Effects of Bifidobacterium longum and Lactobacillus rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-Over Study
Abstract
:1. Introduction
2. Materials and Methods
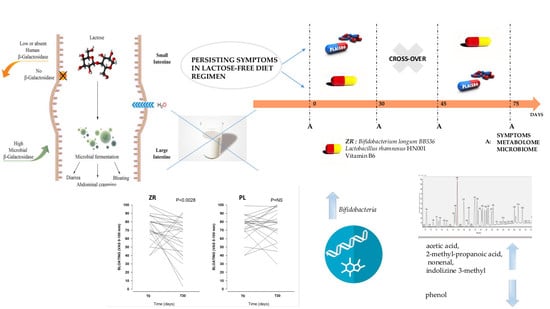
2.1. Patients and Study Design
2.2. Lactose Breath Test
2.3. Randomization and Masking
2.4. Double Blind Cross Over Challenge
2.5. Questionnaires
2.6. Outcomes
2.7. Fecal Collection
2.8. RNA Extraction and Fecal Microbiome
2.9. Fecal Metabolome
2.10. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.1.1. Symptoms
3.1.2. Diet
3.2. Clinical Scores during the Double Blind, Cross-Over Challenge
3.3. Probiotics and Vitamin B6 Affect the Fecal Microbiome of LI Patients
3.4. Probiotics and Vitamin B6 Affect the Fecal Metabolome of LI Patients
3.5. Correlations between Microbiome and Metabolome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drossman, D.A. Functional Gastrointestinal Disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut. Microb. 2014, 5, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Bonfrate, L.; Tack, J.; Grattagliano, I.; Cuomo, R.; Portincasa, P. Microbiota in health and irritable bowel syndrome: Current knowledge, perspectives and therapeutic options. Scand. J. Gastroenterol. 2013, 48, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Moschetta, A.; Baldassarre, G.; Altomare, D.F.; Palasciano, G. Pan-enteric dysmotility, impaired quality of life and alexithymia in a large group of patients meeting ROME II criteria for irritable bowel syndrome. World J. Gastroenterol. 2003, 9, 2293–2299. [Google Scholar] [CrossRef]
- Liebert, A.; Lopez, S.; Jones, B.L.; Montalva, N.; Gerbault, P.; Lau, W.; Thomas, M.G.; Bradman, N.; Maniatis, N.; Swallow, D.M. World-wide distributions of lactase persistence alleles and the complex effects of recombination and selection. Hum. Genet. 2017, 136, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Hill, R.J. The role of food intolerance in functional gastrointestinal disorders in children. Aust. Fam. Phys. 2014, 43, 686–689. [Google Scholar]
- Portincasa, P.; Di Ciaula, A.; Vacca, M.; Montelli, R.; Wang, D.Q.; Palasciano, G. Beneficial effects of oral tilactase on patients with hypolactasia. Eur. J. Clin. Investig. 2008, 38, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Bonfrate, L.; Krawczyk, M.; Lembo, A.; Grattagliano, I.; Lammert, F.; Portincasa, P. Effects of dietary education, followed by a tailored fructose-restricted diet in adults with fructose malabsorption. Eur. J. Gastroenterol. Hepatol. 2015, 27, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Deng, Y.; Chu, H.; Cong, Y.; Zhao, J.; Pohl, D.; Misselwitz, B.; Fried, M.; Dai, N.; Fox, M. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.I. Perceived lactose intolerance in adult Canadians: A national survey. Appl. Physiol. Nutr. Metab. 2013, 38, 830–835. [Google Scholar] [CrossRef]
- Farup, P.G.; Monsbakken, K.W.; Vandvik, P.O. Lactose malabsorption in a population with irritable bowel syndrome: Prevalence and symptoms. A case-control study. Scand. J. Gastroenterol. 2004, 39, 645–649. [Google Scholar] [CrossRef]
- The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2018. [CrossRef]
- Tomasik, P.J.; Tomasik, P. Probiotics and prebiotics. Cereal Chem. 2003, 80, 113–117. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.; Naghibi, M.M.; Garcha, D. The role of bacteria, probiotics and diet in Irritable Bowel Syndrome. Foods 2018, 7, 13. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Honda, H.; Kataoka, F.; Nagaoka, S.; Kawai, Y.; Kitazawa, H.; Itoh, H.; Kimura, K.; Taketomo, N.; Yamazaki, Y.; Tateno, Y.; Saito, T. Beta-galactosidase, phospho-beta-galactosidase and phospho-beta-glucosidase activities in lactobacilli strains isolated from human faeces. Lett. Appl. Microbiol. 2007, 45, 461–466. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Hurst, N.R.; Kendig, D.M.; Murthy, K.S.; Grider, J.R. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol. Motil. 2014, 26, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Ligaarden, S.C.; Farup, P.G. Low intake of vitamin B6 is associated with irritable bowel syndrome symptoms. Nutr. Res. 2011, 31, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, H.; Mooney, S. Vitamin B6: A molecule for human health? Molecules 2010, 15, 442–459. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Corazza, G.R.; Gasbarrini, G.; Montalto, M.; Di Stefano, M.; Basilisco, G.; Parodi, A.; Usai-Satta, P.; Vernia, P.; Anania, C.; et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009, 29, 1–49. [Google Scholar] [PubMed]
- Di Stefano, M.; Missanelli, A.; Miceli, E.; Strocchi, A.; Corazza, G.R. Hydrogen breath test in the diagnosis of lactose malabsorption: Accuracy of new versus conventional criteria. J. Lab. Clin. Med. 2004, 144, 313–318. [Google Scholar] [CrossRef]
- Thompson, D.G.; O’Brien, J.D.; Hardie, J.M. Influence of the oropharyngeal microflora on the measurement of exhaled breath hydrogen. Gastroenterology 1986, 91, 853–860. [Google Scholar] [CrossRef]
- Payne, D.L.; Welsh, J.D.; Claypool, P.L. Breath hydrogen (H2) response to carbohydrate malabsorption after exercise. J. Lab. Clin. Med. 1983, 102, 147–150. [Google Scholar]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Bonfrate, L.; Scaccianoce, G.; Palasciano, G.; Ben-Chetrit, E.; Portincasa, P. A novel cluster of patients with Familial Mediterranean Fever (FMF) in southern Italy (J COVER). Eur. J. Clin. Investig. 2017, 47, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Chao, A.; Bunge, J. Estimating the number of species in a stochastic abundance model. Biometrics 2002, 58, 531–539. [Google Scholar] [CrossRef]
- Tallarida, R.J.; Murray, R.B. Manual of Pharmacologic Calculations with Computer Programs; Springer: New York, NY, USA, 1987. [Google Scholar]
- Arnold, J.; Simpson, J.; Roach, J.; Bruno-Barcena, J.; Azcarate-Peril, M. Prebiotics for Lactose Intolerance: Variability in galacto-oligosaccharide utilization by intestinal Lactobacillus rhamnosus. Nutrients 2018, 10, 1517. [Google Scholar] [CrossRef]
- Szilagyi, A.; Ishayek, N. Lactose Intolerance, dairy avoidance, and treatment options. Nutrients 2018, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- Nolan-Clark, D.; Tapsell, L.C.; Hu, R.; Han, D.Y.; Ferguson, L.R. Effects of dairy products on Crohn’s Disease Symptoms are influenced by fat content and disease location but not lactose content or disease activity status in a New Zealand population. J. Am. Diet. Assoc. 2011, 111, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Mishkin, S. Dairy sensitivity, lactose malabsorption, and elimination diets in inflammatory bowel disease. Am. J. Clin. Nutr. 1997, 65, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Woodford, K.; Kukuljan, S.; Ho, S. Milk Intolerance, beta-casein and lactose. Nutrients 2015, 7, 7285–7297. [Google Scholar] [CrossRef]
- Jianqin, S.; Leiming, X.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr. J. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Gueimonde, M.; Sanz, Y.; Salminen, S. Adhesion properties and competitive pathogen exclusion ability of bifidobacteria with acquired acid resistance. J. Food Prot. 2006, 69, 1675–1679. [Google Scholar] [CrossRef]
- Agustina, R.; Lukito, W.; Firmansyah, A.; Suhardjo, H.N.; Murniati, D.; Bindels, J. The effect of early nutritional supplementation with a mixture of probiotic, prebiotic, fiber and micronutrients in infants with acute diarrhea in Indonesia. Asia Pac. J. Clin. Nutr. 2007, 16, 435–442. [Google Scholar]
- Zhu, Y.; Zheng, X.; Cong, Y.; Chu, H.; Fried, M.; Dai, N.; Fox, M. Bloating and distention in irritable bowel syndrome: The role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am. J. Gastroenterol. 2013, 108, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, G.; Orlando, A.; D’Attoma, B.; Linsalata, M.; Martulli, M.; Russo, F. Randomised double blind placebo controlled trial on Lactobacillus reuteri DSM 17938: Improvement in symptoms and bowel habit in functional constipation. Benef. Microbes 2018, 9, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Yuce, O.; Kalayci, A.G.; Comba, A.; Eren, E.; Caltepe, G. Lactose and Fructose Intolerance in Turkish children with Chronic Abdominal Pain. Ind. Pediatr. 2016, 53, 394–397. [Google Scholar] [CrossRef]
- Use of a Novel Probiotic Formulation to Alleviate Lactose Intolerance Symptoms—A Pilot Study. Probiotics Antimicrob. Proteins 2019. [CrossRef]
- Rosenberg, J.; Ischebeck, T.; Commichau, F.M. Vitamin B6 metabolism in microbes and approaches for fermentative production. Biotechnol. Adv. 2017, 35, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Arola, H.; Tamm, A. Metabolism of lactose in the human body. Scand. J. Gastroenterol. Suppl. 1994, 202, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Azcarate-Peril, M.A.; Ritter, A.J.; Savaiano, D.; Monteagudo-Mera, A.; Anderson, C.; Magness, S.T.; Klaenhammer, T.R. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc. Natl. Acad. Sci. USA 2017, 114, E367–E375. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Vannini, L.; Di Cagno, R.; Cavallo, N.; Minervini, F.; Francavilla, R.; Ercolini, D.; Gobbetti, M. Salivary and fecal microbiota and metabolome of celiac children under gluten-free diet. Int. J. Food Microbiol. 2016, 239, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Setoyama, H.; Imaoka, A.; Ishikawa, H.; Umesaki, Y. Prevention of gut inflammation by Bifidobacterium in dextran sulfate-treated gnotobiotic mice associated with Bacteroides strains isolated from ulcerative colitis patients. Microbes Infect. 2003, 5, 115–122. [Google Scholar] [CrossRef]
- Pakdaman, M.N.; Udani, J.K.; Molina, J.P.; Shahani, M. Erratum to: The effects of the DDS-1 strain of Lactobacillus on symptomatic relief for lactose intolerance—A randomized, double-blind, placebo-controlled, crossover clinical trial. Nutr. J. 2016, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Vipperla, K.; O’Keefe, S.J. The microbiota and its metabolites in colonic mucosal health and cancer risk. Nutr. Clin. Pract. 2012, 27, 624–635. [Google Scholar] [CrossRef]
- Thorburn, A.W.; Storlien, L.H.; Jenkins, A.B.; Khouri, S.; Kraegen, E.W. Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am. J. Clin. Nutr. 1989, 49, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Lembo, A.; De Bari, O.; Di Palo, D.M.; Maggio, A.; Cataldo, I.; Calamita, G. The role of dietary approach in irritable bowel syndrome. Curr. Med. Chem. 2017, 24, 1–8. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; de Bari, O.; Lembo, A.; Ballou, S. Irritable bowel syndrome and diet. Gastroenterol. Rep. 2017, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Sharma, R. Fermentation potential of the gut microbiome: Implications for energy homeostasis and weight management. Nutr. Rev. 2011, 69, 99–106. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut microbiota profiling: Metabolomics based approach to unravel compounds affecting human health. Front. Microbiol. 2016, 7, 1144. [Google Scholar] [CrossRef]
- Moens, F.; Van den Abbeele, P.; Basit, A.W.; Dodoo, C.; Chatterjee, R.; Smith, B.; Gaisford, S. A four-strain probiotic exerts positive immunomodulatory effects by enhancing colonic butyrate production in vitro. Int. J. Pharm. 2019, 555, 1–10. [Google Scholar] [CrossRef]






| Age, years | 48 ± 3.1 (48) |
| Females, n. (%) | 19 (83%) |
| BMI (Kg/m2) | 23.2 ± 0.68 (22.3) |
| Symptoms | |
| Bloating (VAS, mm) | 69 ± 5.4 (80) |
| Abdominal pain (VAS, mm) | 61 ± 4.6 (62) |
| Bowel habits | |
| Bristol Score (range 1–7) | 3 ± 0.38 (2) |
| Compounds | ZR | PL | p-value |
|---|---|---|---|
| 1-Butanol | 0 (0, 0) | 11.22 (0, 12.58) | 0.037 |
| 5-Hepten-2-ol, 6-methyl- | 51.74 (5.98, 72.3) | 30.81 (0.79, 39.28) | 0.029 |
| Phenol | 4.12 (0, 8.56) | 19.78 (3.48, 28.56) | 0.048 |
| 1-Hexadecanol | 4.03 (0, 6.29) | 8.17 (0, 13.94) | 0.043 |
| Acetic Acid | 44.53 (26.62, 63.82) | 23.34 (7.72, 37.47) | 0.042 |
| Propanoic Acid, 2-methyl- | 29.78 (13.4, 45.04) | 11.26 (0.71, 17.35) | 0.009 |
| Hexanal | 5.81 (0, 13.22) | 11.3 (2, 19.38) | 0.009 |
| 2-Nonenal | 5.38 (2.24, 8.9) | 3.35 (0, 4.34) | 0.045 |
| Indolizine, 3-methyl- | 248.96 (87.94, 372.62) | 99.53 (31, 93.91) | 0.045 |
| 5-Hepten-2-One, 6-methyl- | 310.76 (55.31, 396.75) | 124.03 (19.91, 210.84) | 0.048 |
| D-Limonene | 87.43 (11.19, 187.9) | 483.68 (55.46, 804.44) | 0.042 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitellio, P.; Celano, G.; Bonfrate, L.; Gobbetti, M.; Portincasa, P.; De Angelis, M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-Over Study. Nutrients 2019, 11, 886. https://doi.org/10.3390/nu11040886
Vitellio P, Celano G, Bonfrate L, Gobbetti M, Portincasa P, De Angelis M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-Over Study. Nutrients. 2019; 11(4):886. https://doi.org/10.3390/nu11040886
Chicago/Turabian StyleVitellio, Paola, Giuseppe Celano, Leonilde Bonfrate, Marco Gobbetti, Piero Portincasa, and Maria De Angelis. 2019. "Effects of Bifidobacterium longum and Lactobacillus rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-Over Study" Nutrients 11, no. 4: 886. https://doi.org/10.3390/nu11040886