Identification of Six Phytochemical Compounds from Asparagus officinalis L. Root Cultivars from New Zealand and China Using UAE-SPE-UPLC-MS/MS: Effects of Extracts on H2O2-Induced Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Chemicals and Reagents
2.3. Calibration Standard Preparation
2.4. Materials
2.5. UPLC-MS/MS Instrumentation
2.5.1. Liquid Chromatography (LC) Separation
2.5.2. Mass Spectrometry (MS)
2.6. Sample Preparation and Solid-Phase Extraction (SPE) Procedures
2.6.1. Sample Preparation
2.6.2. SPE Preparation
2.7. Method Validation
2.7.1. Residual Amount Calculation
2.7.2. Quantitative Analysis
2.8. Cell Cultures of the HepG2 and L929 Cell Lines
2.8.1. Induction of Oxidative Stress
2.8.2. Determination of Biomarkers of General Cellular Health and Integrity
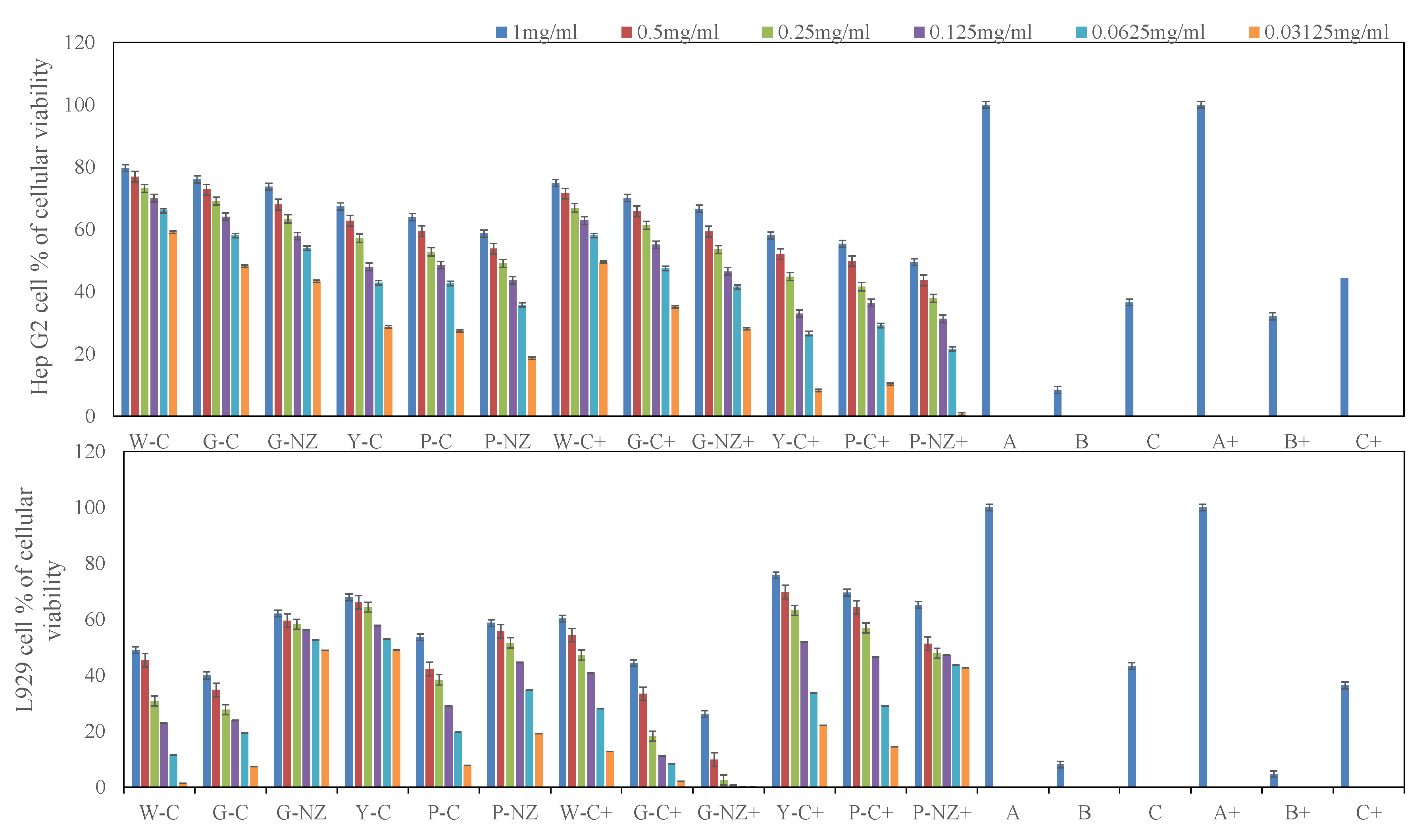
MTS Cell Proliferation Assay
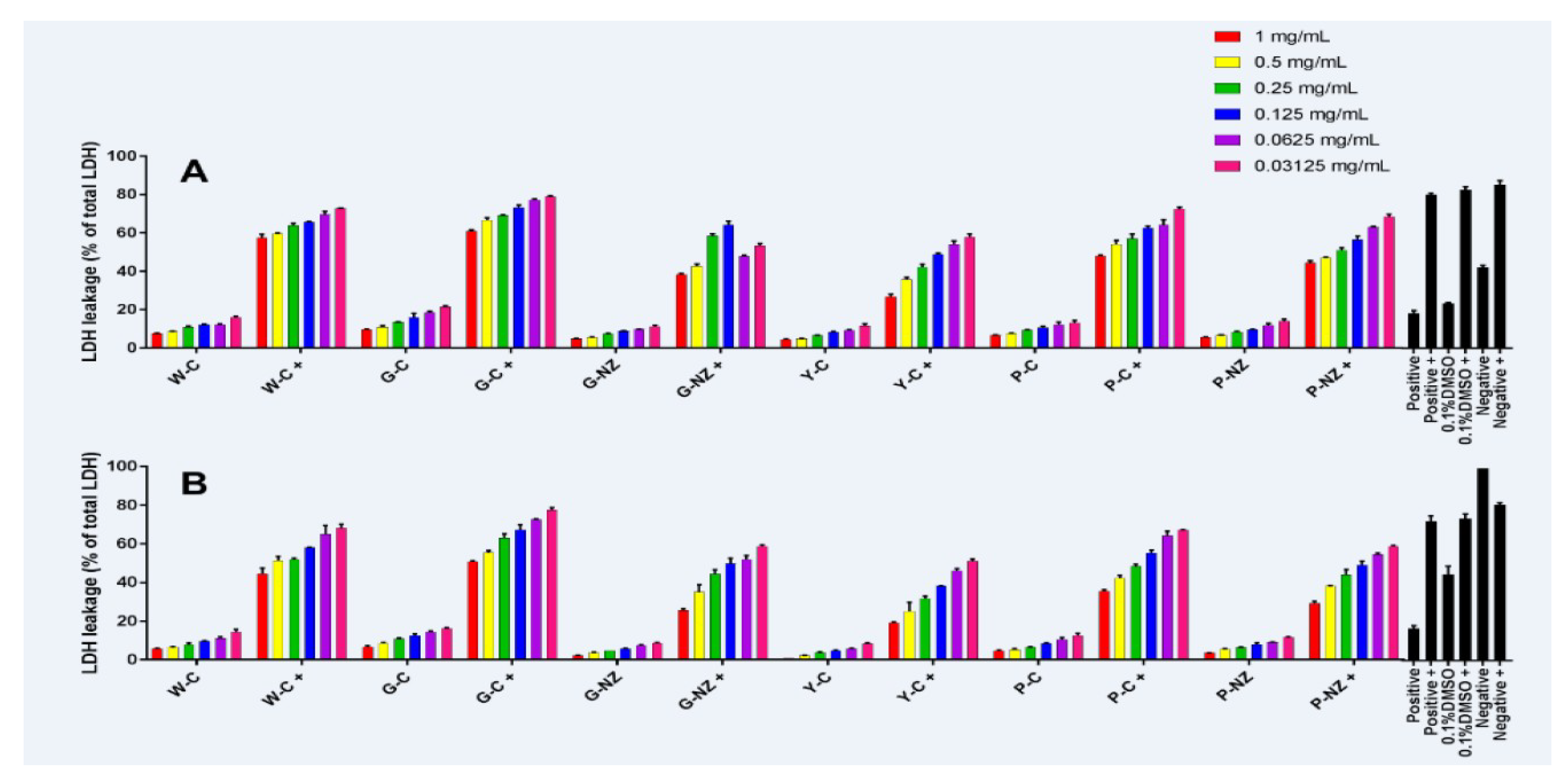
Lactate Dehydrogenase (LDH) Leakage
2.9. Statistical Analysis
3. Results and Discussion
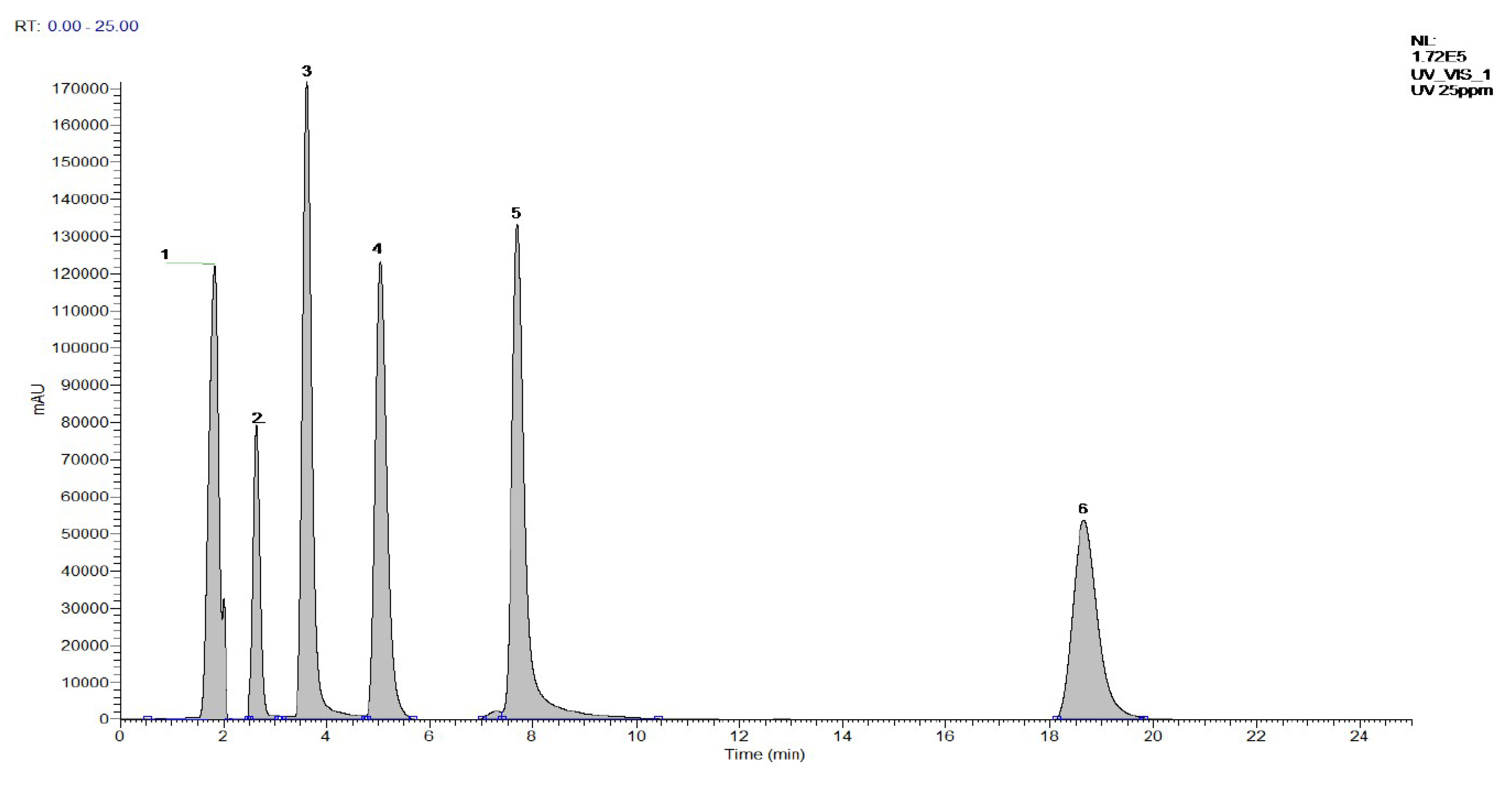
3.1. Determination and Identification of Six Characteristic Compounds in AR
3.1.1. Optimization of Separation of the Six Standard Compounds
3.1.2. Optimization of LC Conditions
3.2. Optimization of Extraction Procedures with a Clean-Up Step
3.2.1. Clean-Up Procedures
3.2.2. Optimization of Extraction Procedures
3.3. Validation
3.4. Calibration
3.5. LC-DAD-MS of AR
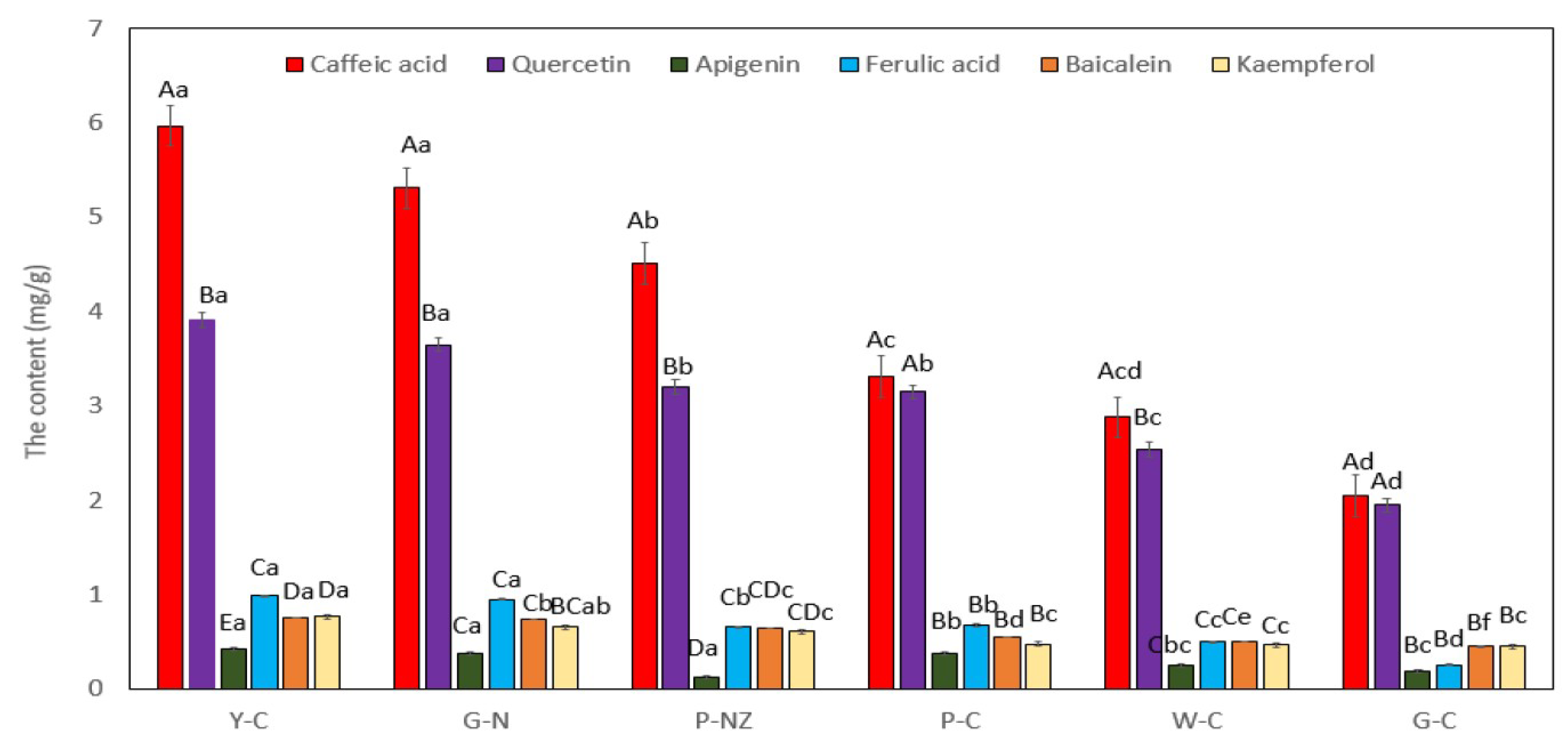
3.6. Concentrations of the Six Compounds in AR
3.7. Bio-Protective Capacity of AR Cultivar Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan, R.; Yuan, F.; Wang, N.; Gao, Y.; Huang, Y. Extraction and analysis of antioxidant compounds from the residues of Asparagus officinalis L. J. Food Sci. Technol. 2015, 52, 2690–2700. [Google Scholar] [CrossRef]
- Huang, X.F.; Luo, J.; Zhang, Y.; Kong, L.Y. Chemical constituents of Asparagus officinalis. Chin J. Nat. Med. 2006, 4, 181–184. [Google Scholar]
- Hartung, A.; Nair, M.; Putnam, A. Isolation and characterization of phytotoxic compounds from asparagus (Asparagus officinalis L.) roots. J. Chem. Ecol. 1990, 16, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Potduang, B.; Meeploy, M.; Giwanon, R.; Benmart, Y.; Kaewduang, M.; Supatanakul, W. Biological activities of Asparagus racemosus. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Singaravadivel, K. Screening of phytochemical and GC-MS analysis of some bioactive constituents of Asparagus racemosus. Screening 2014, 6, 428–432. [Google Scholar]
- Zhou, L.-B.; Chen, T.-H.; Bastow, K.F.; Shibano, M.; Lee, K.-H.; Chen, D.-F. Filiasparosides A–D, cytotoxic steroidal saponins from the roots of Asparagus filicinus. J. Nat. Prod. 2007, 70, 1263–1267. [Google Scholar] [CrossRef]
- Hayes, P.Y.; Jahidin, A.H.; Lehmann, R.; Penman, K.; Kitching, W.; De Voss, J.J. Structural revision of shatavarins I and IV, the major components from the roots of Asparagus racemosus. Tetrahedron Lett. 2006, 47, 6965–6969. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Nair, S.V.; Hettihewa, M.; Rupasinghe, H. Apoptotic and inhibitory effects on cell proliferation of hepatocellular carcinoma HepG2 cells by methanol leaf extract of Costus speciosus. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, Y.; Mahadevan, K.; Kumaraswamy, M.; Vaidya, V.; Manjunatha, H.; Kumar, V.; Satyanarayana, N. Antioxidant, cytotoxic and genotoxic evaluation of alcoholic extract of Polyalthia cerasoides (Roxb.) Bedd. Environ. Toxicol. Pharmacol. 2008, 26, 142–146. [Google Scholar] [CrossRef]
- Thabrew, M.I.; Hughes, R.D.; Mcfarlane, I.G. Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J. Pharm. Pharmacol. 1997, 49, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Birch, J.; Yang, H.; Xie, C.; Kong, L.; Dias, G.; Bekhit, A.E.D. Effect of solvents on polyphenol recovery and antioxidant activity of isolates of Asparagus Officinalis roots from Chinese and New Zealand cultivars. Int. J. Food Sci. Technol. 2018, 53, 2369–2377. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F.; Yang, H.; Kong, L. Determination of chlormequat and mepiquat residues and their dissipation rates in tomato cultivation matrices by ultra-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2017, 1064, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Directive, I.C. 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Commun. 2002, 221, 8–36. [Google Scholar]
- Commission of the European Communities. Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and Interpretation of Results, 2002/657/Ec.2008-05-20. 2002. Available online: http://www/wetgiw.gov.Pl/Old/Ue/Prawo/02?_657/E02657.Pdf (accessed on 12 October 2017).
- Zhang, Y.; Chen, A.Y.; Li, M.; Chen, C.; Yao, Q. Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. J. Surg. Res. 2008, 148, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Horvath, C.R.; Martos, P.A.; Saxena, P.K. Identification and quantification of eight flavones in root and shoot tissues of the medicinal plant Huang-qin (Scutellaria baicalensis Georgi) using high-performance liquid chromatography with diode array and mass spectrometric detection. J. Chromatogr. A 2005, 1062, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Wu, S.; Lu, X.; Zhang, C.; Li, J.; Gong, M.; Wang, M. Quality evaluation of the artemisinin-producing plant Artemisia annua L. based on simultaneous quantification of artemisinin and six synergistic components and hierarchical cluster analysis. Ind. Crops Prod. 2018, 118, 131–141. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, N.; Xian, H.; Wei, D.; Shi, L.; Feng, X. A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1429, 22–29. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. TrAC Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [PubMed]
- Wei, Q.; Wang, S.-Y.; Tang, F.; Zhang, H.-X.; Yu, J.; Yue, Y.-D. Simultaneous Determination of 13 flavonoids in Bamboo Leaves by HPLC. Sci. Sile Sci. 2015, 51, 81–87. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Zhang, W.; Chao, S.-W. Extraction of ferulic acid and caffeic acid with ionic liquids. Chin. J. Anal. Chem. 2007, 35, 1726–1730. [Google Scholar] [CrossRef]
- Cacace, J.; Mazza, G. Extraction of anthocyanins and other phenolics from black currants with sulfured water. J. Agric. Food Chem. 2002, 50, 5939–5946. [Google Scholar] [CrossRef] [PubMed]
- Symes, A.; Shavandi, A.; Zhang, H.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Bekhit, A.E. Antioxidant activities and Caffeic acid content in New Zealand Asparagus (Asparagus officinalis) roots extracts. Antioxidants 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Liu, Y.; Xia, Y.; Tang, T. Analysis of flavonoids in Portulaca oleracea L. by UV–vis spectrophotometry with comparative study on different extraction technologies. Food Anal. Meth. 2010, 3, 90–97. [Google Scholar] [CrossRef]
- Hvattum, E.; Ekeberg, D. Study of the collision-induced radical cleavage of flavonoid glycosides using negative electrospray ionization tandem quadrupole mass spectrometry. J. Mass Spectrom. 2003, 38, 43–49. [Google Scholar] [CrossRef]
- Zhang, H.; Birch, J.; Xie, C.; Yang, H.; Dias, G.; Kong, L.; Bekhit, A.E.-D. Optimization of extraction parameters of antioxidant activity of extracts from New Zealand and Chinese Asparagus officinalis L. root cultivars. Ind. Crops Prod. 2018, 119, 191–200. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jaramillo, S.; Rodríguez, G.; Espejo, J.A.; Guillén, R.; Fernández-Bolaños, J.; Jiménez, A. Antioxidant activity of ethanolic extracts from several asparagus cultivars. J. Agric. Food Chem. 2005, 53, 5212–5217. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Kmiecik, D.; Gramza-Michałowska, A.; Golczak, D.; Korczak, J. Antiradical capacity and polyphenol composition of asparagus spears varieties cultivated under different sunlight conditions. Acta Sci. Pol. Technol. Aliment. 2016, 15, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.R.; Jeyanthimala, K.; Ramachandran, S. Caffeic acid modulates ultraviolet radiation-B induced oxidative damage in human blood lymphocytes. J. Photochem. Photobiol. B 2009, 95, 196–203. [Google Scholar] [CrossRef]
- Pari, L.; Prasath, A. Efficacy of caffeic acid in preventing nickel induced oxidative damage in liver of rats. Chem.-Biol. Interact. 2008, 173, 77–83. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Talcott, S.T.; Safe, S.; Mertens-Talcott, S. Absorption and biological activity of phytochemical-rich extracts from acai (Euterpe oleracea Mart.) pulp and oil in vitro. J. Agric Food Chem. 2008, 56, 3593–3600. [Google Scholar] [CrossRef]
- Gaube, F.; Wolfl, S.; Pusch, L.; Kroll, T.C.; Hamburger, M. Gene expression profiling reveals effects of Cimicifuga racemosa (L.) NUTT.(black cohosh) on the estrogen receptor positive human breast cancer cell line MCF-7. BMC Pharmacol. 2007, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.; Wang, L.; See, P.; Grayer, R.J.; Chan, S.-Y.; Lee, S.T. Phenolic compounds of Chromolaena odorata protect cultured skin cells from oxidative damage: Implication for cutaneous wound healing. Biol. Pharm. Bull. 2001, 24, 1373–1379. [Google Scholar] [CrossRef]
| Time (min) | Solvent A (%) | Solvent B (%) |
|---|---|---|
| 0.00 | 95 | 5 |
| 2.00 | 85 | 15 |
| 3.00 | 75 | 25 |
| 4.00 | 40 | 60 |
| 6.25 | 55 | 45 |
| 8.25 | 20 | 80 |
| 20.50 | 15 | 95 |
| 25.00 | 95 | 5 |
| Components | Parent Ion (m/z) | Retention Time (min) | Product Ions for Identification (m/z) | Collision Energy (V) | DP * (V) |
|---|---|---|---|---|---|
| Caffeic acid | 180.15 | 1.83 | 91/88 | 20/20 | 80/80 |
| Quercetin | 302.24 | 7.71 | 153/299 | 40/40 | 135/135 |
| Apigenin | 270.24 | 5.05 | 153/119 | 35/30 | 80/80 |
| Ferulic acid | 194.187 | 2.64 | 72/123 | 20/20 | 80/80 |
| Baicalein | 271 | 3.63 | 123/169 | 35/35 | 80/80 |
| Kaempferol | 287 | 18.86 | 153/258 | 35/30 | 100/100 |
| Analyte | Regression Equation | Correlation Coefficient (R2) | Linear Range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|
| Caffeic acid | Y = 5814.9X + 94,5763 | 0.977 | 70–500 | 23 | 70 |
| Quercetin | Y = 23,142.3X + 4 × 106 | 0.937 | 150–500 | 50 | 150 |
| Apigenin | Y = 69,288X + 694,300 | 0.996 | 30–500 | 10 | 30 |
| Ferulic acid | Y = 24,691X + 522,209 | 0.986 | 50–500 | 18 | 54 |
| Baicalein | Y = 73,699X + 1 × 106 | 0.991 | 43.6–500 | 14.4 | 43.6 |
| Kaempferol | Y = 75,732X + 425,150 | 0.998 | 22.5–500 | 7.5 | 22.5 |
| Standards | Original Quantity (mg/g) | Addition Quantity (μg/g) | Amount Found (mg/g) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Caffeic acid | 2.02 | 140 | 2.16 | 81.80 | 4.15 |
| 280 | 2.30 | 87.50 | 3.93 | ||
| 500 | 2.49 | 88.21 | 6.58 | ||
| Ferulic acid | 0.24 | 108 | 0.34 | 75.00 | 7.37 |
| 200 | 0.40 | 71.90 | 7.68 | ||
| 400 | 0.54 | 72.00 | 8.09 | ||
| Quercetin | 3.05 | 30 | 3.10 | 87.20 | 6.88 |
| 60 | 3.12 | 88.30 | 7.44 | ||
| 120 | 3.18 | 95.17 | 8.17 | ||
| Apigenin | 0.23 | 60 | 0.25 | 101.70 | 5.52 |
| 120 | 0.27 | 68.10 | 9.50 | ||
| 240 | 0.36 | 72.10 | 6.34 | ||
| Baicalein | 0.45 | 87.6 | 0.52 | 75.20 | 5.83 |
| 175.2 | 0.58 | 74.70 | 7.44 | ||
| 350.4 | 0.70 | 80.34 | 9.85 | ||
| Kaempferol | 0.43 | 45 | 0.48 | 76.70 | 7.46 |
| 90 | 0.52 | 73.20 | 6.37 | ||
| 180 | 0.58 | 73.50 | 7.52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Birch, J.; Pei, J.; Mohamed Ahmed, I.A.; Yang, H.; Dias, G.; Abd El-Aty, A.M.; Bekhit, A.E.-D. Identification of Six Phytochemical Compounds from Asparagus officinalis L. Root Cultivars from New Zealand and China Using UAE-SPE-UPLC-MS/MS: Effects of Extracts on H2O2-Induced Oxidative Stress. Nutrients 2019, 11, 107. https://doi.org/10.3390/nu11010107
Zhang H, Birch J, Pei J, Mohamed Ahmed IA, Yang H, Dias G, Abd El-Aty AM, Bekhit AE-D. Identification of Six Phytochemical Compounds from Asparagus officinalis L. Root Cultivars from New Zealand and China Using UAE-SPE-UPLC-MS/MS: Effects of Extracts on H2O2-Induced Oxidative Stress. Nutrients. 2019; 11(1):107. https://doi.org/10.3390/nu11010107
Chicago/Turabian StyleZhang, Hongxia, John Birch, Jinjin Pei, Isam A. Mohamed Ahmed, Haiyan Yang, George Dias, A. M. Abd El-Aty, and Alaa El-Din Bekhit. 2019. "Identification of Six Phytochemical Compounds from Asparagus officinalis L. Root Cultivars from New Zealand and China Using UAE-SPE-UPLC-MS/MS: Effects of Extracts on H2O2-Induced Oxidative Stress" Nutrients 11, no. 1: 107. https://doi.org/10.3390/nu11010107






