Exclusive Enteral Nutrition: Clinical Effects and Changes in Mucosal Cytokine Profile in Pediatric New Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Examinations and Tests
2.2. Clinical Assessment of Disease Activity and Disease Extension
2.3. Endoscopic Scoring
2.4. Histopathology Scoring
2.5. Exclusive Enteral Nutrition (EEN)
2.6. Cytokine-Selection
2.7. RNA Extraction and Gene Expression by Quantitative Real-Time PCR
2.8. Ethical Approval
2.9. Statistics
3. Results
3.1. EEN Treatment
3.2. Clinical Activity Scoring
3.3. Laboratory Values
3.4. Anthropometric Data
3.5. Endoscopic Healing
3.6. Clinical Outcome of the Two UC Patients
3.7. Mucosal Healing
3.8. Adverse Events
3.9. Mucosal Cytokine Profile
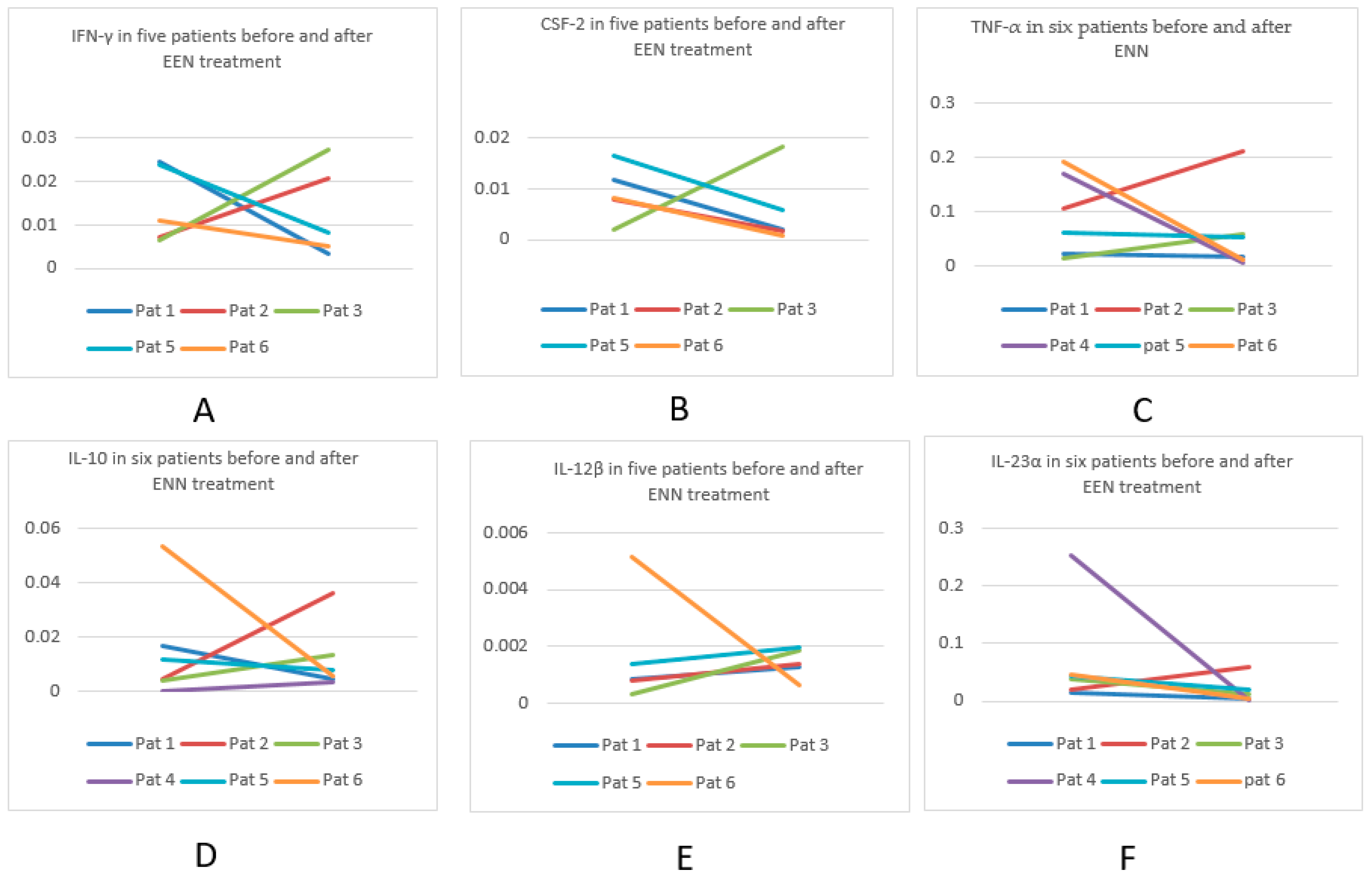
3.9.1. Cytokine Profiles in Six Patients Pre- and Post EEN
3.9.2. Cytokine Comparison between IBD Patients and Non-IBD Controls
4. Discussion
MCP
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Segm. 1 | Segm. 2 | Segm. 3 | Segm. 4 | Segm. 5 | Segm. 6 | Segm. 7 | Segm. 8 | Segm. 9 | Segm. 10 | Segm. 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Endoscopy1|Endoscopy2 | |||||||||||
| Pat 1 CD | mult.g|g | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 |
| Pat 2 UC | <3| 3:3 | 5|3:7 | 3:7|3:7 | 3:3|3:3 | 3:3|3:7 | 5|3:7 | 5|3:3 | 4|3:7 | 3:7|3:7 | <3|3:7 | |
| Pat 3 CD | <3|<3 | <3+g|<3 | 5+b|<3+e | <3|mult.g | <3|<3+e | <3|<3+e | <3|<3 | <3|<3+g | <3|<3 | <3|<3 | <3|<3 |
| Pat 4 CD | 3:3+b+g|3:1 | 3:3+b+g|3:1 | 3:3+b+g|3:1 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | 3:3|<3 | <3|<3 | <3|<3 | <3|<3 |
| Pat 5 CD | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 |
| Pat 6 CD | 3:3+e|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | 4+g|<3 |
| Pat 7 UC | <3|<3 | 3:7|3:3 | 3:7+e|3:3 | 3:7|3:3 | 3:7|3:3 | 3:3|3:3 | 3:7|3:7 | 3:7|3:3 | 4+e|3:7 | 3:7+c|3:3 | 3:7|3:3 |
| Pat 8 CD | infl.polyp|3:7 | <3|<3+g | <3+g|<3 | <3+e|3:3 | <3+g|3:3 | 3:2|<3 | 3:2+g|3:1 | <3|<3 | <3+g|<3 | <3+g|<3 | <3+g|<3 |
| Pat 9 I-BDU | <3|<3 | <3|<3 | <3|<3 | 3:2|<3 | <3|<3 | <3|<3 | 3:1|<3 | 3:1|<3 | 3:1|<3 | <3|<3 | <3|<3 |
| Pat 10 CD | <3+g|<3+g | <3+g|<3+g | <3|<3+g | <3|<3 | <3+g|<3 | <3|<3 | <3|<3+g | <3+g|<3 | <3|<3 | <3+g|<3 | <3+g|<3 |
| Pat 11 | <3|<3 | <3|<3 | 3:4|<3 | 3:7+e|<3 | 3:7+e|<3 | 4+e|<3 | 4|<3:4 | 4|<3 | 4|3:4 | 3:7+e|<3 | 4|<3 |
| Pat 12 | 5+e|3:2 | 3:3|3:2 | 3:3|3:2 | <3|<3 | <3|3:7 | <3|3:7 | <3|<3 | <3|<3 | <3|<3 | <3|<3 | 3:7|<3 |
| Patient | Improvement | No Change | Aggravated Inflammation | No Inflammation |
|---|---|---|---|---|
| 1 | 1 | 10 | ||
| 2 | 4 | 4 | 3 | |
| 3 | 2 | 4 | 5 | |
| 4 | 4 | 7 | ||
| 5 | 11 | |||
| 6 | 2 | 1 | 8 | |
| 7 | 8 | 3 | ||
| 8 | 7 | 2 | 1 | 1 |
| 9 | 4 | 7 | ||
| 10 | 4 | 5 | 2 | |
| 11 | 9 | 2 | ||
| 12 | 4 | 2 | 5 |
| Patient | CSF-2 RQ pre-post EEN | IFN-γ RQ pre-post EEN | TNF-α RQ pre-post EEN | IL1-β:1 RQ pre-post EEN | IL-4:1 RQ pre-post EEN | IL-6:1 RQ pre-post EEN |
| 1 | 0.011651–0.001997 | 0.024588–0.003389 | 0.024577–0.016915 | 0.573893–0.020431 | 0.000275–4.72 × 10–5 | 0.001001–0.001825 |
| 2 | 0.007851–0.001629 | 0.007228–0.020651 | 0.106451–0.21017 | 0.051899–0.924653 | 0.001325–######## | 0.020791–0.172768 |
| 3 | 0.001862–0.018126 | 0.00664–0.02729 | 0.014612–0.059869 | 0.218129–0.408354 | 0.000282–8.68 × 10–5 | 0.008237–0.029142 |
| 4 | ########–######## | ########–0.00048 | 0.168562–0.007131 | 1.021516–0.010524 | ########–######## | ########–0.003238 |
| 5 | 0.016589–0.005784 | 0.023729–0.008329 | 0.06296–0.054617 | 2.175898–0.061581 | 0.000737–0.000148 | 0.042764–0.022326 |
| 6 | 0.008264–0.000895 | 0.010918–0.00516 | 0.190799–0.012544 | 3.225579–0.108797 | ########–######## | 0.32355–0.00282 |
| Mean for group pre-post EEN | 0.0092434–0.005686 | 0.014621–0.010883 | 0.09466–0.060208 | 1.211152–0.255723 | 0.0006548–9.39959 × 10−5 | 0.158537–0.038687 |
| SD for group pre-post EEN | 0.0048404–0.006446 | 0.007929–0.009732 | 0.09466–0.060208 | 1.137297–0.328006 | 0.0004296–4.14339 × 10−5 | 0.122961–0.06088 |
| Patient | IL–10:1 RQ pre–post EEN | IL–12β:1 RQ pre–post EEN | IL–22:1 RQ pre–post EEN | IL–23α:1 RQ pre–post EEN | IL–36γ:1 RQ pre–post EEN | TGFβ1:1 RQ pre–post EEN |
| 1 | 0.016987–0.004407 | 0.000851–0.001291 | #######–0.002077 | 0.015346–0.005568 | 0.000435–0.000911 | 0.653298–0.657096 |
| 2 | 0.004295–0.03603 | 0.000767–0.001354 | ########–0.016029 | 0.020307–0.060188 | 0.002836–0.002475 | 1.013262–4.188852 |
| 3 | 0.003799–0.013653 | 0.000296–0.001842 | 0.000458–0.004207 | 0.037239–0.012223 | 0.002853–0.002125 | 0.425452–0.726292 |
| 4 | ########–0.003523 | ########–######## | ########–####### | 0.254504–0.003021 | ########–######## | 1.497149–0.271375 |
| 5 | 0.01185–0.007582 | 0.001391–0.001984 | 0.014448–0.008729 | 0.044249–0.019889 | 0.00407–0.000911 | 0.988215–1.261974 |
| 6 | 0.053329–0.005778 | 0.005143–0.000639 | 0.008147–8.46 × 10−5 | 0.046621–0.004837 | 0.001272–0.000563 | 0.83872–0.52586 |
| Mean for group pre-post EEN | 0.018052–0.011829 | 0.00169–0.001422 | 0.007684–0.006225 | 0.069711–0.017621 | 0.002293–0.001397 | 0.902683–1.271908 |
| SD for group pre-post EEN | 0.018311–0.011312 | 0.001761–0.000475 | 0.005721–0.005681 | 0.083447–0.019867 | 0.001285–0.000756 | 0.333408–1.338034 |
References
- Van Limbergen, J.; Russell, R.K.; Drummond, H.E.; Aldhous, M.C.; Round, N.K.; Nimmo, E.R.; Smith, L.; Gillett, P.M.; McGrogan, P.; Weaver, L.T.; et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008, 135, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohns Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef] [PubMed]
- Zachos, M.; Tondeur, M.; Griffiths, A.M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2008, 4, Cd000542. [Google Scholar]
- Dziechciarz, P.; Horvath, A.; Shamir, R.; Szajewska, H. Meta-analysis: Enteral nutrition in active Crohn’s disease in children. Aliment. Pharmacol. Ther. 2007, 26, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Whitten, K.E.; Lemberg, D.A.; Clarkson, C.; Vitug-Sales, M.; Jackson, R.; Bohane, T.D. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: A feasible and effective approach. J. Gastroenterol. Hepatol. 2006, 21, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Cuiv, P.O.; Begun, J.; Keely, S.; Lewindon, P.J.; Morrison, M. Towards an integrated understanding of the therapeutic utility of exclusive enteral nutrition in the treatment of Crohn’s disease. Food Funct. 2016, 7, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Kunze, W.A.; Clerc, N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: Neural, endocrine and immune responses. Am. J. Physiol. 1999, 277 Pt 1, G922–G928. [Google Scholar] [PubMed]
- Korzenik, J.R.; Dieckgraefe, B.K. Is Crohn’s disease an immunodeficiency? A hypothesis suggesting possible early events in the pathogenesis of Crohn’s disease. Dig. Dis. Sci. 2000, 45, 1121–1129. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Fell, J.M.; Paintin, M.; Arnaud-Battandier, F.; Beattie, R.M.; Hollis, A.; Kitching, P.; Donnet-Hughes, A.; MacDonald, T.T.; Walker-Smith, J.A. Mucosal healing and a fall in mucosal proinflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2000, 14, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakahigashi, M.; Umegae, S.; Kitagawa, T.; Matsumoto, K. Impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: Cytokine production and endoscopic and histological findings. Inflamm. Bowel Dis. 2005, 11, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Schwerd, T.; Frivolt, K.; Clavel, T.; Lagkouvardos, I.; Katona, G.; Mayr, D.; Uhlig, H.H.; Haller, D.; Koletzko, S.; Bufler, P. Exclusive enteral nutrition in active pediatric Crohn disease: Effects on intestinal microbiota and immune regulation. J. Allergy Clin. Immunol. 2016, 138, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Griffiths, A.M.; Walters, T.D.; Seah, T.; Markowitz, J.; Pfefferkorn, M.; Keljo, D.; Waxman, J.; Otley, A.; LeLeiko, N.S.; et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm. Bowel Dis. 2012, 18, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Geboes, K.; Riddell, R.; Ost, A.; Jensfelt, B.; Persson, T.; Lofberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Day, A.S.; Lopez, R.N. Exclusive enteral nutrition in children with Crohn’s disease. World J. Gastroenterol. 2015, 21, 6809–6816. [Google Scholar] [CrossRef]
- Bannerjee, K.; Camacho-Hubner, C.; Babinska, K.; Dryhurst, K.M.; Edwards, R.; Savage, M.O.; Sanderson, I.R.; Croft, N.M. Antiinflammatory and growth-stimulating effects precede nutritional restitution during enteral feeding in Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 270–275. [Google Scholar] [CrossRef]
- Gonzalez-Huix, F.; Fernandez-Banares, F.; Esteve-Comas, M.; Abad-Lacruz, A.; Cabre, E.; Acero, D.; Sanderson, I.R.; Croft, N.M. Enteral versus parenteral nutrition as adjunct therapy in acute ulcerative colitis. Am. J. Gastroenterol. 1993, 88, 227–232. [Google Scholar] [PubMed]
- Shaoul, R.; Brown, S.; Day, A.S. Reasoning Beyond the Potential Use of Exclusive Enteral Nutrition and Other Specified Diets in Children with Ulcerative Colitis. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 378–382. [Google Scholar] [CrossRef]
- Guan, Q.; Zhang, J. Recent Advances: The Imbalance of Cytokines in the Pathogenesis of Inflammatory Bowel Disease. Mediators Inflamm. 2017, 2017, 4810258. [Google Scholar] [CrossRef] [PubMed]
- Dock, J.; Ramirez, C.M.; Hultin, L.; Hausner, M.A.; Hultin, P.; Elliott, J.; Yang, O.O.; Anton, P.A.; Jamieson, B.D.; Effros, R.B. Distinct aging profiles of CD8+ T cells in blood versus gastrointestinal mucosal compartments. PLoS ONE 2017, 12, e0182498. [Google Scholar] [CrossRef]
- Roggenbuck, D.; Reinhold, D.; Baumgart, D.C.; Schierack, P.; Conrad, K.; Laass, M.W. Autoimmunity in Crohn’s Disease-A Putative Stratification Factor of the Clinical Phenotype. Adv. Clin. Chem. 2016, 77, 77–101. [Google Scholar] [PubMed]
- Cleynen, I.; Boucher, G.; Jostins, L.; Schumm, L.P.; Zeissig, S.; Ahmad, T.; Andersen, V.; Andrews, J.M.; Annese, V.; Brand, S.; et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: A genetic association study. Lancet 2016, 387, 156–167. [Google Scholar] [CrossRef]
- Ek, W.E.; D’Amato, M.; Halfvarson, J. The history of genetics in inflammatory bowel disease. Ann. Gastroenterol. 2014, 27, 294–303. [Google Scholar] [PubMed]
- Duerr, R.H.; Taylor, K.D.; Brant, S.R.; Rioux, J.D.; Silverberg, M.S.; Daly, M.J.; Steinhart, A.H.; Abraham, C.; Regueiro, M.; Griffiths, A.; et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006, 314, 1461–1463. [Google Scholar] [CrossRef]
- Waschke, K.A.; Villani, A.C.; Vermeire, S.; Dufresne, L.; Chen, T.C.; Bitton, A.; Cohen, A.; Thomson, A.B.; Wild, G.E. Tumor necrosis factor receptor gene polymorphisms in Crohn’s disease: Association with clinical phenotypes. Am. J. Gastroenterol. 2005, 100, 1126–1133. [Google Scholar] [CrossRef]
- Bamias, G.; Cominelli, F. Cytokines and intestinal inflammation. Curr. Opin. Gastroenterol. 2016, 32, 437–442. [Google Scholar] [CrossRef]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Antiinflammatory and proinflammatory roles of TGF-beta, IL-10 and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Egea, L.; Hirata, Y.; Kagnoff, M.F. GM-CSF: A role in immune and inflammatory reactions in the intestine. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Dabritz, J. Granulocyte macrophage colony-stimulating factor and the intestinal innate immune cell homeostasis in Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G455–G465. [Google Scholar] [CrossRef] [PubMed]
| Sex (Girls/Boys) | 6/7 |
|---|---|
| Age in years, median (IQR) range | 12.5 (10.5–14.5) 7.8–16.4 |
| Symptom duration in months, median (IQR) range | 6 (3.1–11.1) 1.5–12 |
| Paris classification | No (=13) |
| A1aL1B1G1 | 1 |
| A1bL1B1G0 | 4 |
| A1bL2B1G0 | 4 |
| A1bL2L4bB3G1 | 1 |
| A1bL3L4aB1G0 | 1 |
| A1bL2L4aB1G0 | 1 |
| A1bL3L4aB1G0p | 1 |
| Value | At Inclusion Median (IQR) Range | After EEN Treatment Median (IQR) Range |
|---|---|---|
| Weight (kg) | 37.9 (31.5–52.6) 27.5–59.7 | 40.5 (32.8–55.4) 29.8–59.1 |
| ESR (mm/h) | 28 (17.3–27.5) 8–53 | 9 (8–11) 2–21 |
| CRP (mg/L) | 14 (2–49.3) 1–86 | 2.5 (1–8.3) 1–20 |
| Albumin (g/L) | 33 (25–36) 24–41 | 36.3 (33.5–40.5) 31–44 |
| Hemoglobin (g/L) | 113 (101–119.5) 83–142 | 129 (121–135) 116–144 |
| F-calprotectin (mg/kg) | 2640 (1191–3169) 580–6360 | 620 (198–1556) 5–2256 |
| CYTOKINE | Decreased | Unchanged | Increased |
|---|---|---|---|
| CSF-2 | 4 | 1 | |
| IFN-γ | 3 | 2 | |
| TNF-α | 4 | 2 | |
| IL-1β | 4 | 2 | |
| IL-4 | 3 | ||
| IL-6 | 2 | 2 | 2 |
| IL-10 | 3 | 3 | |
| IL-12β | 1 | 4 | |
| IL-22 | 2 | ||
| IL-23α | 5 | 1 | |
| IL-36γ | 4 | 1 | |
| TGF-β1 | 2 | 1 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolandsdotter, H.; Jönsson-Videsäter, K.; L. Fagerberg, U.; Finkel, Y.; Eberhardson, M. Exclusive Enteral Nutrition: Clinical Effects and Changes in Mucosal Cytokine Profile in Pediatric New Inflammatory Bowel Disease. Nutrients 2019, 11, 414. https://doi.org/10.3390/nu11020414
Rolandsdotter H, Jönsson-Videsäter K, L. Fagerberg U, Finkel Y, Eberhardson M. Exclusive Enteral Nutrition: Clinical Effects and Changes in Mucosal Cytokine Profile in Pediatric New Inflammatory Bowel Disease. Nutrients. 2019; 11(2):414. https://doi.org/10.3390/nu11020414
Chicago/Turabian StyleRolandsdotter, Helena, Kerstin Jönsson-Videsäter, Ulrika L. Fagerberg, Yigael Finkel, and Michael Eberhardson. 2019. "Exclusive Enteral Nutrition: Clinical Effects and Changes in Mucosal Cytokine Profile in Pediatric New Inflammatory Bowel Disease" Nutrients 11, no. 2: 414. https://doi.org/10.3390/nu11020414





