 ± 28 | 157 ± 5 | 238 ± 8 | 10.6 ± 0.7 |
| Onion | 1067 ± 32 | 246 ± 19 | 4780 ± 299 | 147 ± 4 | 105 ± 1 | 8.7 ± 1.4 |
| Red pepper | 1159 ± 22 | 348 ± 5 | 14,436 ± 807 | 4503 ± 382 | 403 ± 8 | 14.7 ± 1.23 |
| Green pea | 737 ± 14 | 229 ± 4 | 12,464 ± 484 | 80 ± 12 | 185 ± 1 | 9.6 ± 0.1 |
| Tomato | 1232 ± 33 | 305 ± 4 | 39,091 ± 538 | 1794 ± 200 | 231 ± 18 | 13.9 ± 1.23 |
| Patty Type | Lutein (µg 100 g−1) | β-cryptoxanthin (µg 100 g−1) | Lycopene (µg 100 g−1) | α-carotene (µg 100 g−1) | β-carotene (µg 100 g−1) |
|---|---|---|---|---|---|
| Plain | 0.5 ± 0.1 | ND | ND | ND | ND |
| Carrot | 1.8 ± 0.2 | 1.0 ± 0.1 | 2.0 ± 0.1 | 13 ± 4 | 22 ± 1 |
| Swede | 5.0 ± 0.1 | ND | 4.0 ± 0.3 | ND | 1.0 ± 0.1 |
| Broccoli | 87 ± 5 | ND | 3.6 ± 0.2 | 2 ± 0.3 | 58 ± 4 |
| Celery | 0.7 ± 0.05 | ND | ND | ND | ND |
| Beetroot | 1.1 ± 0.1 | ND | ND | 2 ± 0.2 | 1.0 ± 0.1 |
| Spinach | 499 ± 36 | ND | 12 ± 0.8 | 1.8 ± 0.1 | 236 ± 7 |
| Yellow pea | 2.0 ± 0.1 | ND | ND | ND | ND |
| Onion | 0.9 ± 0.2 | ND | ND | ND | ND |
| Red pepper | 33 ± 0.2 | 26 ± 8 | ND | 4.5 ± 0.4 | 115 ± 3 |
| Green pea | 18 ± 0.1 | ND | 10 ± 0.2 | ND | 8.6 ± 0.1 |
| Tomato | 39 ± 2 | ND | 1842 ± 48 | 1.7 ± 0.1 | 206 ± 2 |
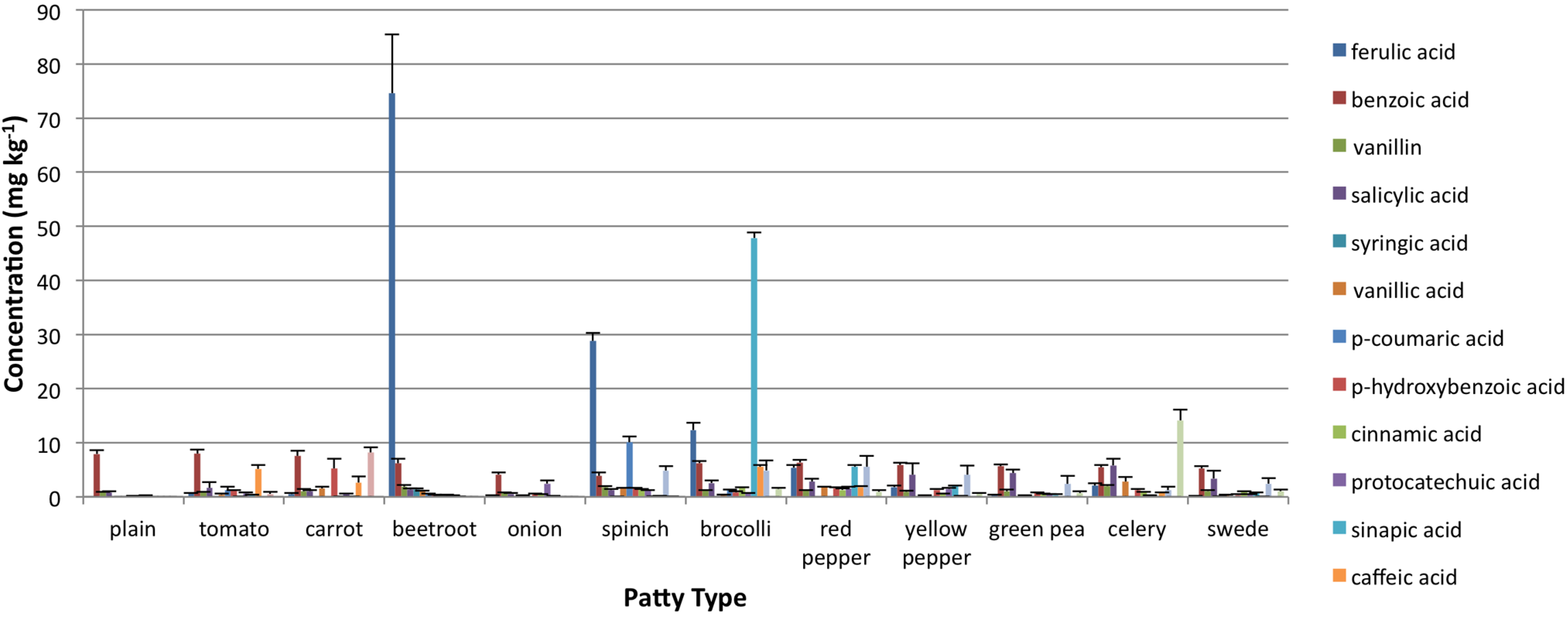
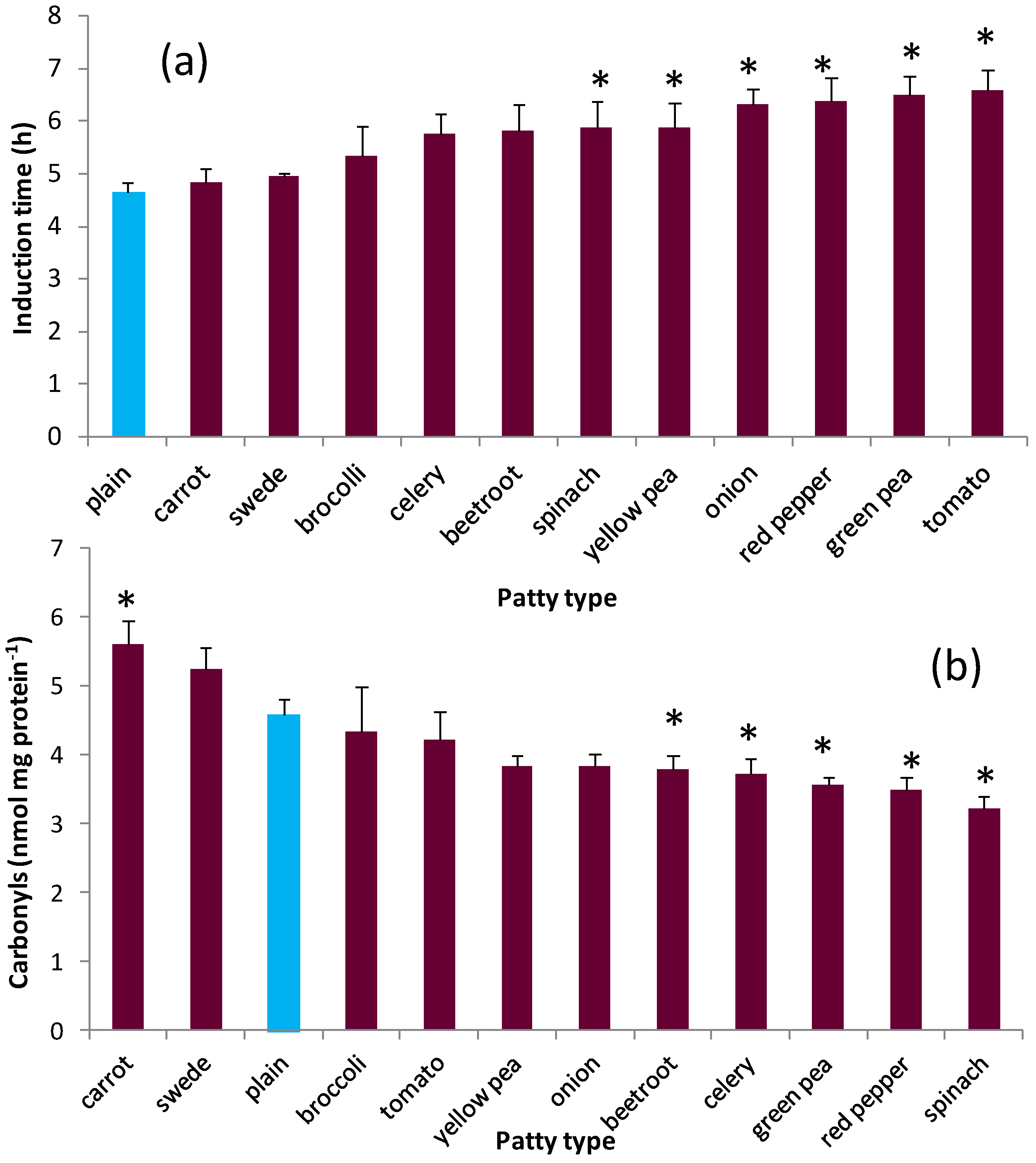
3.2. Oxidative Stability
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Kanner, J.; Rosenthal, I. An assessment of lipid oxidation in foods. Pure Appl. Chem. 1992, 64, 1959–1964. [Google Scholar] [CrossRef]
- Poli, G.; Schaur, R.J.; Siems, W.G.; Leonarduzzi, G. 4-Hydroxynonenal: A membrane lipid oxidation product of medical interest. Med. Res. Rev. 2007, 28, 569–631. [Google Scholar]
- West, J.D.; Marnett, L.J. Alterations in gene expression induced by the lipid peroxidation product, 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005, 18, 1642–1653. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Daniels, J.S.; Rouzer, C.A.; Greene, R.E.; Marnett, L.J. Malondialdehyde, a product of lipid peroxidation is mutagenic in human cells. J. Biol. Chem. 2003, 278, 31426–31433. [Google Scholar]
- Esterbauer, H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993, 57, 779S–788S. [Google Scholar]
- Penumetcha, M.; Khan, N.; Parthasarathy, S. Dietary oxidised fatty acids: An atherogenic risk? J. Lipid Res. 2000, 41, 1473–1480. [Google Scholar]
- Kanner, J. Dietary advanced lipid oxidation end products are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef]
- Chloe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Comp. Rev. Food Sci. Food Safety 2006, 5, 178–186. [Google Scholar]
- Khokhar, S.; Owusu Apenten, R.K. Iron binding characteristics of phenolic compounds: Some tentative structure-activity relations. Food Chem. 2003, 81, 133–140. [Google Scholar] [CrossRef]
- McPhail, D.B.; Hartley, R.C.; Gardner, P.T.; Duthie, G.G. Kinetic and stoichiometric assessment of the antioxidant activity of flavonoids by electron spin resonance spectroscopy. J. Agric. Food Chem. 2003, 51, 1684–1690. [Google Scholar] [CrossRef]
- Duthie, G.G. Lipid peroxidation. Eur. J. Clin. Nutr. 1993, 47, 759–764. [Google Scholar]
- Pokornỳ, J.P. Are natural antioxidants better—And safer—Than synthetic antioxidants. Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Thongtan, K.; Toma, R.B.; Rieboldt, W.; Dahoud, A.Z. Effect of rosemary extract on lipid oxidation and sensory evaluation of frozen, precooked beef patties. Foodserv. Res. Int. 2005, 16, 93–104. [Google Scholar] [CrossRef]
- El-Alim, S.S.L.A.; Lugasi, A.; Hóvári, A.; Dworschák, E. Culinary herbs inhibit lipid oxidation in raw and cooked minced meat patties during storage. J. Sci. Food Agric. 1999, 79, 277–285. [Google Scholar] [CrossRef]
- McCarthy, T.L.; Kerry, J.P.; Kerry, J.F.; Lynch, P.B.; Buckley, D.J. Assessment of the antioxidant potential of natural food and plant extracts in fresh and previously frozen pork patties. Meat Sci. 2001, 57, 177–184. [Google Scholar] [CrossRef]
- Brettonnet, A.; Hewavitarana, A.; Dejong, S.; Lanari, M.C. Phenolic acids composition and antioxidant activity of canola extracts in cooked beef, chicken and pork. Food Chem. 2010, 121, 927–933. [Google Scholar] [CrossRef]
- DeJong, S.; Lanari, M.C. Extracts of olive polyphenols improve lipid stability in cooked beef and pork: Contribution of individual phenolics to the antioxidant activity of the extract. Food Chem. 2009, 116, 892–897. [Google Scholar] [CrossRef]
- Vuorela, S.; Salminen, H.; Mäkela, M.; Kivikari, R.; Karonen, M.; Heinonen, M. Effect of plant phenolics on protein and lipid oxidation in cooked meat patties. J. Agric. Food Chem. 2005, 53, 8492–8497. [Google Scholar]
- Tang, S.Z.; Ou, S.Y.; Huang, X.S.; Li, W.; Perry, J.P.; Buckley, D.J. Effects of added tea catechins on colour stability and lipid oxidation in minced beef patties held under aerobic and modified atmospheric packaging conditions. J. Food Eng. 2006, 77, 248–253. [Google Scholar] [CrossRef]
- Jongberg, S.; Skov, S.H.; Tørngren, M.A.; Skibsted, L.H.; Lund, M.N. Effect of white grape extract and modified atmosphere packaging on lipid and protein oxidation in chill stored beef patties. Food Chem. 2011, 128, 276–283. [Google Scholar] [CrossRef]
- Ganhão, R.; Estēvez, M.; Kylli, P.; Heinonen, M.; Morcuende, D. Characterization of selected wild Mediterranean fruits and comparative efficacy as inhibitors of oxidative reactions in emulsified raw pork burger patties. J. Agric. Food Chem. 2010, 58, 8854–8861. [Google Scholar]
- Dauchet, L.; Amouyel, P.; Dallongeville, J. Fruits, vegetables and coronary heart disease. Nat. Rev. Cardiol. 2009, 6, 599–608. [Google Scholar] [CrossRef]
- Vainio, H.; Weiderpass, E. Fruit and vegetables in cancer prevention. Nutr. Cancer 2006, 54, 111–142. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations, Methods of Food Analysis. In Food Energy—Methods of Analysis and Conversion Factors; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2003; Chapter 2.
- Liu, K.S. Preparation of fatty acid methyl esters for gas-chromatographic analysis of lipids in biological materials. J. Am. Oil. Chem. Soc. 1994, 71, 1179–1187. [Google Scholar] [CrossRef]
- Hess, D.; Keller, H.E.; Oberlin, B.; Bonfanti, R.; Schuep, W. Simultaneous determination of retinol, tocopherols, carotene, lycopene, and xanthophylls in plasma by means of reversed-phase high-performance liquid chromatogray. Int. J. Vit. Nutr. Res. 1991, 61, 232–238. [Google Scholar]
- Ross, M.A. Determination of ascorbic acid and uric acid in plasma by high-performance liquid chromatography. J. Chromatogr. B 1994, 657, 197–200. [Google Scholar] [CrossRef]
- Vinson, J.A.; Hao, Y.; Su, W.; Zubik, L. Phenol antioxidant quantity and quality in foods. J. Agric. Food Chem. 1998, 46, 3630–3643. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High protein, reduced carbohydrate weight loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- Metrohm Stability Application Note R-12. Oxidative Stability of Different Solid Foodstuffs. Available online: http://partners.metrohm.com/GetDocument?action=get_dms_document&docid=873882 (accessed on 27 November 2012).
- Robinson, C.E.; Keshavarzian, A.; Pasco, D.S.; Frommel, T.O.; Winship, D.H.; Holmes, E.W. Determination of protein carbonyl groups by immunoblotting. Anal. Biochem. 1999, 266, 48–57. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woddil, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Res. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables—The millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Hall, J.N.; Moore, S.; Harper, S.B.; Lynch, J.L. Global variability in fruit and vegetable consumption. Am. J. Prev. Med. 2009, 36, 402–409. [Google Scholar] [CrossRef]
- Krokida, M.K.; Karathanos, V.T.; Maroulis, Z.B. Effect of freeze-drying conditions on shrinkage and porosity of dehydrated agricultural products. J. Food Eng. 1998, 35, 369–380. [Google Scholar] [CrossRef]
- Hooper, L.; Cassidy, A. A review of the health care potential of bioactive compounds. J. Sci. Food Agric. 2006, 86, 1805–1813. [Google Scholar] [CrossRef]
- Jackson, M. Potential Mechanisms of Action of Bioactive Substances Found in Foods. In Plants: Diet and Health; Goldberg, G., Ed.; Blackwell Publishing: Oxford, UK, 2003; pp. 65–75. [Google Scholar]
- Russell, W.R.; Scobbie, L.; Chesson, A.; Richardson, A.J.; Stewart, C.S.; Duncan, S.H.; Drew, J.E.; Duthie, G.G. Anti-inflammatory implications of the microbial transformation of dietary phenolic compounds. Nutr. Cancer 2008, 60, 636–642. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Widened horizon of vitamin E research. Mol. Nutr. Food Res. 2010, 54, 581–582. [Google Scholar] [CrossRef]
- Pischetsrieder, M. Are dietary AGEs/ALEs a risk to human health and, if so, what is the mechanism of action? Mo. Nutr. Food Res. 2007, 51, 1069–1070. [Google Scholar]
- Šebeková, K.; Somoza, V. Dietary advanced glycation products (AGEs) and their health effects. Mol. Nutr. Food Res. 2007, 51, 1079–1084. [Google Scholar] [CrossRef]
- Gardner, P.T.; White, A.C.; McPhail, D.B.; Duthie, G.G. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000, 68, 471–474. [Google Scholar] [CrossRef]
- Ames, J.M. Dietary Maillard reaction products: Implications for human health and disease. Czech J. Food Sci. 2009, 27, S66–S69. [Google Scholar]
- Augustyniak, A.; Bartosz, G.; Ćipak, A.; Duburs, G.; Horáková, L.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, L.; et al. Natural and synthetic antioxidants: An updated review. Free Radiac. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Miková, K. The Regulation of Antioxidants in Food. In Antioxidant in Foods: Practical Applications; Pokornỳ, J.P., Yanishlieva, N., Gordon, M., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2001; pp. 267–284. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Soneson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste: Extent, Causes and Prevention; Food and Agriculture Organisation of the United Nations (FAO): Rome, Italy, 2001; p. 29. [Google Scholar]
- Sheeran, P. Intention-behaviour relations: A conceptual and empirical view. Eur. Rev. Soc. Pyschol. 2002, 12, 1–36. [Google Scholar] [CrossRef]
- P.A.; Fong, G.T.; Epp, L.J.; Elias, L. Executive function moderates the intention-behaviour link for physical activity and dietary behavior. Pyschol. Health 2008, 23, 309–326. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Duthie, G.; Campbell, F.; Bestwick, C.; Stephen, S.; Russell, W. Antioxidant Effectiveness of Vegetable Powders on the Lipid and Protein Oxidative Stability of Cooked Turkey Meat Patties: Implications for Health. Nutrients 2013, 5, 1241-1252. https://doi.org/10.3390/nu5041241
Duthie G, Campbell F, Bestwick C, Stephen S, Russell W. Antioxidant Effectiveness of Vegetable Powders on the Lipid and Protein Oxidative Stability of Cooked Turkey Meat Patties: Implications for Health. Nutrients. 2013; 5(4):1241-1252. https://doi.org/10.3390/nu5041241
Chicago/Turabian StyleDuthie, Garry, Fiona Campbell, Charles Bestwick, Sylvia Stephen, and Wendy Russell. 2013. "Antioxidant Effectiveness of Vegetable Powders on the Lipid and Protein Oxidative Stability of Cooked Turkey Meat Patties: Implications for Health" Nutrients 5, no. 4: 1241-1252. https://doi.org/10.3390/nu5041241





