Immunological Function of Sphingosine 1-Phosphate in the Intestine
Abstract
:1. Introduction
2. Relationship Between S1P and Dietary Lipids
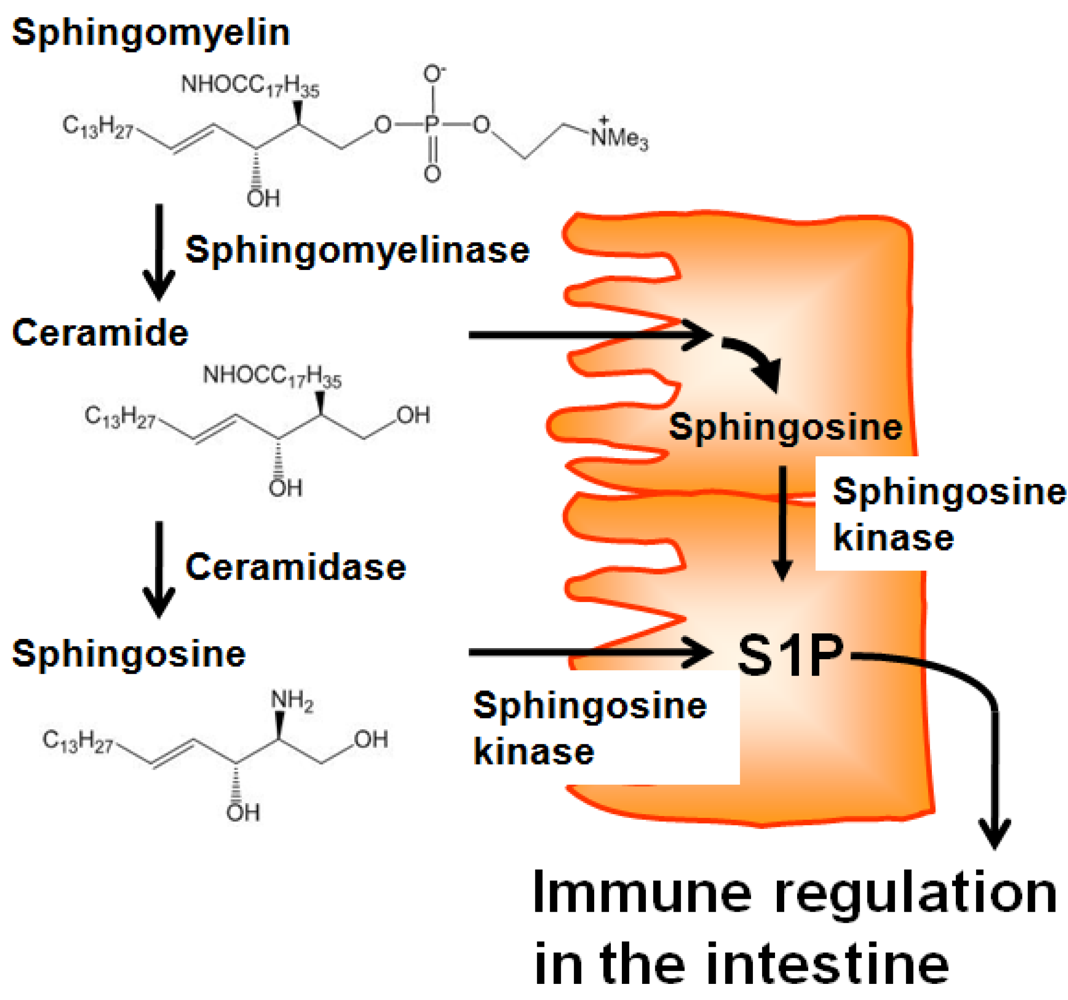
3. Regulation of S1P Metabolism by Dietary Materials
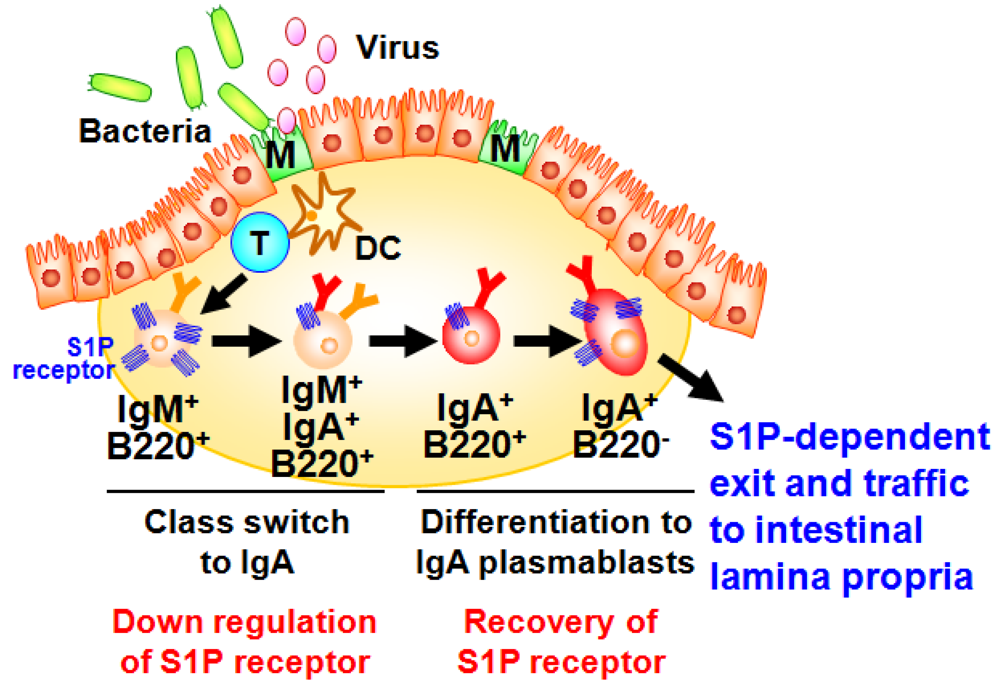
4. S1P Regulates Innate and Acquired Phases of Intestinal IgA Responses
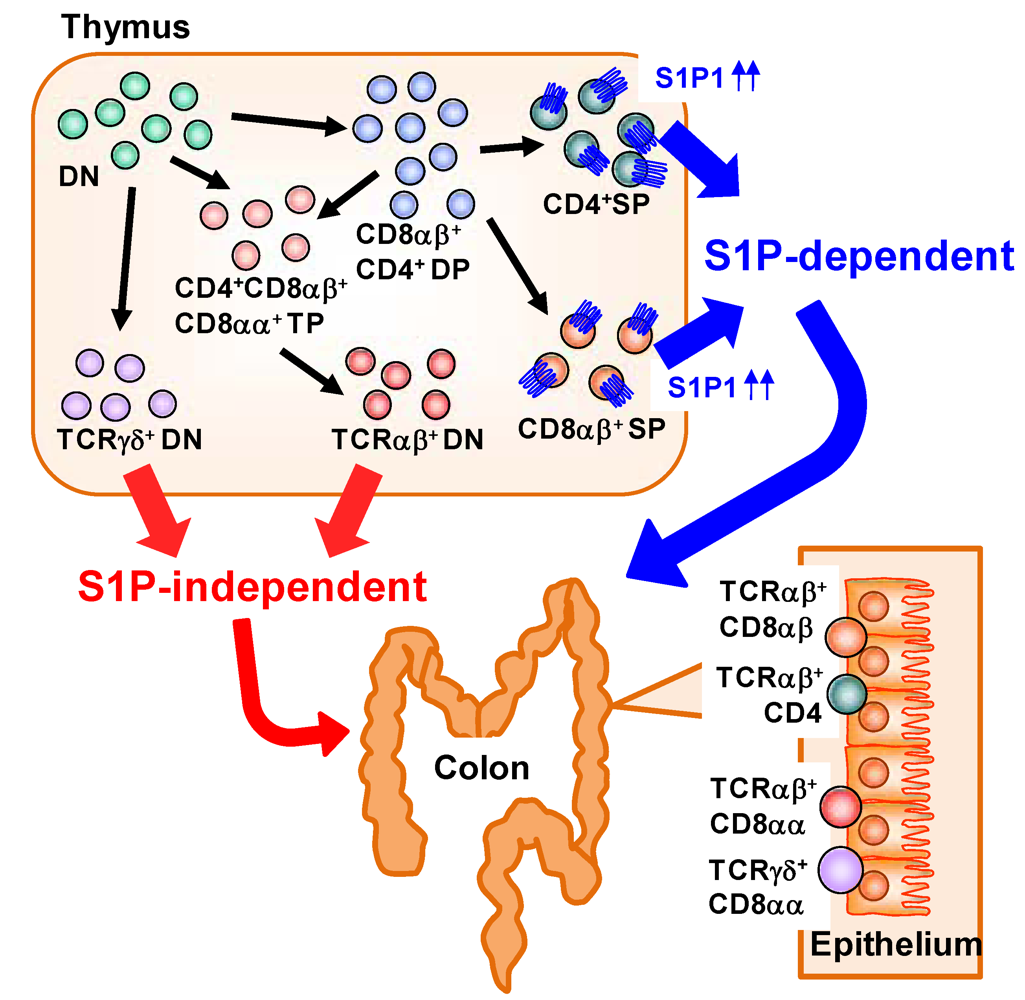
5. Distinct S1P Dependency of Trafficking of Intraepithelial T-Lymphocytes in the Gut
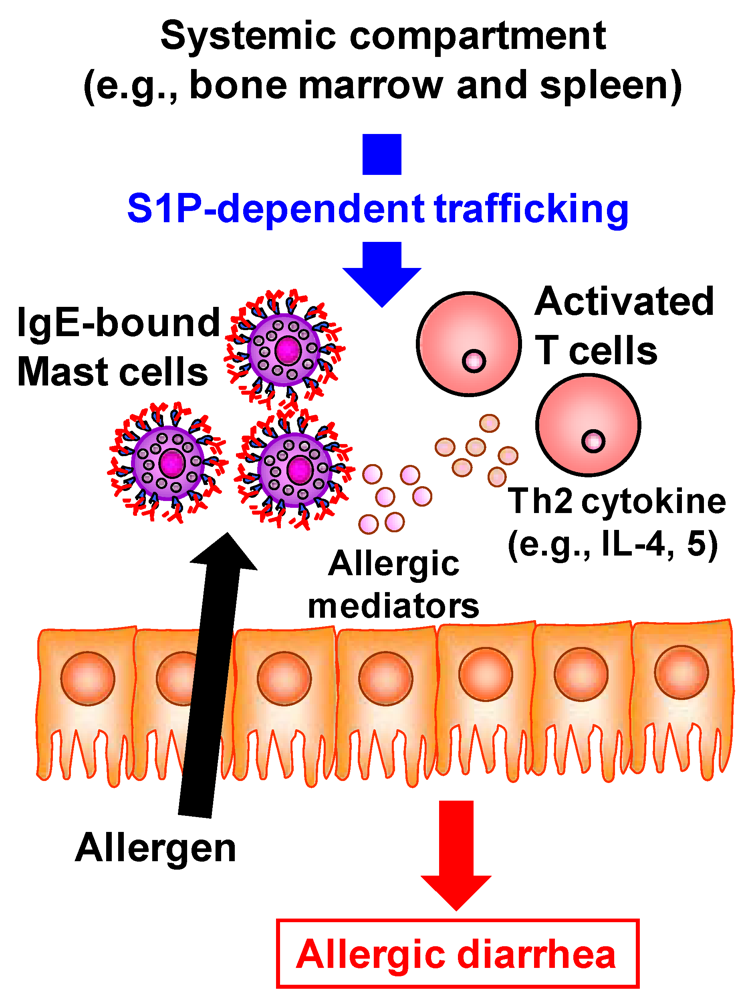
6. S1P-Mediated Regulation in the Development of Intestinal Immune Diseases
7. Conclusion
Acknowledgements
Conflict of Interest
References
- Kiyono, H.; Kunisawa, J.; McGhee, J.R.; Mestecky, J. The Mucosal Immune System. In Fundamental Immunology; Paul, W.E., Ed.; Lippincott-Raven: Philadelphia, PA, USA, 2008; Volume 6, pp. 983–1030. [Google Scholar]
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar]
- Margioris, A.N. Fatty acids and postprandial inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 129–137. [Google Scholar]
- Chi, H. Sphingosine-1-phosphate and immune regulation: Trafficking and beyond. Trends Pharmacol. Sci. 2011, 32, 16–24. [Google Scholar]
- Rivera, J.; Proia, R.L.; Olivera, A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008, 8, 753–763. [Google Scholar]
- Pappu, R.; Schwab, S.R.; Cornelissen, I.; Pereira, J.P.; Regard, J.B.; Xu, Y.; Camerer, E.; Zheng, Y.W.; Huang, Y.; Cyster, J.G.; et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007, 316, 295–298. [Google Scholar]
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005, 309, 1735–1739. [Google Scholar]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar]
- Michaud, J.; Im, D.S.; Hla, T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J. Immunol. 2010, 184, 1475–1483. [Google Scholar]
- Maeda, Y.; Matsuyuki, H.; Shimano, K.; Kataoka, H.; Sugahara, K.; Chiba, K. Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J. Immunol. 2007, 178, 3437–3446. [Google Scholar]
- Walzer, T.; Chiossone, L.; Chaix, J.; Calver, A.; Carozzo, C.; Garrigue-Antar, L.; Jacques, Y.; Baratin, M.; Tomasello, E.; Vivier, E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 2007, 8, 1337–1344. [Google Scholar] [CrossRef]
- Liu, G.; Yang, K.; Burns, S.; Shrestha, S.; Chi, H. The S1P1-mTOR axis directs the reciprocal differentiation of Th1 and Treg cells. Nat. Immunol. 2010, 11, 1047–1056. [Google Scholar]
- Wang, W.; Huang, M.C.; Goetzl, E.J. Type 1 sphingosine 1-phosphate G protein-coupled receptor (S1P1) mediation of enhanced IL-4 generation by CD4 T cells from S1P1 transgenic mice. J. Immunol. 2007, 178, 4885–4890. [Google Scholar]
- Huang, M.C.; Watson, S.R.; Liao, J.J.; Goetzl, E.J. Th17 augmentation in OTII TCR plus T cell-selective type 1 sphingosine 1-phosphate receptor double transgenic mice. J. Immunol. 2007, 178, 6806–6813. [Google Scholar]
- Oskeritzian, C.A.; Price, M.M.; Hait, N.C.; Kapitonov, D.; Falanga, Y.T.; Morales, J.K.; Ryan, J.J.; Milstien, S.; Spiegel, S. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J. Exp. Med. 2010, 207, 465–474. [Google Scholar] [CrossRef]
- Hughes, J.E.; Srinivasan, S.; Lynch, K.R.; Proia, R.L.; Ferdek, P.; Hedrick, C.C. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ. Res. 2008, 102, 950–958. [Google Scholar]
- Bouhours, D.; Bouhours, J.F. Developmental changes of monohexosylceramide and free ceramide in the large intestine of the rat. J. Biochem. 1985, 98, 1359–1366. [Google Scholar]
- Gustafsson, B.E.; Karlsson, K.A.; Larson, G.; Midtvedt, T.; Stromberg, N.; Teneberg, S.; Thurin, J. Glycosphingolipid patterns of the gastrointestinal tract and feces of germ-free and conventional rats. J. Biol. Chem. 1986, 261, 15294–15300. [Google Scholar]
- Vesper, H.; Schmelz, E.M.; Nikolova-Karakashian, M.N.; Dillehay, D.L.; Lynch, D.V.; Merrill, A.H., Jr. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J. Nutr. 1999, 129, 1239–1250. [Google Scholar]
- Nilsson, A. Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim. Biophys. Acta 1968, 164, 575–584. [Google Scholar]
- Schmelz, E.M.; Crall, K.J.; Larocque, R.; Dillehay, D.L.; Merrill, A.H., Jr. Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J. Nutr. 1994, 124, 702–712. [Google Scholar]
- Nilsson, A.; Duan, R.D. Alkaline sphingomyelinases and ceramidases of the gastrointestinal tract. Chem. Phys. Lipids 1999, 102, 97–105. [Google Scholar]
- Tani, M.; Ito, M.; Igarashi, Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell. Signal. 2007, 19, 229–237. [Google Scholar]
- Duan, R.D.; Nilsson, A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog. Lipid Res. 2009, 48, 62–72. [Google Scholar]
- Mazzei, J.C.; Zhou, H.; Brayfield, B.P.; Hontecillas, R.; Bassaganya-Riera, J.; Schmelz, E.M. Suppression of intestinal inflammation and inflammation-driven colon cancer in mice by dietary sphingomyelin: Importance of peroxisome proliferator-activated receptor gamma expression. J. Nutr. Biochem. 2011, 22, 1160–1171. [Google Scholar]
- Fischbeck, A.; Leucht, K.; Frey-Wagner, I.; Bentz, S.; Pesch, T.; Kellermeier, S.; Krebs, M.; Fried, M.; Rogler, G.; Hausmann, M.; et al. Sphingomyelin induces cathepsin D-mediated apoptosis in intestinal epithelial cells and increases inflammation in DSS colitis. Gut 2011, 60, 55–65. [Google Scholar]
- Sjoqvist, U.; Hertervig, E.; Nilsson, A.; Duan, R.D.; Ost, A.; Tribukait, B.; Lofberg, R. Chronic colitis is associated with a reduction of mucosal alkaline sphingomyelinase activity. Inflamm. Bowel Dis. 2002, 8, 258–263. [Google Scholar]
- Maines, L.W.; Fitzpatrick, L.R.; French, K.J.; Zhuang, Y.; Xia, Z.; Keller, S.N.; Upson, J.J.; Smith, C.D. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig. Dis. Sci. 2008, 53, 997–1012. [Google Scholar]
- Nyberg, L.; Duan, R.D.; Nilsson, A. A mutual inhibitory effect on absorption of sphingomyelin and cholesterol. J. Nutr. Biochem. 2000, 11, 244–249. [Google Scholar]
- Allende, M.L.; Bektas, M.; Lee, B.G.; Bonifacino, E.; Kang, J.; Tuymetova, G.; Chen, W.; Saba, J.D.; Proia, R.L. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 2011, 286, 7348–7358. [Google Scholar]
- Bagdanoff, J.T.; Donoviel, M.S.; Nouraldeen, A.; Tarver, J.; Fu, Q.; Carlsen, M.; Jessop, T.C.; Zhang, H.; Hazelwood, J.; Nguyen, H.; et al. Inhibition of sphingosine-1-phosphate lyase for the treatment of autoimmune disorders. J. Med. Chem. 2009, 52, 3941–3953. [Google Scholar]
- Bandhuvula, P.; Honbo, N.; Wang, G.Y.; Jin, Z.Q.; Fyrst, H.; Zhang, M.; Borowsky, A.D.; Dillard, L.; Karliner, J.S.; Saba, J.D. S1p lyase: A novel therapeutic target for ischemia-reperfusion injury of the heart. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1753–H1761. [Google Scholar]
- Kunisawa, J.; McGhee, J.; Kiyono, H. Mucosal S-IgA Enhancement: Development of Safe and Effective Mucosal Adjuvants and Mucosal Antigen Delivery Vehicles. In Mucosal Immune Defense: Immunoglobulin A; Kaetzel, C., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2007; pp. 346–389. [Google Scholar]
- Fagarasan, S.; Kawamoto, S.; Kanagawa, O.; Suzuki, K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010, 28, 243–273. [Google Scholar]
- Kunisawa, J.; Nochi, T.; Kiyono, H. Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol. 2008, 29, 505–513. [Google Scholar]
- Neutra, M.R.; Mantis, N.J.; Kraehenbuhl, J.P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2001, 2, 1004–1009. [Google Scholar]
- Gohda, M.; Kunisawa, J.; Miura, F.; Kagiyama, Y.; Kurashima, Y.; Higuchi, M.; Ishikawa, I.; Ogahara, I.; Kiyono, H. Sphingosine 1-phosphate regulates the egress of IgA plasmablasts from Peyer’s patches for intestinal IgA responses. J. Immunol. 2008, 180, 5335–5343. [Google Scholar]
- Graler, M.H.; Goetzl, E.J. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004, 18, 551–553. [Google Scholar]
- Kunisawa, J.; Kiyono, H. A marvel of mucosal T cells and secretory antibodies for the creation of first lines of defense. Cell Mol. Life Sci. 2005, 62, 1308–1321. [Google Scholar]
- Kunisawa, J.; Kurashima, Y.; Gohda, M.; Higuchi, M.; Ishikawa, I.; Miura, F.; Ogahara, I.; Kiyono, H. Sphingosine 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood 2007, 109, 3749–3756. [Google Scholar]
- Kunisawa, J.; Gohda, M.; Kurashima, Y.; Ishikawa, I.; Higuchi, M.; Kiyono, H. Sphingosine 1-phosphate-dependent trafficking of peritoneal B cells requires functional NFκB-inducing kinase in stromal cells. Blood 2008, 111, 4646–4652. [Google Scholar]
- Kunisawa, J.; Takahashi, I.; Kiyono, H. Intraepithelial lymphocytes: Their shared and divergent immunological behaviors in the small and large intestine. Immunol. Rev. 2007, 215, 136–153. [Google Scholar]
- Vivier, E.; Tomasello, E.; Paul, P. Lymphocyte activation via NKG2D: Towards a new paradigm in immune recognition? Curr. Opin. Immunol. 2002, 14, 306–311. [Google Scholar] [CrossRef]
- Natarajan, K.; Dimasi, N.; Wang, J.; Mariuzza, R.A.; Margulies, D.H. Structure and function of natural killer cell receptors: Multiple molecular solutions to self, nonself discrimination. Annu. Rev. Immunol. 2002, 20, 853–885. [Google Scholar]
- Gangadharan, D.; Lambolez, F.; Attinger, A.; Wang-Zhu, Y.; Sullivan, B.A.; Cheroutre, H. Identification of pre- and postselection TCRαβ+ intraepithelial lymphocyte precursors in the thymus. Immunity 2006, 25, 631–641. [Google Scholar]
- Kunisawa, J.; Kurashima, Y.; Higuchi, M.; Gohda, M.; Ishikawa, I.; Ogahara, I.; Kim, N.; Shimizu, M.; Kiyono, H. Sphingosine 1-phosphate dependence in the regulation of lymphocyte trafficking to the gut epithelium. J. Exp. Med. 2007, 204, 2335–2348. [Google Scholar]
- Sharma, S.; Mathur, A.G.; Pradhan, S.; Singh, D.B.; Gupta, S. Fingolimod (FTY720): First approved oral therapy for multiple sclerosis. J. Pharmacol. Pharmacother. 2011, 2, 49–51. [Google Scholar]
- Kweon, M.N.; Yamamoto, M.; Kajiki, M.; Takahashi, I.; Kiyono, H. Systemically derived large intestinal CD4+ Th2 cells play a central role in STAT6-mediated allergic diarrhea. J. Clin. Invest. 2000, 106, 199–206. [Google Scholar]
- Kurashima, Y.; Kunisawa, J.; Higuchi, M.; Gohda, M.; Ishikawa, I.; Takayama, N.; Shimizu, M.; Kiyono, H. Sphingosine 1-phosphate-mediated trafficking of pathogenic Th2 and mast cells for the control of food allergy. J. Immunol. 2007, 179, 1577–1585. [Google Scholar]
- Gurish, M.F.; Boyce, J.A. Mast cell growth, differentiation, and death. Clin. Rev. Allergy Immunol. 2002, 22, 107–118. [Google Scholar] [CrossRef]
- Mizushima, T.; Ito, T.; Kishi, D.; Kai, Y.; Tamagawa, H.; Nezu, R.; Kiyono, H.; Matsuda, H. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm. Bowel Dis. 2004, 10, 182–192. [Google Scholar]
- Fujii, R.; Kanai, T.; Nemoto, Y.; Makita, S.; Oshima, S.; Okamoto, R.; Tsuchiya, K.; Totsuka, T.; Watanabe, M. FTY720 suppresses CD4+ CD44high CD62L− effector memory T cell-mediated colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G267–G274. [Google Scholar]
- Deguchi, Y.; Andoh, A.; Yagi, Y.; Bamba, S.; Inatomi, O.; Tsujikawa, T.; Fujiyama, Y. The S1P receptor modulator FTY720 prevents the development of experimental colitis in mice. Oncol. Rep. 2006, 16, 699–703. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kunisawa, J.; Kiyono, H. Immunological Function of Sphingosine 1-Phosphate in the Intestine. Nutrients 2012, 4, 154-166. https://doi.org/10.3390/nu4030154
Kunisawa J, Kiyono H. Immunological Function of Sphingosine 1-Phosphate in the Intestine. Nutrients. 2012; 4(3):154-166. https://doi.org/10.3390/nu4030154
Chicago/Turabian StyleKunisawa, Jun, and Hiroshi Kiyono. 2012. "Immunological Function of Sphingosine 1-Phosphate in the Intestine" Nutrients 4, no. 3: 154-166. https://doi.org/10.3390/nu4030154



