Bone Density Testing: An Under-Utilised and Under-Researched Health Education Tool for Osteoporosis Prevention?
Abstract
:1. Introduction
2. Experimental Section
2.1. Interventions
2.1.1. Bone density feedback
2.1.2. Education
2.2. Outcome Measures
2.3. Other Factors
2.4. Statistical Analysis
- (1) available data analysis, i.e., including all participants who reached 2 years of follow-up;
- (2) intention to treat, in which we imputed missing data at 2 years using the method of last observation carried forward [24] for measured variables, and imputed no change for self-reported behavioral change variables; and
- (3) per protocol analysis defined in two ways: firstly, by whether subjects attended at least one educational session of the OPSMC; and secondly, by whether they attended all four OPSMC sessions.
3. Results and Discussion
3.1. Results: Primary Outcomes—BMD and Behaviour Change
| Multivariate β a,b (95% CI) | ||
|---|---|---|
| Femoral Neck BMD change (% p.a.) | ||
| Intervention group | ||
| High vs. normal risk feedback | +0.86 | (+0.39, +1.34) |
| OPSMC v leaflet | +0.14 | (−0.32, +0.62) |
| Behaviour change | ||
| Commenced calcium supplements | +1.33 | (+0.49, +2.17) |
| Calcium intake change (per 100 mg) | −0.03 | (−0.08, +0.01) |
| Persistent smoking cessation | −0.04 | (−1.16, +1.08) |
| Persistent self-reported physical activity change | +0.72 | (+0.22, +1.22) |
| Persistent increase in strenuous activity | +0.11 | (−0.45, +0.67) |
| Change in work capacity (per W) | −0.06 | (−0.48, +0.36) |
| Change in leg strength (per SD) | +0.02 | (−0.22, +0.26) |
| Lumbar Spine BMD change (% p.a.) | ||
| Intervention group | ||
| High vs. normal risk feedback | −0.01 | (−0.32, +0.30) |
| OPSMC v leaflet | +0.09 | (−0.21, +0.40) |
| Behaviour change | ||
| Commenced calcium supplements | +0.18 | (−0.37, +0.73) |
| Calcium intake change (per 100 mg) | −0.01 | (−0.04, +0.02) |
| Persistent smoking cessation | +0.11 | (−0.62, +0.85) |
| Persistent self-reported physical activity change | −0.05 | (−0.38, +0.28) |
| Persistent increase in strenuous activity | −0.16 | (−0.53, +0.21) |
| Change in work capacity (per W) | +0.31 | (+0.03, +0.59) |
| Change in leg strength (per SD) | +0.05 | (−0.10, +0.22) |
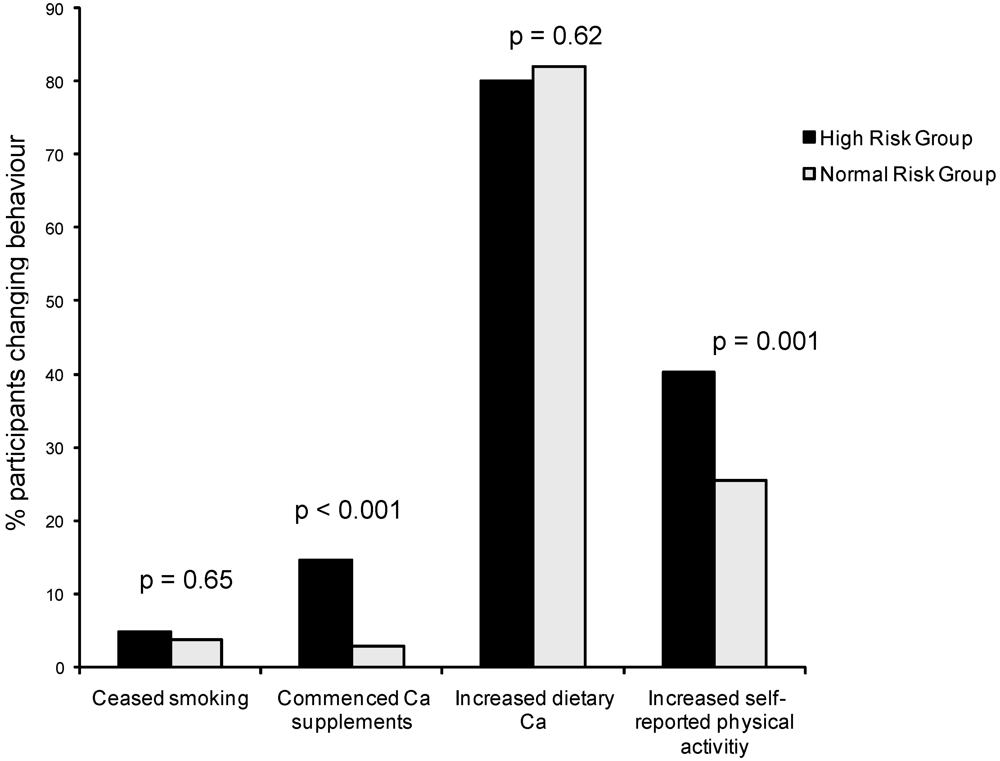
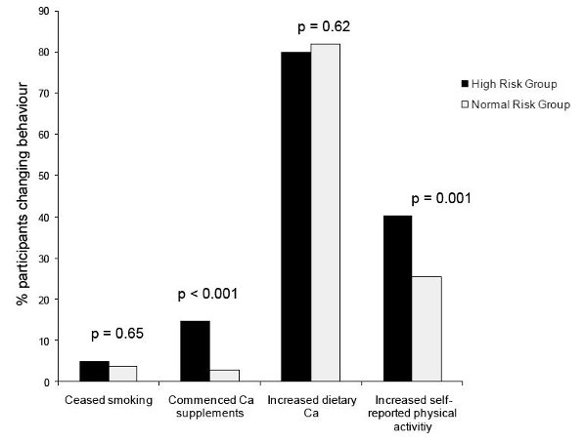
3.2. Results: Secondary Outcomes—Maternal Report of Changing Children’s Behaviour
| Univariable OR (95% CI) | Multivariable a OR (95% CI) | |
|---|---|---|
| Predictors of calcium intake increase | ||
| Youngest child <18 yrs old | 4.30 (1.20, 15.42) | 4.32 (1.11, 16.75) |
| T-score group | 2.16 (1.38, 3.41) | 1.97 (1.19, 3.27) |
| OPSMC | 1.88 (1.20, 2.95) | 2.28 (1.37, 3.80) |
| Mother commenced Ca supplements (2-years) | 3.38 (1.39, 8.26) | 2.55 (0.97, 6.72) b |
| Mother Increased physical activity | 2.70 (1.68, 4.36) | 2.21 (1.32, 3.72) |
| Predictors of physical activity increase | ||
| Youngest child <18 yrs old | 2.65 (0.72, 9.74) | 1.58 (0.38, 6.68) |
| T-score group | 0.94 (0.57, 1.55) | 0.87 (0.48, 1.57) |
| OPSMC | 1.07 (0.65, 1.78) | 1.12 (0.63, 2.01) |
| Mother commenced Ca supplements (2-years) | 1.03 (0.38, 2.75) | 0.79 (0.26, 2.86) |
| Mother Increased physical activity | 2.41 (1.42, 4.10) | 2.74 (1.51, 4.98) |
3.3. Discussion
4. Conclusions
Acknowledgements
References
- Britt, H.; Miller, G.; Charles, J.; Henderson, J.; Bayram, C.; Pan, Y.; Valenti, L.; Harrison, C.; Fahridin, S.; O’Halloran, J. General Practice Activity in Australia, 2008-09; General practice series no. 25. Cat. no. GEP 25; AIHW: Canberra, Australia, 2009. [Google Scholar]
- Access Economics, The Burden of Brittle Bones: Costing Osteoporosis in Australia; Osteoporosis Australia: Sydney, Australia, 2001.
- Pasco, J.A.; Sanders, K.M.; Hoekstra, F.M.; Henry, M.J.; Nicholson, G.C.; Kotowicz, M.A. The human cost of fracture. Osteoporos. Int. 2005, 16, 2046–2052. [Google Scholar]
- Marshall, D.; Johnell, O.; Wedel, H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996, 312, 1254–1259. [Google Scholar]
- Nguyen, T.; Sambrook, P.; Kelly, P.; Jones, G.; Lord, S.; Freund, J.; Eisman, J. Prediction of osteoporotic fractures by postural instability and bone density. BMJ 1993, 307, 1111–1115. [Google Scholar]
- Riis, B.J.; Hansen, M.A.; Jensen, A.M.; Overgaard, K.; Christiansen, C. Low bone mass and fast rate of bone loss at menopause: equal risk factors for future fracture: a 15-year follow-up study. Bone 1996, 19, 9–12. [Google Scholar]
- Recker, R.R.; Davies, K.M.; Hinders, S.M.; Heaney, R.P.; Stegman, M.R.; Kimmel, D.B. Bone gain in young adult women. JAMA 1992, 268, 2403–2408. [Google Scholar]
- Recker, R.; Lappe, J.; Davies, K.; Heaney, R. Characterization of perimenopausal bone loss: a prospective study. J. Bone Miner. Res. 2000, 15, 1965–1973. [Google Scholar]
- Welten, D.C.; Kemper, H.C.; Post, G.B.; van Staveren, W.A. A meta-analysis of the effect of calcium intake on bone mass in young and middle aged females and males. J. Nutr. 1995, 125, 2802–2813. [Google Scholar]
- Wolff, I.; van Croonenborg, J.J.; Kemper, H.C.; Kostense, P.J.; Twisk, J.W. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos. Int. 1999, 9, 1–12. [Google Scholar]
- Edwards, A.; Hood, K.; Matthews, E.; Russell, D.; Russell, I.; Barker, J.; Bloor, M.; Burnard, P.; Covey, J.; Pill, R.; Wilkinson, C.; Stott, N. The effectiveness of one-to-one risk communication interventions in health care: a systematic review. Med. Decis. Making 2000, 20, 290–297. [Google Scholar]
- Torley, D.; Zwar, N.; Comino, E.J.; Harris, M. GPs’ views of absolute cardiovascular risk and its role in primary prevention. Aust. Fam. Physician 2005, 34, 503-504, 507. [Google Scholar]
- Group, R.O.W. Clinical Guideline for the Prevention and Treatment of Osteoporosis in Postmenopausal Women and Older Men; Royal Australian College of General Practitioners: Melbourne, Australia, 2010. [Google Scholar]
- Ewald, D.P.; Eisman, J.A.; Ewald, B.D.; Winzenberg, T.M.; Seibel, M.J.; Ebeling, P.R.; Flicker, L.A.; Nash, P.T. Population rates of bone densitometry use in Australia, 2001-2005, by sex and rural versus urban location. Med. J. Aust. 2009, 190, 126–128. [Google Scholar] [PubMed]
- Cook, B.; Noteloviz, M.; Rector, C.; Krischer, J. An Osteoporosis Patient Education and Screening Program:Results and Implications. Patient Educ. Couns. 1991, 17, 135–145. [Google Scholar]
- Jamal, S.A.; Ridout, R.; Chase, C.; Fielding, L.; Rubin, L.A.; Hawker, G.A. Bone mineral density testing and osteoporosis education improve lifestyle behaviors in premenopausal women: a prospective study. J. Bone Miner. Res. 1999, 14, 2143–2149. [Google Scholar]
- Jones, G.; Scott, F. Low bone mass in premenopausal parous women: identification and the effect of an information and bone density feedback program. J. Clin. Densitom. 1999, 2, 109–115. [Google Scholar]
- Winzenberg, T.M.; Oldenburg, B.; Frendin, S.; De Wit, L.; Jones, G. Effects of bone density feedback and group education on osteoporosis knowledge and osteoporosis self-efficacy in premenopausal women: a randomized controlled trial. J. Clin. Densitom. 2005, 8, 95–103. [Google Scholar]
- Winzenberg, T.; Oldenburg, B.; Frendin, S.; De Wit, L.; Riley, M.; Jones, G. The effect on behavior and bone mineral density of individualized bone mineral density feedback and educational interventions in premenopausal women: a randomized controlled trial [NCT00273260]. BMC Public Health 2006, 6, 12. [Google Scholar] [Green Version]
- Angus, R.M.; Sambrook, P.N.; Pocock, N.A.; Eisman, J.A. A simple method for assessing calcium intake in Caucasian women. J. Am. Diet. Assoc. 1989, 89, 209–214. [Google Scholar]
- Aaron, D.J.; Kriska, A.M.; Dearwater, S.R.; Cauley, J.A.; Metz, K.F.; LaPorte, R.E. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am. J. Epidemiol. 1995, 142, 191–201. [Google Scholar]
- Jones, G.; Scott, F.S. A cross-sectional study of smoking and bone mineral density in premenopausal parous women: effect of body mass index, breastfeeding, and sports participation. J. Bone Miner. Res. 1999, 14, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Withers, R.T.; Davies, G.J.; Crouch, R.G. A comparison of 3 W170 protocols. Eur. J. Appl. Physiol. 1977, 37, 123–128. [Google Scholar]
- Streiner, D.L. The case of the missing data: methods of dealing with dropouts and other research vagaries. Can. J. Psychiatry 2002, 47, 68–75. [Google Scholar]
- Winzenberg, T.M.; Oldenburg, B.; Frendin, S.; De Wit, L.; Jones, G. A mother-based intervention trial for osteoporosis prevention in children. Prev. Med. 2006, 42, 21–26. [Google Scholar]
- Cranney, A.; Wells, G.; Willan, A.; Griffith, L.; Zytaruk, N.; Robinson, V.; Black, D.; Adachi, J.; Shea, B.; Tugwell, P.; Guyatt, G. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr. Rev. 2002, 23, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Paganini-Hill, A.; Chao, A.; Ross, R.K.; Henderson, B.E. Exercise and other factors in the prevention of hip fracture: the Leisure World study. Epidemiology 1991, 2, 16–25. [Google Scholar]
- Heinonen, A.; Kannus, P.; Sievanen, H.; Oja, P.; Pasanen, M.; Rinne, M.; Uusi-Rasi, K.; Vuori, I. Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet 1996, 348, 1343–1347. [Google Scholar]
- McDermott, M.T.; Christensen, R.S.; Lattimer, J. The effects of region-specific resistance and aerobic exercises on bone mineral density in premenopausal women. Mil. Med. 2001, 166, 318–321. [Google Scholar]
- Friedlander, A.L.; Genant, H.K.; Sadowsky, S.; Byl, N.N.; Gluer, C.C. A two-year program of aerobics and weight training enhances bone mineral density of young women. J. Bone Miner. Res. 1995, 10, 574–585. [Google Scholar]
- Winzenberg, T.; Hansen, E.; Jones, G. How do women change osteoporosis-preventive behaviours in their children? Eur. J. Clin. Nutr. 2008, 62, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Blalock, S.J.; Currey, S.S.; DeVellis, R.F.; DeVellis, B.M.; Giorgino, K.B.; Anderson, J.J.; Dooley, M.A.; Gold, D.T. Effects of educational materials concerning osteoporosis on women’s knowledge, beliefs, and behavior. Am. J. Health Promot. 2000, 14, 161–169. [Google Scholar] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Winzenberg, T.; Oldenburg, B.; Jones, G. Bone Density Testing: An Under-Utilised and Under-Researched Health Education Tool for Osteoporosis Prevention? Nutrients 2010, 2, 985-996. https://doi.org/10.3390/nu2090985
Winzenberg T, Oldenburg B, Jones G. Bone Density Testing: An Under-Utilised and Under-Researched Health Education Tool for Osteoporosis Prevention? Nutrients. 2010; 2(9):985-996. https://doi.org/10.3390/nu2090985
Chicago/Turabian StyleWinzenberg, Tania, Brian Oldenburg, and Graeme Jones. 2010. "Bone Density Testing: An Under-Utilised and Under-Researched Health Education Tool for Osteoporosis Prevention?" Nutrients 2, no. 9: 985-996. https://doi.org/10.3390/nu2090985