The Effect of Spirulina platensis versus Soybean on Insulin Resistance in HIV-Infected Patients: A Randomized Pilot Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Protocol
2.2.1. Randomization, Treatment Allocation and Follow-Up
| Spirulina | Soybean | |
|---|---|---|
| Quantity per 100 g | Quantity per 100 g | |
| Protein | 65 g | 27 g |
| Carbohydrates | 15 g | 58 g |
| Lipids | 6 g | 8.6 g |
| Calcium | 1000 mg | 106.3 mg |
| Iron | 180 mg | 6.3 mg |
| Magnesium | 400 mg | - |
| Minerals | 7 mg | - |
2.2.2. Clinical Assessment
2.2.3. Biochemical Assays
2.2.4. Outcome Measures
2.2.5. Sample Size
2.3. Statistical Methods
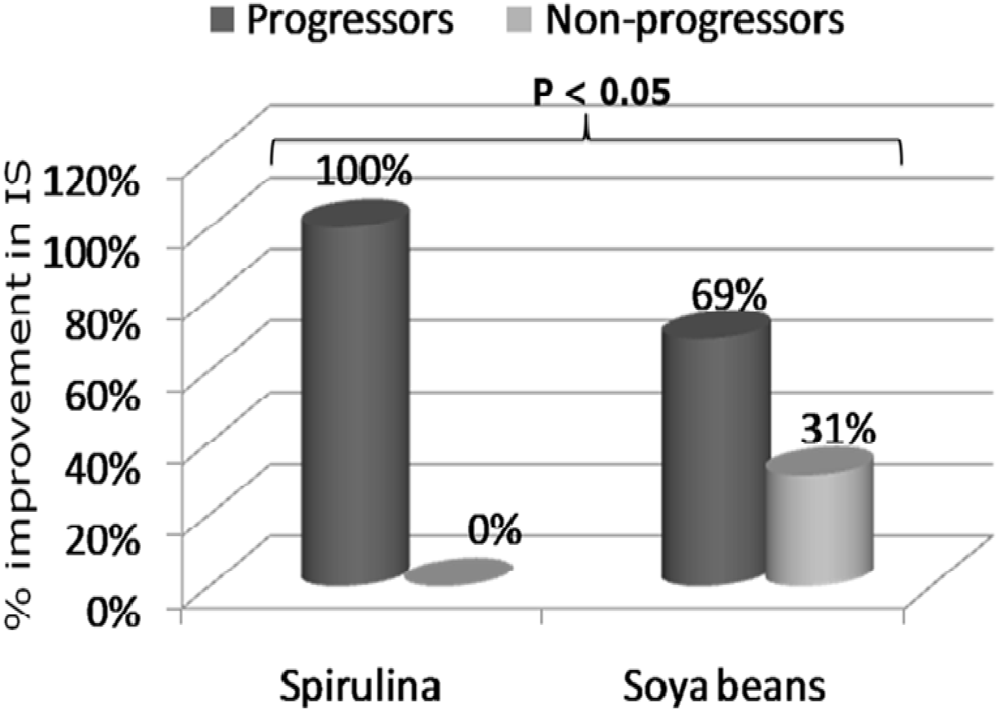
3. Results and Discussion
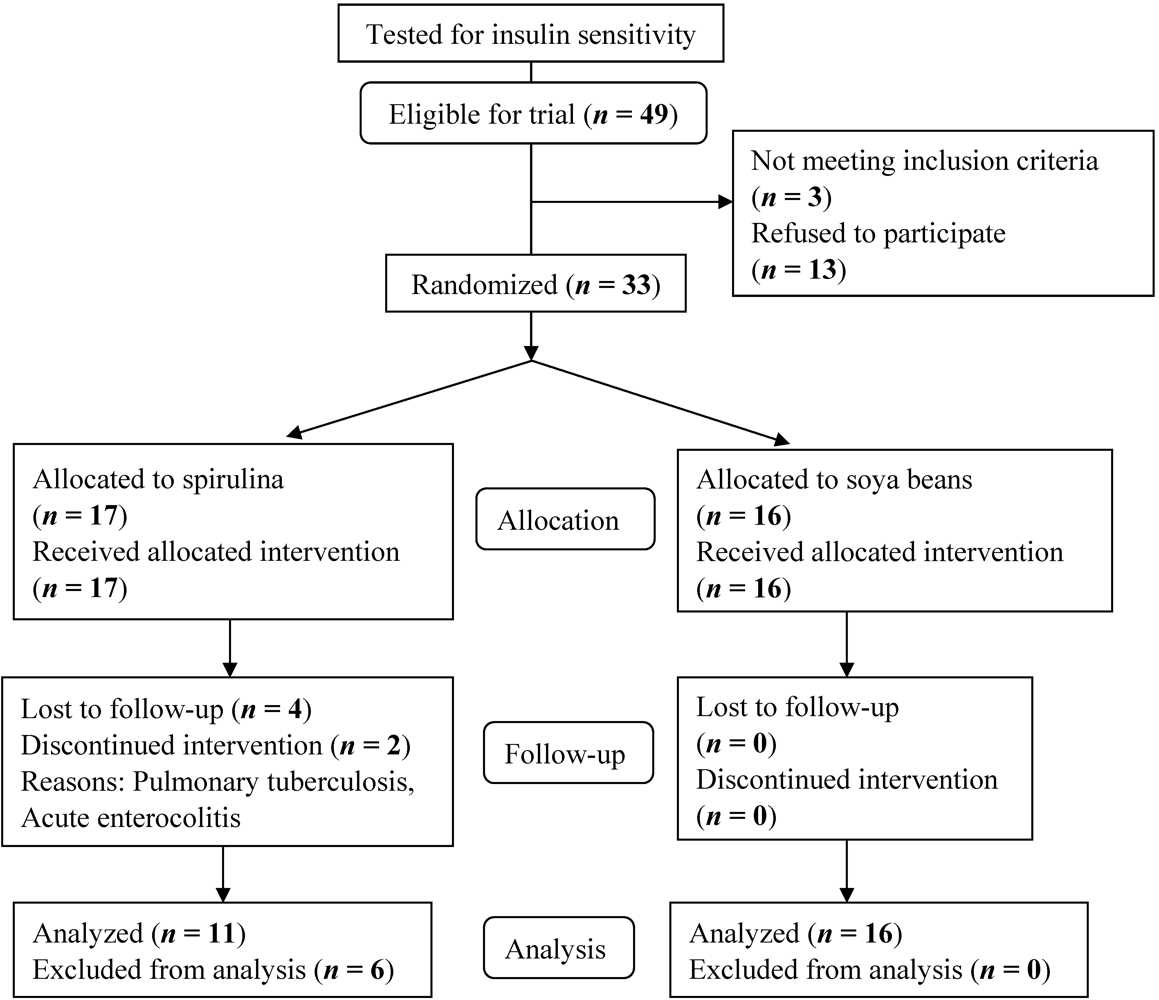
| Characteristics | Spirulina (n = 17) | Soya bean (n = 16) | p value | |
|---|---|---|---|---|
| Demographic and Clinical | ||||
| Sex, n | Men | 4 | 3 | 0.74 |
| Women | 13 | 13 | ||
| Treatment regiment | ZDV+ | 12 | 12 | 0.95 |
| D4T+ | 4 | 3 | ||
| Treatment naive | 1 | 1 | ||
| Age, mean (SD), years | 36 (11) | 39 (7) | 0.45 | |
| HAART duration, mean (SD), months | 22 (14) | 27 (17) | 0.23 | |
| BMI, mean (SD), kg/m2 | 23.8 (3.2) | 24.7 (2.8) | 0.40 | |
| Systolic BP, mean (SD), mmHg | 127 (17) | 121 (11) | 0.20 | |
| Diastolic BP, mean (SD), mmHg | 78 (10) | 80 (7) | 0.63 | |
| Waist Circumference, mean (SD), cm | 82.2 (9.1) | 82.3 (7.3) | 0.98 | |
| Fat-free mass, mean (SD), kg | 38.2 (11.5) | 37.1 (10.9) | 0.78 | |
| Percent Body Fat, mean (SD), % | 39.1 (17.4) | 41.6 (16.3) | 0.45 | |
| Biological | ||||
| CD4 count, median (Q1–Q3), µL | 277 (75.5–472.3) | 226 (112–402) | 1.0 | |
| Total cholesterol, mean (SD), g/L | 1.7 (0.49) | 1.8 (0.43) | 0.54 | |
| Triglycerides, mean (SD), g/L | 0.7 (0.53) | 0.6 (0.28) | 0.77 | |
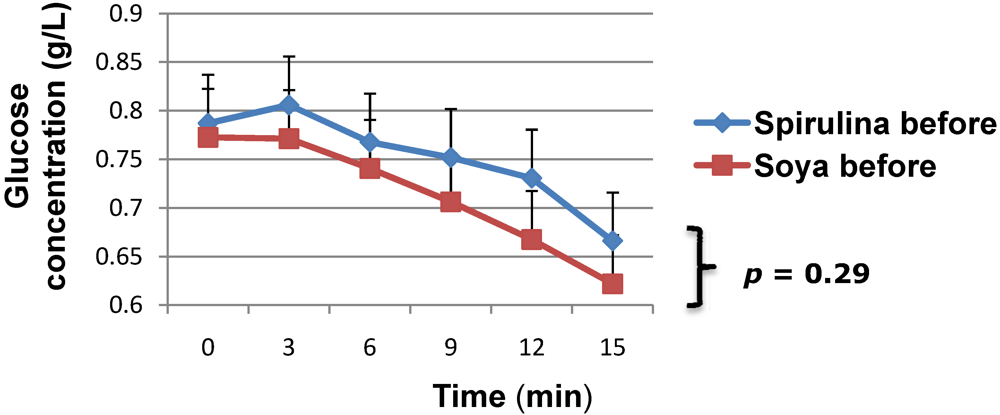


| Spirulina (n = 11) | Soya bean (n = 16) | |
|---|---|---|
| Waist Circumference, mean (SD), cm | −0.40 (3.16) | +0.01 (2.34) |
| Total Body Fat, mean (SD), kg | +0.36 (4.52) | +0.38 (3.30) |
| Fat-Free Mass, mean (SD), kg | +0.35 (2.01) | −1.59 (1.57) |
| Fasting glycemia, mean (SD), g/L | +1.3 (0.9) | +1.8 (2.1) |
| Total cholesterol, mean (SD), g/L | +0.63 (0.53) | +0.87 (0.79) |
| Triglycerides, mean (SD), g/L | +0.48 (0.52) | +0.61 (0.70) |
4. Conclusions
Acknowledgements
References
- Nolan, D. Metabolic complications associated with HIV protease inhibitor therapy. Drugs 2003, 63, 2556–2574. [Google Scholar]
- Vigouroux, C.; Gharakhanian, S.; Salhi, Y.; Nguyen, T.H.; Adda, N.; Rozenbaum, W.; Capeau, J. Adverse metabolic disorders during highly active antiretroviral treatments (HAART) of HIV disease. Diabetes Metab. 1999, 25, 383–392. [Google Scholar]
- Martinez, E.; Mocroft, A.; García-Viejo, M.A.; Pérez-Cuevas, J.B.; Blanco, J.L.; Mallolas, J.; Bianchi, L.; Conget, I.; Blanch, J.; Phillips, A.; Gatell, J.M. Risk of lipodystrophy in HIV-1 patients treated with protease inhibitors: a prospective cohort study. Lancet 2001, 357, 592–598. [Google Scholar]
- Carr, A.; Samaras, K.; Burton, S.; Freund, J.; Chisholm, D.J.; Cooper, D.A. A syndrome of peripheral lipodystrophy and insulin resistance due to HIV protease inhibitors. Aids 1998, 12 (Suppl.), F51–F58. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.T.; Cofrancesco, J. Metabolic Abnormalities in HIV-infected Patients: An Update. Curr. Infect. Dis. Rep. 2006, 8, 497–504. [Google Scholar]
- Behrens, G.; Dejam, A.; Schmidt, H.; Balks, H.; Brabant, G.; Korner, T.; Stoll, M.; Schimdt, R.E. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. Aids 1999, 13, F63–F70. [Google Scholar]
- Arendt, B.M.; Aghdassi, E.; Mohammed, S.S.; Fung, L.Y.; Jalali, P.; Salit, I.E.; Allard, J.P. Dietary intake and physical activity in a Canadian population sample of male patients with HIV infection and metabolic abnormalities. Curr. HIV Res. 2008, 6, 82–90. [Google Scholar]
- Dube, M. Defects of glucose metabolism in patients with human immunodeficiency virus. Clin. Infect. Dis. 2000, 31, 1467–1475. [Google Scholar]
- Laws, A.; Reavens, G. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J. Intern. Med. 1992, 231, 25–30. [Google Scholar]
- Murata, H.; Hruz, P.W.; Mueckler, M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J. Biol. Chem. 2000, 275, 20251–20254. [Google Scholar]
- Jensen, M.D.; Miles, J.M. The roles of diet and exercise in the management of patients with insulin-dependent diabetes mellitus. Mayo Clin. Proc. 1986, 61, 813–819. [Google Scholar]
- Paquot, N. Diet and nutrition principles in type 2 diabetes. Rev. Med. Liege 2005, 60, 391–394. (in French).. [Google Scholar]
- Wolever, T.M. Carbohydrate and the regulation of blood glucose and metabolism. Nutr. Rev. 2003, 61, S40–S48. [Google Scholar]
- Malita, F.M.; Karelis, A.D.; Toma, E.; Rabasa-Lhoret, R. Effects of different types of exercise on body composition and fat distribution in HIV-infected patients: a brief review. Can. J. Appl. Physiol. 2005, 30, 233–245. [Google Scholar]
- Hasler, C. Functional foods: benefits, concerns and challenges—a position paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar]
- Annapuna, V.; Shah, N.; Bhaskaram, P. Bioavailability of Spirulina carotenes in pre-school children. J. Clin. Biochem. Nutr. 1991, 10, 145–151. [Google Scholar]
- Parikh, P.; Mani, U.; Iyer, U. Role of Spirulina in the Control of Glycemia and Lipidemia in Type 2 Diabetes Mellitus. J. Med. Food 2001, 4, 193–199. [Google Scholar]
- Torres-Duran, P.V.; Ferreira-Hermosillo, A.; Juarez-Oropeza, M.A. Antihyperlipemic and antihypertensive effects of Spirulina maxima in an open sample of Mexican population: a preliminary report. Lipids Health Dis. 2007, 6, 33. [Google Scholar]
- Maranesi, M.; Barzanti, V.; Carenini, G.; Gentili, P. Nutritional studies on Spirulina maxima. Acta Vitaminol. Enzymol. 1984, 6, 295–304. [Google Scholar]
- Oliva, M.E.; Chicco, A.G.; Lombardo, Y.B. Soya protein reverses dyslipidaemia and the altered capacity of insulin-stimulated glucose utilization in the skeletal muscle of sucrose-rich diet-fed rats. Br. J. Nutr. 2009, 102, 60–68. [Google Scholar]
- Gobert, C.P.; Pipe, E.A.; Capes, S.E.; Darlington, G.A.; Lampe, J.W.; Duncan, A.M. Soya protein does not affect glycaemic control in adults with type 2 diabetes. Br. J. Nutr. 2010, 103, 412–421. [Google Scholar]
- Chen, S.W.; Zhang, H.M.; Zhang, L.S.; Feng, X.F. Effects of soy isoflavone on gene expression of resistin in insulin-resistance rats. Sichuan Da Xue Xue Bao Yi Xue Ban 2006, 37, 717–720. (in Chinese).. [Google Scholar] [PubMed]
- The Helsinki Declaration on human experimentation. Available online: http://ohsr.od.nih.gov/guidelines/Helsinki.html (accessed on 31 October 2009).
- Chumlea, W.C.; Guo, S.S.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Heymsfield, S.B.; Lukaski, H.C.; Friedl, K. Body composition estimates from NHANES III bioelectric impedance data. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1596–1609. [Google Scholar]
- Lundbaek, K. Intravenous glucose tolerance as a tool in definition and diagnosis of diabetes mellitus. Br. Med. J. 1962, 5291, 1507–1513. [Google Scholar]
- Sobngwi, E.; Effoe, V.; Boudou, P.; Njamen, D.; Gautier, J.F.; Mbanya, J.C. Waist circumference does not predict circulating adiponectin levels in sub-Saharan women. Cardiovasc. Diabetol. 2007, 6, 31. [Google Scholar]
- Elise, J.W. Statistics review 4: Sample size calculations. Crit. Care 2002, 6, 335–341. [Google Scholar]
- Park, H.J.; Lee, Y.J.; Ryu, H.K.; Kim, M.H.; Chung, H.W.; Kim, Y.W. A randomized double-blind, placebo-controlled study to establish the effects of spirulina in elderly Koreans. Ann. Nutr. MeTable 2008, 52, 322–328. [Google Scholar]
- Sutinen, J.; Hakkinen, A.M.; Westerbacka, J.; Seppala-Lindroos, A.; Vehkavaara, S.; Halavaara, J.; Jarvinen, A.; Ristola, M.; Yki-Jarvinen, H. Increased fat accumulation in the liver in HIV-infected patients with antiretroviral therapy-associated lipodystrophy. Aids 2002, 16, 2183–2193. [Google Scholar]
- George, P.; Ludvik, B. Lipids and diabetes. J. Clin. Basic Cardiol. 2000, 3, 159–162. [Google Scholar]
- Nordentoft, I.; Jeppesen, P.B.; Hong, J.; Abudula, R.; Hermansen, K. Increased insulin sensitivity and changes in the expression profile of key insulin regulatory genes and beta cell transcription factors in diabetic KKAy-mice after feeding with a soy bean protein rich diet high in isoflavone content. J. Agric. Food Chem. 2008, 56, 4377–4385. [Google Scholar]
- Chen, S.W.; Zhang, H.M.; Zhang, L.S.; Feng, X.F.; Peng, X. Effects of soy isoflavone on gene expression of adiponectin in insulin-resistance rats induced by high-fat and high-sugar diet. Wei Sheng Yan Jiu 2006, 35, 46–49. (in Chinese).. [Google Scholar] [PubMed]
- Nagasawa, A.; Fukui, K.; Funahashi, T.; Maeda, N.; Shimomura, I.; Kihara, S.; Waki, M.; Takamatsu, K.; Matsuzawa, Y. Effects of soy protein diet on the expression of adipose genes and plasma adiponectin. Horm. Metab. Res. 2002, 34, 635–639. [Google Scholar]
- Iritani, N.; Sugimoto, T.; Fukuda, H.; Komiya, M.; Ikeda, H. Dietary soybean protein increases insulin receptor gene expression in Wistar fatty rats when dietary polyunsaturated fatty acid level is low. J. Nutr. 1997, 127, 1077–1083. [Google Scholar]
- Feres, N.H.; Reis, S.R.; Veloso, R.V.; Arantes, V.C.; Souza, L.M.; Carneiro, E.M.; Boschero, A.C.; Reis, M.A.; Latorraca, M.Q. Soybean diet alters the insulin-signaling pathway in the liver of rats recovering from early-life malnutrition. Nutrition 2010, 26, 441–448. [Google Scholar]
- Tsai, A.C.; Mott, E.L.; Owen, G.M.; Bennick, M.R.; Lo, G.S.; Steinke, F.S. Effects of soy polysaccharide on gastrointestinal function, nutrient balance, steroid excretion, glucose tolerance, serum lipids and other parameters in humans. Am. J. Clin. Nutr. 1983, 38, 504–511. [Google Scholar] [PubMed]
- Liu, Z.M.; Chen, Y.M.; Ho, S.C.; Ho, Y.P.; Woo, J. Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: a 6-month double-blind, randomized, placebo-controlled trial in postmenopausal Chinese women with prediabetes or untreated early diabetes. Am. J. Clin. Nutr. 2010, 91, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marcel, A.-K.; Ekali, L.G.; Eugene, S.; Arnold, O.E.; Sandrine, E.D.; Von der Weid, D.; Gbaguidi, E.; Ngogang, J.; Mbanya, J.C. The Effect of Spirulina platensis versus Soybean on Insulin Resistance in HIV-Infected Patients: A Randomized Pilot Study. Nutrients 2011, 3, 712-724. https://doi.org/10.3390/nu3070712
Marcel A-K, Ekali LG, Eugene S, Arnold OE, Sandrine ED, Von der Weid D, Gbaguidi E, Ngogang J, Mbanya JC. The Effect of Spirulina platensis versus Soybean on Insulin Resistance in HIV-Infected Patients: A Randomized Pilot Study. Nutrients. 2011; 3(7):712-724. https://doi.org/10.3390/nu3070712
Chicago/Turabian StyleMarcel, Azabji-Kenfack, Loni G. Ekali, Sobngwi Eugene, Onana E. Arnold, Edie D. Sandrine, Denis Von der Weid, Emmanuel Gbaguidi, Jeanne Ngogang, and Jean C. Mbanya. 2011. "The Effect of Spirulina platensis versus Soybean on Insulin Resistance in HIV-Infected Patients: A Randomized Pilot Study" Nutrients 3, no. 7: 712-724. https://doi.org/10.3390/nu3070712




