Gastric Emptying and Dynamic In Vitro Digestion of Drinkable Yogurts: Effect of Viscosity and Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viscosity Analysis
2.2. Gastric Emptying Assessment by Gamma-Scintigraphy on Pigs
2.3. Yogurt Dynamic In Vitro Digestion
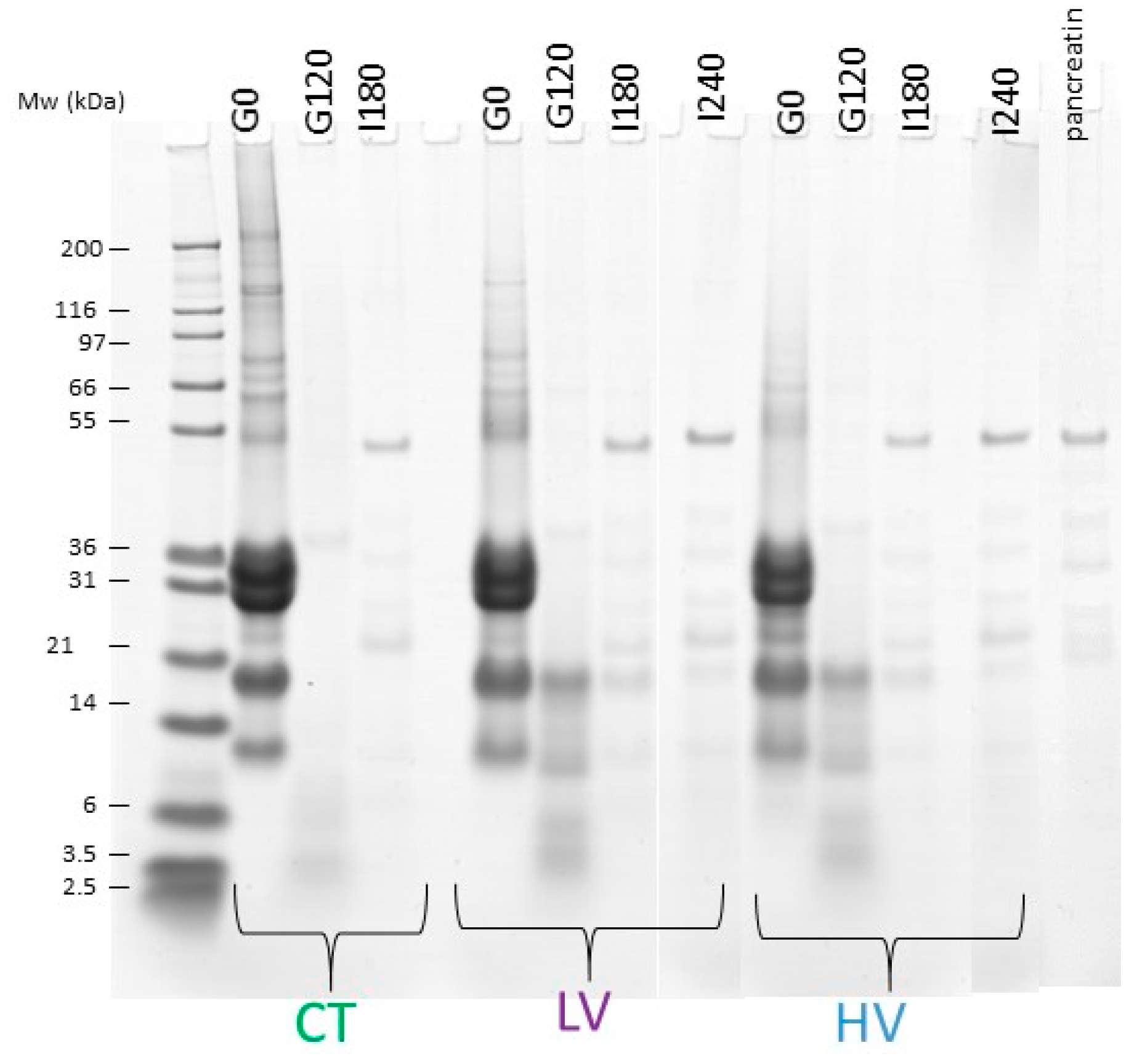
2.4. SDS-PAGE
2.5. Statistics
3. Results
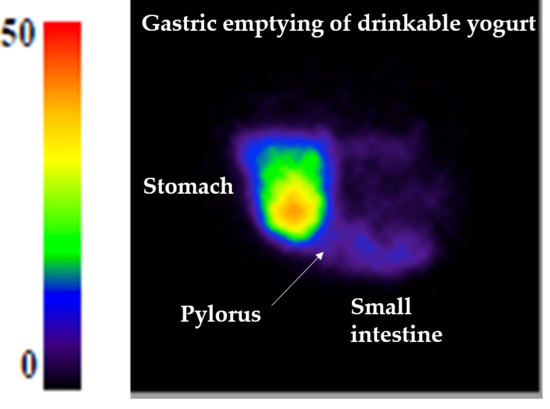
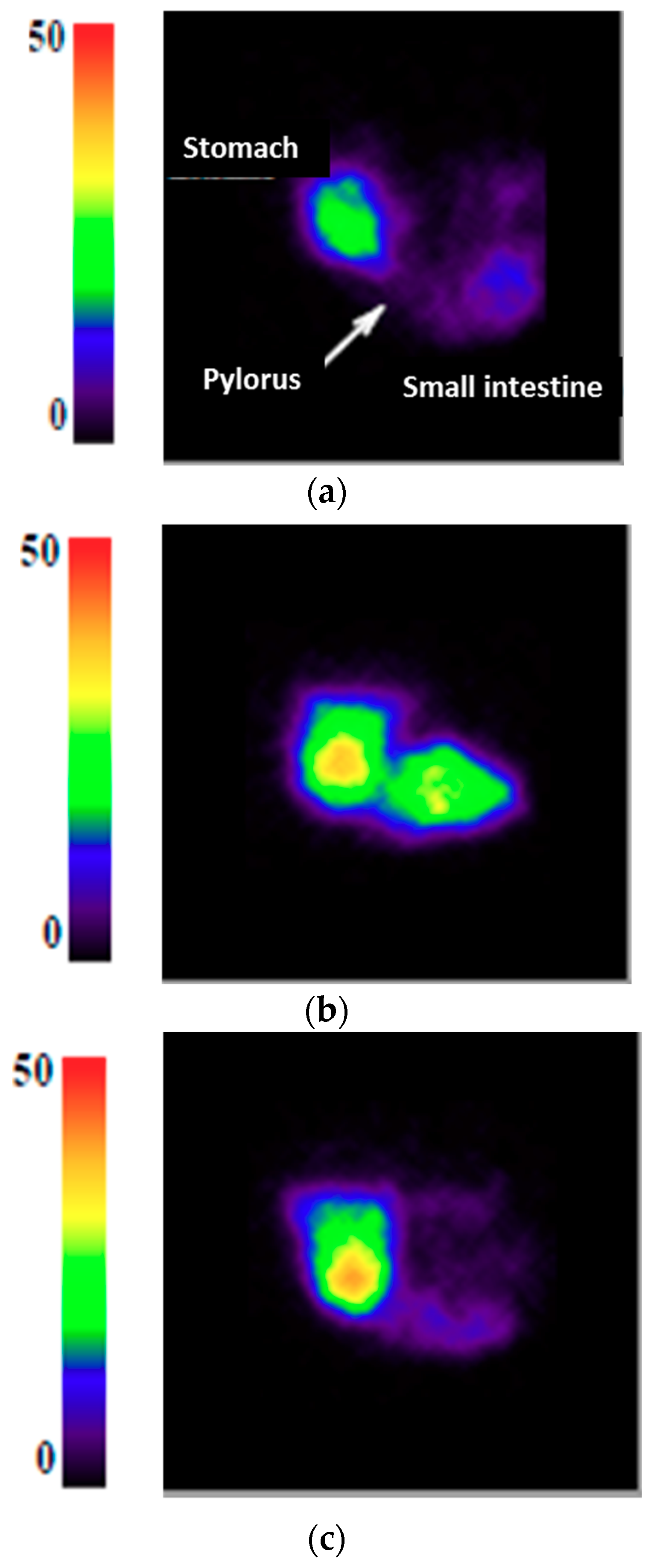
3.1. Gastric Emptying
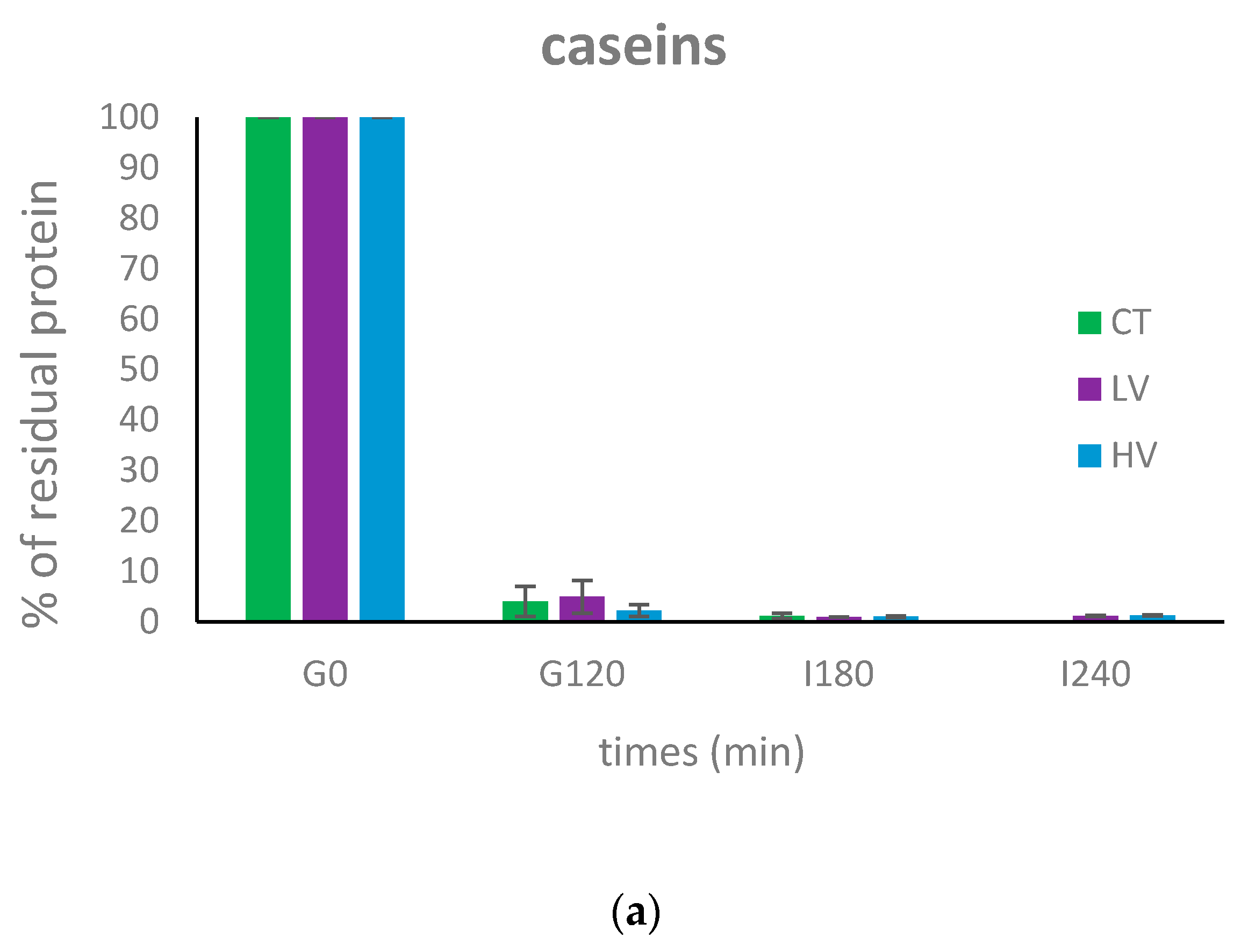
3.2. Dynamic In Vitro Digestion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turgeon, S.L.; Rioux, L.E. Food matrix impact on macronutrients nutritional properties. Food Hydrocoll. 2011, 25, 1915–1924. [Google Scholar] [CrossRef]
- Mao, L.K.; Miao, S. Structuring food emulsions to improve nutrient delivery during digestion. Food Eng. Rev. 2015, 7, 439–451. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.Q.; Bellissimo, N.; Singh, H.; Rousseau, D. Modulating fat digestion through food structure design. Prog. Lipid Res. 2017, 68, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Le Feunteun, S.; Marze, S.; Souchon, I. Structuring food to control its disintegration in the gastrointestinal tract and optimize nutrient bioavailability. Innov. Food Sci. Emerg. Technol. 2018, 46, 83–90. [Google Scholar] [CrossRef]
- Bensaid, A.; Tome, D.; Gietzen, D.; Even, P.; Morens, C.; Gausseres, N.; Fromentin, G. Protein is more potent than carbohydrate for reducing appetite in rats. Physiol. Behav. 2002, 75, 577–582. [Google Scholar] [CrossRef]
- Anderson, G.H.; Moore, S.E. Dietary proteins in the regulation of food intake and body weight in humans. J. Nutr. 2004, 134, 974S–979S. [Google Scholar] [CrossRef] [PubMed]
- Dougkas, A.; Ostman, E. Protein-enriched liquid preloads varying in macronutrient content modulate appetite and appetite-regulating hormones in healthy adults. J. Nutr. 2016, 146, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Trahair, L.G.; Luscombe-Marsh, N.D.; Hausken, T.; Standfield, S.; Jones, K.L.; Lange, K.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of randomized whey-protein loads on energy intake, appetite, gastric emptying, and plasma gut-hormone concentrations in older men and women. Am. J. Clin. Nutr. 2017, 106, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; van der Burgh, Y.; Lange, K.; Hatzinikolas, S.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of substitution, and adding of carbohydrate and fat to whey-protein on energy intake, appetite, gastric emptying, glucose, insulin, ghrelin, cck and glp-1 in healthy older men—A randomized controlled trial. Nutrients 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Aimutis, W.R. Bioactive properties of milk proteins with particular focus on anticariogenesis. J. Nutr. 2004, 134, 989S–995S. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldhorst, M.A.B.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; Van Vught, A.J.A.H.; Westerterp, K.R.; Engelen, M.P.K.J.; Brummer, R.J.M.; Deutz, N.E.P.; Westerterp-Plantenga, M.S. Dose-dependent satiating effect of whey relative to casein or soy. Physiol. Behav. 2009, 96, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, L.Q.; Lorenzen, J.K.; Bendsen, N.T.; Rasmussen, C.; Astrup, A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: A review of the evidence from controlled clinical trials. Adv. Nutr. 2013, 4, 418–438. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.J.; Slavin, J.L. The effect of fiber on satiety and food intake: A systematic review. J. Am. Coll. Nutr. 2013, 32, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.; Green, H. Dietary fibre and satiety. Nutr. Bull. 2007, 32, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Wanders, A.J.; van den Borne, J.; de Graaf, C.; Hulshof, T.; Jonathan, M.C.; Kristensen, M.; Mars, M.; Schols, H.A.; Feskens, E.J.M. Effects of dietary fibre on subjective appetite, energy intake and body weight: A systematic review of randomized controlled trials. Obes. Rev. 2011, 12, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Clegg, M.E.; Ranawana, V.; Shafat, A.; Henry, C.J. Soups increase satiety through delayed gastric emptying yet increased glycaemic response. Eur. J. Clin. Nutr. 2013, 67, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Ritter, R.C. Gastrointestinal mechanisms of satiation for food. Physiol. Behav. 2004, 81, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Roe, L.S.; Rolls, B.J. Sensory-specific satiety is affected more by volume than by energy content of a liquid food. Physiol. Behav. 2003, 78, 593–600. [Google Scholar] [CrossRef]
- Andrews, J.M.; Doran, S.M.; Hebbard, G.S.; Malbert, C.H.; Horowitz, M.; Dent, J. Nutrient-induced spatial patterning of human duodenal motor function. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G501–G509. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Castellanos, V.H.; Halford, J.C.; Kilara, A.; Panyam, D.; Pelkman, C.L.; Smith, G.P.; Thorwart, M.L. Volume of food consumed affects satiety in men. Am. J. Clin. Nutr. 1998, 67, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Tomasi, D.; Backus, W.; Wang, R.; Telang, F.; Geliebter, A.; Korner, J.; Bauman, A.; Fowler, J.S.; Thanos, P.K.; et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage 2008, 39, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.R.; Rafiee, H.; Malcolm, P.; Salt, L.; van Aken, G. Specific food structures supress appetite through reduced gastric emptying rate. Am. J. Physiol Gastrointest. Liver Physiol. 2013, 304, G1038–G1043. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, N.; Mars, M.; de Wijk, R.A.; Westerterp-Plantenga, M.S.; de Graaf, C. The effect of viscosity on ad libitum food intake. Int. J. Obes. (Lond.) 2008, 32, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Wanders, A.J.; Jonathan, M.C.; van den Borne, J.; Mars, M.; Schols, H.A.; Feskens, E.J.M.; de Graaf, C. The effects of bulking, viscous and gel-forming dietary fibres on satiation. Br. J. Nutr. 2013, 109, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Bertenshaw, E.J.; Lluch, A.; Yeomans, M.R. Perceived thickness and creaminess modulates the short-term satiating effects of high-protein drinks. Br. J. Nutr. 2013, 110, 578–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. The impact of food viscosity on eating rate, subjective appetite, glycemic response and gastric emptying rate. PLoS ONE 2013, 8, e67482. [Google Scholar] [CrossRef] [PubMed]
- Clegg, M.E.; Shafat, A. The effect of agar jelly on energy expenditure, appetite, gastric emptying and glycaemic response. Eur. J. Nutr. 2014, 53, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Camps, G.; Mars, M.; de Graaf, C.; Smeets, P.A.M. Empty calories and phantom fullness: A randomized trial studying the relative effects of energy density and viscosity on gastric emptying determined by MRI and satiety. Am. J. Clin. Nutr. 2016, 104, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ménard, O.; Cattenoz, T.; Guillemin, H.; Souchon, I.; Deglaire, A.; Dupont, D.; Picque, D. Validation of a new in vitro dynamic system to simulate infant digestion. Food Chem. 2014, 145, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Camps, G.; Mars, M.; Witteman, B.J.M.; de Graaf, C.; Smeets, P.A.M. Indirect vs direct assessment of gastric emptying: A randomized crossover trial comparing c-isotope breath analysis and MRI. Neurogastroenterol. Motil. 2018, 30, e13317. [Google Scholar] [CrossRef] [PubMed]
- Herschel, W.H.; Bulkley, R. Konsistenzmessungen von gummi-benzollosungen. Colloid Polym. Sci. 1926, 39, 291–300. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Guerin, S.; Malbert, C.H. Slower eating rate is independent to gastric emptying in obese minipigs. Physiol. Behav. 2010, 101, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Elashoff, J.D.; Reedy, T.J.; Meyer, J.H. Analysis of gastric-emptying data. Gastroenterology 1982, 83, 1306–1312. [Google Scholar] [PubMed]
- Egger, L.; Ménard, O.; Baumann, C.; Duerr, D.; Schlegel, P.; Stoll, P.; Vergères, G.; Dupont, D.; Portmann, R. Digestion of milk proteins: Comparing static and dynamic in vitro digestion systems with in vivo data. Food Res. Int. 2017, in press. [Google Scholar] [CrossRef]
- Blundell, J.E.; Macdiarmid, J.I. Fat as a risk factor for overconsumption: Satiation, satiety, and patterns of eating. J. Am. Diet. Assoc. 1997, 97, S63–S69. [Google Scholar] [CrossRef]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Veldhorst, M.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Masic, U.; Yeomans, M.R. Does monosodium glutamate interact with macronutrient composition to influence subsequent appetite? Physiol. Behav. 2013, 116, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.L.; Gratz, S.W.; Peinado, D.I.; Thomson, L.M.; Garden, K.E.; Williams, P.A.; Richardson, A.J.; Ross, A.W. Effects of dietary fibre (pectin) and/or increased protein (casein or pea) on satiety, body weight, adiposity and caecal fermentation in high fat diet-induced obese rats. PLoS ONE 2016, 11, e0155871. [Google Scholar] [CrossRef] [PubMed]
- Barbe, F.; Menard, O.; Le Gouar, Y.; Buffiere, C.; Famelart, M.H.; Laroche, B.; Le Feunteun, S.; Dupont, D.; Remond, D. The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids. Food Chem. 2013, 136, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Mandalari, G.; Molle, D.; Jardin, J.; Rolet-Repecaud, O.; Duboz, G.; Leonil, J.; Mills, E.N.C.; Mackie, A.R. Food processing increases casein resistance to simulated infant digestion. Mol. Nutr. Food Res. 2010, 54, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Macierzanka, A.; Sancho, A.I.; Mills, E.N.C.; Rigby, N.M.; Mackie, A.R. Emulsification alters simulated gastrointestinal proteolysis of beta-casein and beta-lactoglobulin. Soft Matter 2009, 5, 538–550. [Google Scholar] [CrossRef]
- Mandalari, G.; Adel-Patient, K.; Barkholt, V.; Baro, C.; Bennett, L.; Bublin, M.; Gaier, S.; Graser, G.; Ladics, G.; Mierzejewska, D.; et al. In vitro digestibility of beta-casein and beta-lactoglobulin under simulated human gastric and duodenal conditions: A multi-laboratory evaluation. Regul. Toxicol. Pharmacol. 2009, 55, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Digestibility and antigenicity of beta-lactoglobulin as affected by heat, pH and applied shear. Food Chem. 2017, 217, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rivera, L.; Menard, O.; Recio, I.; Dupont, D. Peptide mapping during dynamic gastric digestion of heated and unheated skimmed milk powder. Food Res. Int. 2015, 77, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.K.; Oiseth, S.K.; Lundin, L.; Day, L. Influence of heat and shear induced protein aggregation on the in vitro digestion rate of whey proteins. Food Funct. 2014, 5, 2686–2698. [Google Scholar] [CrossRef] [PubMed]
- Mathai, J.K.; Liu, Y.H.; Stein, H.H. Values for digestible indispensable amino acid scores (diaas) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (pdcaas). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.; Mahé, S.; Gaudichon, C.; Benamouzig, R.; Gausserès, N.; Luengo, C.; Ferrière, F.; Rautureau, J.; Tomé, D. Assessment of net postprandial protein utilization of 15N-labelled milk nitrogen in human subjects. Br. J. Nutr. 1999, 81, 221–226. [Google Scholar] [PubMed]


| Control | Low Viscosity | High Viscosity | |
|---|---|---|---|
| Protein (g/100 g) | 3.1 | 8.1 | 8.1 |
| Lipid (g/100 g) | 0.1 | 0.2 | 0.2 |
| Sugar (g/100 g) | 17.3 | 11.6 | 11.6 |
| Fiber (g/100 g) | 0 | 2.5 | 2.5 |
| Starch (%) | 0.53 | 0.18 | 0.18 |
| Energy (kcal/100 g) | 82 | 85 | 85 |
| Texture | standard | liquid | thick liquid |
| Viscosityapp (Pa∙s) | CT | LV | HV |
|---|---|---|---|
| D + 4 | 1.26 ± 0.12 | 0.32 ± 0.04 | 2.20 ± 0.11 |
| D + 15 | 1.30 ± 0.05 | 0.37 ± 0.04 | 2.07 ± 0.20 |
| Gastric Conditions (37 °C) | |||
| Simulated Gastric Fluid (SGF) (stock solution adjusted at pH 6.5) | Na+ | 100 mmol/L | |
| Ca2+ | 1 mmol/L | ||
| Fasted state/initial conditions | SGF | 24 mL | |
| pH | 1.8 | ||
| Yogurt | Ingested amount | 150 g | |
| Gastric pH (acidification curve) | pH = 1.68 + 3.82(−t/42) (with t: time after ingestion in min) | ||
| SGF + pepsin (porcine) | Pepsin | 2000 U/mL of gastric content | |
| Flow rate | 1 mL/min from 0 to 5 min | ||
| Flow rate | 0.5 mL/min from 5 to 180 min | ||
| Gastric emptying (Elashoff fitting) | CT | T1/2 * | 58 min |
| β | 1.1 | ||
| LV | T1/2 | 73 min | |
| β | 1.0 | ||
| HV | T1/2 | 65 min | |
| β | 1.1 | ||
| Intestinal Conditions (37 °C) | |||
| Simulated Intestinal Fluid (SIF) (stock solution adjusted at pH 6.2) | Na+ | 100 mmol/L | |
| Ca2+ | 1 mmol/L | ||
| Intestinal pH | pH | 6.6 | |
| SIF + bile (bovine) | Bile | 4% from 0 to 30 min | |
| Bile | 2% from 30 min to the end | ||
| Flow rate | 0.5 mL/min from 0 to the end | ||
| SIF + pancreatin (porcine) | Pancreatin | 7% | |
| Flow rate | 0.25 mL/min from 0 to the end | ||
| Intestinal emptying (Elashoff fitting) | T1/2 | 160 min | |
| β | 1.6 | ||
| T1/2 (min) | β | |
|---|---|---|
| Control | 57.7 ± 3.9 | 1.1 ± 0.05 |
| Low viscosity | 72.7 ± 5.1 * | 1.0 ± 0.04 |
| High viscosity | 65.3 ± 3.5 | 1.1 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ménard, O.; Famelart, M.-H.; Deglaire, A.; Le Gouar, Y.; Guérin, S.; Malbert, C.-H.; Dupont, D. Gastric Emptying and Dynamic In Vitro Digestion of Drinkable Yogurts: Effect of Viscosity and Composition. Nutrients 2018, 10, 1308. https://doi.org/10.3390/nu10091308
Ménard O, Famelart M-H, Deglaire A, Le Gouar Y, Guérin S, Malbert C-H, Dupont D. Gastric Emptying and Dynamic In Vitro Digestion of Drinkable Yogurts: Effect of Viscosity and Composition. Nutrients. 2018; 10(9):1308. https://doi.org/10.3390/nu10091308
Chicago/Turabian StyleMénard, Olivia, Marie-Hélène Famelart, Amélie Deglaire, Yann Le Gouar, Sylvie Guérin, Charles-Henri Malbert, and Didier Dupont. 2018. "Gastric Emptying and Dynamic In Vitro Digestion of Drinkable Yogurts: Effect of Viscosity and Composition" Nutrients 10, no. 9: 1308. https://doi.org/10.3390/nu10091308