Risk Assessment to Underpin Food Regulatory Decisions: An Example of Public Health Nutritional Epidemiology
Abstract
:1. Introduction
2. Overview of the Food Regulation Context in Which Epidemiological Data are Used
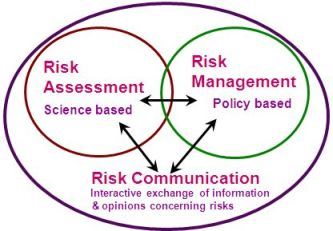
The Risk Analysis Framework
| Type | Example of Food Chemical | Source and Effect | References |
|---|---|---|---|
| Adulterants (prohibited substances) | Melamine | Deliberate adulteration; renal failure and death in infants fed adulterated infant formula | [7] |
| Nutrients | Fluoride | Found naturally. Also added to water and toothpaste. Potential exposure to high levels following the Icelandic volcano | [8] |
| Iodine | Very high levels in a soy drink due to use of a seaweed concentrate lead to hospital admissions in adults and breastfeeding neonates; the product was recalled in several countries | [9] | |
| Bioactives | Caffeine | Found in coffee and lesser amounts in tea & chocolate. Added to some energy drinks; new research suggesting pregnant women with higher intakes were more likely to have a low birth weight infant | [10] |
| Lutein | Marigold petals and some other foods; alleged to improve eye health and therefore possibly desirable to add to infant formula | [11] | |
| Substances formed during cooking | Acrylamide | Formed during high temperature cooking, such as roasting or frying, from protein and sugars present in food; a carcinogen | [12] |
| Polycyclic aromatic hydrocarbons (PAH) | Found naturally and also produced by industrial processes and by some cooking methods (e.g., barbequing, smoking). A number of PAH are known or suspected carcinogens | [13] | |
| Additives | Certain artificial colors | Used in various foods; alleged to cause behavior problems in children | [14] |
| Benzene | In the presence of acid, sodium benzoate (a preservative) can break down to benzene, a carcinogen. Benzene from this source was detected in carbonated beverages in several countries | [15] | |
| Packaging migrants | Bisphenol A | Monomer found in polycarbonate plastics and epoxy resins used to line cans, variable effects on hormonal activity in laboratory animals | [16] |
| Environmental contaminants | Perchlorates | Found naturally and also man-made (e.g., in rocket fuel), in high doses, it interferes with uptake of iodine by the thyroid | [17] |
| Nitrates | Found naturally in leafy vegetables but also derived from fertilizers and is used as a food additive; can be converted into nitrosamines, a carcinogen, in the body | [18] | |
| Dioxins | Environmental contamination from industrial sources although there are a small number of natural sources; long term exposure linked to immune system impairment | [19] | |
| Naturally occurring toxicants | Cyanogenic glycosides | In improperly prepared cassava chips (crisps); can cause cyanide poisoning | [20] |

3. Information Required for Dietary Modeling

3.1. Reference Health Standards for Food Chemicals
| Food chemical | Focus | Terminology | Abbreviation | Basis |
|---|---|---|---|---|
| Nutrient | Adequacy | Average nutrient requirement | ANR * | Total daily amount with separate values by age, sex, life stage |
| Excess ** | Upper Level of Intake | UL | Total daily amount with separate values by age, sex, life stage | |
| Additives | Excess | Acceptable daily intake | ADI | per kg body weight/day |
| Agricultural and veterinary chemical residues | Excess (chronic) | Acceptable daily intake | ADI | per kg body weight/day |
| Excess (short term) | Acute Reference Dose | ARfD | per kg body weight/day | |
| Contaminants and naturally- occurring toxicants | Excess (chronic) | Provisional tolerable daily/weekly/monthly intake | PTDI/PTWI/PTMI | per kg body weight/day, week or month |
| Excess (short term) | Acute Reference Dose | ARfD | per kg body weight/day |
3.2. Food Consumption Data
3.3. Food Chemical Concentration Data
4. Some Specific Considerations in Dietary Modeling
Estimating Usual (Long-Term) Intake in Semi-Probabilistic and Probabilistic Models
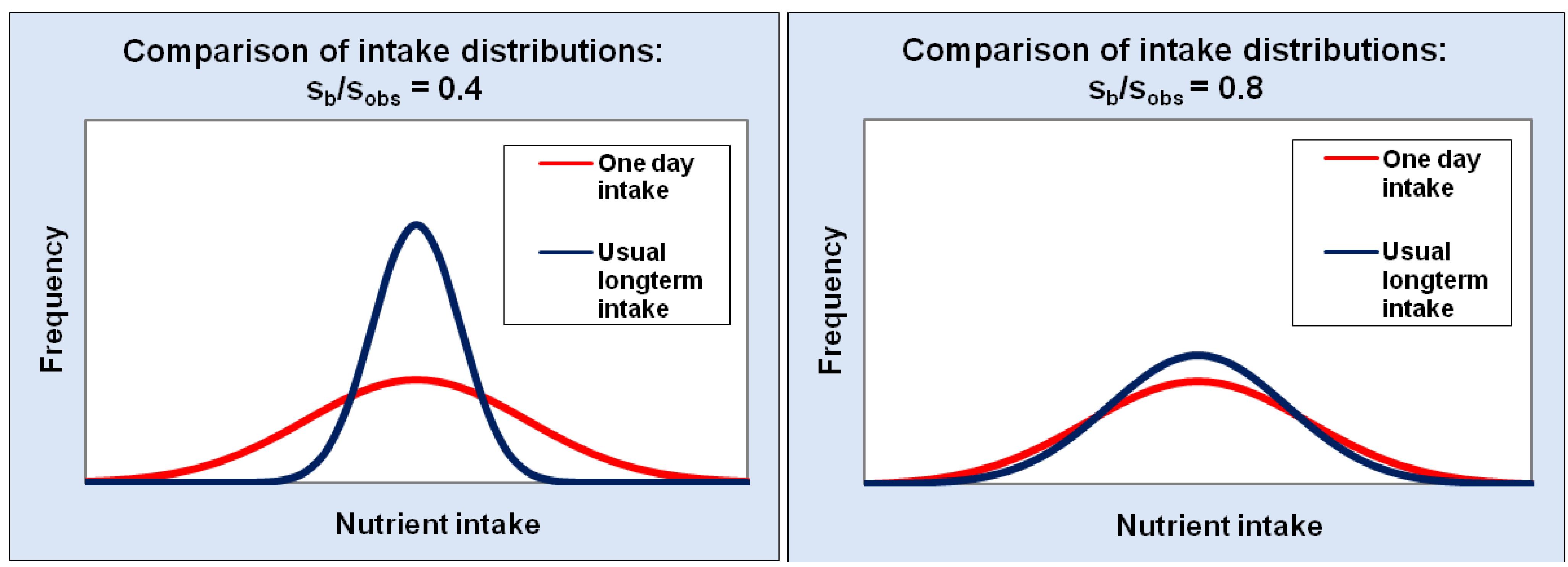
5. Presentation of Results
6. Risk Management
Maximum Limits for Food Chemicals in Food Regulations
| Food Chemical | Terminology | Abbreviation |
|---|---|---|
| Naturally occurring toxicants | Maximum Level | ML |
| Agricultural and Veterinary chemical residues | Maximum Residue Limit | MRL |
| Additives | Maximum Permitted Level | MPL |
| Contaminants | Maximum Level | ML |
7. Risk Communication
8. Case Studies from Australia and New Zealand
8.1. Folic Acid Fortification [45]
8.2. Iodine Fortification [46,47]
8.3. Ferric Sodium EDTA [48]
8.4. Erythrosine in Craft Food Colorings [49]
8.5. Cyanogenic Glycosides in Cassava Chips [20]
8.6. International Modeling
9. Conclusions
References
- Hennekens, C.H.; Buring, J.E. Epidemiology in Medicine; Mayrent, S.L., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1987. [Google Scholar]
- Kaldor, J. Public health epidemiology versus research epidemiology—does it have to be one or the other? Australas. Epidemiologist 1997, 4, 1–2. [Google Scholar]
- Willett, W. Nutritional Epidemiology, 2nd ed; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Design Concepts in Nutritional Epidemiology, 2nd; Margetts, B.M.; Nelson, M. (Eds.) Oxford University Press: New York, NY, USA, 1997.
- Food Standards Australia New Zealand. The Analysis of Food Related Health Risks; FSANZ: Canberra, Australia, 2009. Available online: http://www.foodstandards.gov.au/_srcfiles/Food%20Related%20Health%20Risks%20WEB_FA.pdf (accessed on September 30, 2010).
- Food Standards Australia New Zealand. Principles and Practices of Dietary Exposure Assessment for Food Regulatory Purposes; FSANZ: Canberra, Australia, 2009. Available online: http://www.foodstandards.gov.au/_srcfiles/Principles%20&%20practices%20exposure%20assessment%202009.pdf (accessed on September 30, 2010).
- World Health Organization. Melamine-Contaminated Powdered Infant Formula in China—Update; WHO: Geneva, Switzerland, 2008. Available online: http://www.who.int/csr/don/2008_09_22/en/index.html (accessed on September 30, 2010).
- European Food Safety Authority. Statement of EFSA on the Possible Risks for Public and Animal Health from the Contamination of the Feed and Food Chain due to Possible Ash-Fall Following the Eruption of the Eyjafjallajökull Volcano in Iceland; EFSA: Parma, Italy, 2010. Available online: http://www.efsa.europa.eu/en/scdocs/scdoc/1593.htm (accessed on September 30, 2010).
- Food Standards Australia New Zealand. National Product Recall—Bonsoy Soy Milk with Very High Levels of Iodine; FSANZ: Canberra, Australia, 2010. Available online: http://www.foodstandards.gov.au/scienceandeducation/newsroom/mediareleases/mediareleases2009/nationalproductrecal4651.cfm (accessed on September 30, 2010).
- Food Standards Agency (UK). Food Standards Agency Publishes New Caffeine Advice for Pregnant Women; FSA: London, UK, 2008. Available online: http://www.food.gov.uk/news/pressreleases/2008/nov/caffeineadvice (accessed on September 30, 2010).
- Food Standards Australia New Zealand. A594—Lutein as a Nutritive Substance in Infant Formula. First Review Report; FSANZ: Canberra, Australia, 2009. Available online: http://www.foodstandards.gov.au/_srcfiles/A594%20Lutein%20FRR%20FINAL.pdf (accessed on September 30, 2010).
- Food and Drug Administration (US). Acrylamide Questions and Answers; FDA: Washington, DC, USA, 2009. Available online: http://www.fda.gov/food/foodsafety/foodcontaminantsadulteration/chemicalcontaminants/acrylamide/ucm053569.htm (accessed on September 30, 2010).
- Food Standards Australia New Zealand. Survey of Polycyclic Aromatic Hydrocarbons (PAH) in Australian Foods. Dietary Exposure Assessment and Risk Characterization; FSANZ: Canberra, Australia, 2010. Available online: http://www.foodstandards.gov.au/_srcfiles/PAH%20Survey%20for%20website.pdf (accessed on December 1, 2010).
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; Sonuga-Barke, E.; Warner, J.O.; Stevenson, J. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567, Erratum in: Lancet 2007, 370, 1542.. [Google Scholar]
- Food and Drug Administration (US). Questions and Answers on the Occurrence of Benzene in Soft Drinks and Other Beverages; FDA: Washington, DC, USA, 2009. Available online: http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Benzene/ucm055131.htm (accessed on September 30, 2010).
- Food and Agriculture Organization/World Health Organization. Joint FAO/WHO Meeting to Review Toxicological and Health Aspects of Bisphenol A. Summary Report Including Report of Stakeholder Meeting on Bisphenol A. 1–5 November 2010, Ottawa, Canada; WHO: Geneva, Switzerland, 2010. Available online: http://www.who.int/foodsafety/chem/chemicals/BPA_Summary2010.pdf (accessed on December 1, 2010).
- Food and Drug Administration (US). Collection and Analysis of Food for Perchlorate—High Priority—DFP # 05–09; FDA: Washington, DC, USA, 2005. Available online: http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Perchlorate/ucm077709.htm (accessed on September 30, 2010).
- World Health Organization, Nitrate (and Potential Endogenous Formation of N-Nitroso Compounds); WHO Food Additive Series: 50; WHO: Geneva, Switzerland, 2002.
- Food Safety Authority of Ireland. Dioxins in Pork and Bacon Products; FSAI: Dublin, Ireland, 2008. Available online: http://www.fsai.ie/details.aspx?id=7875&LangType=1033&terms=pork+dioxin (accessed on September 30, 2010).
- Food Standards Australia New Zealand. Proposal P1002—Hydrocyanic Acid in Ready-to-Eat Cassava Chips. First Review Report; FSANZ: Canberra, Australia, 2009. Available online: http://www.foodstandards.gov.au/_srcfiles/P1002%20Hydrocyanic%20acid%20in%20cassava%20chips%20FRR%20FINAL.pdf (accessed on September 30, 2010).
- World Health Organization. About Risk Analysis in Food; WHO: Geneva, Switzerland, 2010. Available online: http://www.who.int/foodsafety/micro/riskanalysis/en/ (accessed on September 30, 2010).
- World Health Organization. WHO/FNU/FOS/95.3. Application of Risk Analysis to Food Standards Issues, a Joint FAO/WHO Expert Consultation, Geneva, Switzerland, 13–17 March 1995; WHO: Geneva, Switzerland, 1995. Available online: http://www.who.int/foodsafety/publications/micro/march1995/en/index.html (accessed on September 30, 2010).
- Codex Alimentarius Commission. Working Principles for Risk Analysis for Food Safety for Application by Governments; FAO: Rome, Italy, 2010, 1st ed. Available online: ftp://ftp.fao.org/codex/Publications/Booklets/Risk/Risk_EN_FR_ES.pdf (accessed on September 30, 2010).
- Codex Alimentarius Commission. Procedural Manual; FAO: Rome, Italy, 2010, 19th ed. Available online: ftp://ftp.fao.org/codex/Publications/ProcManuals/Manual_19e.pdf (accessed on December 6, 2010).
- Armstrong, B.K.; White, E.; Saracci, R. Principles of Exposure Measurement in Epidemiology; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Food and Agriculture Organization/World Health Organization. Joint FAO/WHO Consultation on Food Consumption and Exposure Assessment of Chemicals, Geneva, Switzerland, 10–14 February 1997; WHO: Geneva, Switzerland, 1997. Available online: http://www.who.int/foodsafety/publications/chem/exposure_feb1997/en/index.html (accessed on December 6, 2010).
- Food and Agriculture Organization/World Health Organization. Project to Update the Principles and Methods of Assessment of Chemicals in Food; WHO: Geneva, Switzerland, 2008. Available online: http://www.who.int/ipcs/food/update_project/en/index.html (accessed on September 30, 2010).
- World Health Organization. Estimating Appropriate Levels of Vitamins and Minerals for Food Fortification Programmes: the WHO Intake Monitoring, Assessment and Planning Program (IMAPP): Meeting Report; WHO: Geneva, Switzerland, 2010. Available online: http://www.who.int/nutrition/publications/micronutrients/9789241599603.pdf (accessed on November 29, 2010).
- Shen, D.D. Toxicokinetics. In Casarett and Doull’s Toxicology: the Basic Science of Poisons, 7th; Klaassen, C.D, Ed.; McGraw-Hill Professional: New York, NY, USA, 2008. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants. Fifty-Seventh Report; WHO Technical Report Series 909; WHO: Geneva, Switzerland, 2002. Available online: http://whqlibdoc.who.int/trs/WHO_TRS_909.pdf (accessed on September 30, 2010).
- Joint FAO/WHO Expert Committee on Food Additives. Seventy-Third Meeting, Geneva, 8–17 June 2010. Summary and Conclusions; WHO: Geneva, Switzerland, 2010. Available online: http://www.fao.org/ag/agn/agns/jecfa/JECFA73%20Summary%20Report%20Final.pdf (accessed on September 30, 2010).
- Joint FAO/WHO Expert Committee on Food Additives Home Page. Available online: http://www.codexalimentarius.net/web/jecfa.jsp (accessed on September 30, 2010).
- Joint FAO/WHO Meetings on Pesticide Residues Home Page. Available online: http://www.codexalimentarius.net/web/jmpr.jsp (accessed on September 30, 2010).
- Beaton, G.H. Uses and limits of the use of the Recommended Dietary Allowances for evaluating dietary intake data. Am. J. Clin. Nutr. 1985, 41, 155–164. [Google Scholar]
- Subcomittee on Dietary Evaluation, Food and Nutrition Board. Nutrient Adequacy.Assessment Using Food Consumption Surveys; National Academy Press: Washington, DC, USA, 1986. Available online: http://www.nap.edu/openbook.php?record_id=618 (accessed on September 30, 2010).
- Food and Nutrition Board Institute of Medicine. Dietary Reference Intakes. Applications in Dietary Planning; National Academy Press: Washington, DC, USA, 2003. Available online: http://www.nap.edu/openbook.php?isbn=0309088534 (accessed on September 30, 2010).
- Mackerras, D.; Powers, J.; Boorman, J.; Loxton, D.; Giles, G. Estimating the impact of mandatory fortification of bread with iodine on pregnant and post-partum women. J. Epidemiol. Community Health 2010. [Google Scholar]
- Food Standards Australia New Zealand. 22nd Australian Total Diet Study. A Total Diet Study of Five Trace Elements: Iodine, Selenium, Chromium, Molybdenum, and Nickel; FSANZ: Canberra, Australia, 2008. Available online: http://www.foodstandards.gov.au/scienceandeducation/publications/22ndaustraliantotaldietstudy/ (accessed on September 30, 2010).
- New Zealand Food Safety Authority. New Zealand Total Diet Study; NZFSA: Wellington, New Zealand, 2009. Available online: http://www.nzfsa.govt.nz/science/research-projects/total-diet-survey/index.htm (accessed on September 30, 2010).
- World Health Organization, Guidelines for the Study of Dietary Intakes of Chemical Contaminants; WHO: Geneva, Switzerland, 1985.
- Australian Bureau of Statistics, Confidentialised Unit Record File; ABS: Canberra, Australia, 1998.
- Subar, A.F.; Dodd, K.W.; Guenther, P.M.; Kipnis, V.; Midthune, D.; McDowell, M.; Tooze, J.A.; Freedman, L.S.; Krebs-Smith, S.M. The food propensity questionnaire: concept, development, and validation for use as a covariate in a model to estimate usual food intake. J. Am. Diet. Assoc. 2006, 106, 1556–1563. [Google Scholar]
- Freedman, L.S.; Midthune, D.; Carroll, R.J.; Krebs-Smith, S.; Subar, A.F.; Troiano, R.P.; Dodd, K.; Schatzkin, A.; Bingham, S.A.; Ferrari, P.; Kipnis, V. Adjustments to improve the estimation of usual dietary intake distributions in the population. J. Nutr. 2004, 134, 1836–1843, Erratum in: J. Nutr. 2005, 135, 1524.. [Google Scholar]
- Kipnis, V.; Midthune, D.; Buckman, D.W.; Dodd, K.W.; Guenther, P.M.; Krebs-Smith, S.M.; Subar, A.F.; Tooze, J.A.; Carroll, R.J.; Freedman, L.S. Modeling data with excess zeros and measurement error: application to evaluating relationships between episodically consumed foods and health outcomes. Biometrics 2009, 65, 1003–1010. [Google Scholar]
- Food Standards Australia New Zealand. Proposal P295—Consideration of Mandatory Fortification with Folic Acid. Final Assessment Report; FSANZ: Canberra, Australia, 2006. Available online: http://www.foodstandards.gov.au/foodstandards/proposals/proposalp295considerationofmandatoryfortificationwithfolicacid/p295finalassessmentr3568.cfm (accessed on September 30, 2010).
- Food Standards Australia New Zealand. Proposal P1003—Mandatory Iodine Fortification for Australia. Approval Report; FSANZ: Canberra, Australia, 2008. Available online: http://www.foodstandards.gov.au/foodstandards/proposals/proposalp295considerationofmandatoryfortificationwithfolicacid/p295finalassessmentr3568.cfm (accessed on September 30, 2010).
- Thoma, C.; Seal, J.; Mackerras, D.; Hunt, A. Iodine fortification of bread: experiences from Australia and New Zealand. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK, 2011. [Google Scholar]
- Food Standards Australia New Zealand. Application A570—Ferric Sodium EDTA as a Permitted Form of Iron. Final Assessment Report; FSANZ: Canberra, Australia, 2008. Available online: http://www.foodstandards.gov.au/_srcfiles/A570%20Ferric%20Sodium%20Edetate%20FAR%20FINAL.pdf (accessed on September 30, 2010).
- Food Standards Australia New Zealand. Application A603—Red 3 Erythrosine in Food Colouring Preparations. Final Assessment Report; FSANZ: Canberra, Australia, 2010. Available online: http://www.foodstandards.gov.au/_srcfiles/A603%20Erythrosine%20FAR%20FINAL.pdf (accessed on September 30, 2010).
- World Health Organization. GEMS/Food Consumption Cluster Diets; WHO: Geneva, Switzerland, 2006. Available online: http://www.who.int/foodsafety/chem/gems/en/index1.html (accessed on September 30, 2010).
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Baines, J.; Cunningham, J.; Leemhuis, C.; Hambridge, T.; Mackerras, D. Risk Assessment to Underpin Food Regulatory Decisions: An Example of Public Health Nutritional Epidemiology. Nutrients 2011, 3, 164-185. https://doi.org/10.3390/nu3010164
Baines J, Cunningham J, Leemhuis C, Hambridge T, Mackerras D. Risk Assessment to Underpin Food Regulatory Decisions: An Example of Public Health Nutritional Epidemiology. Nutrients. 2011; 3(1):164-185. https://doi.org/10.3390/nu3010164
Chicago/Turabian StyleBaines, Janis, Judy Cunningham, Christel Leemhuis, Tracy Hambridge, and Dorothy Mackerras. 2011. "Risk Assessment to Underpin Food Regulatory Decisions: An Example of Public Health Nutritional Epidemiology" Nutrients 3, no. 1: 164-185. https://doi.org/10.3390/nu3010164