Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Nanoemulsions
2.2. Preparation of Nanoemulsions
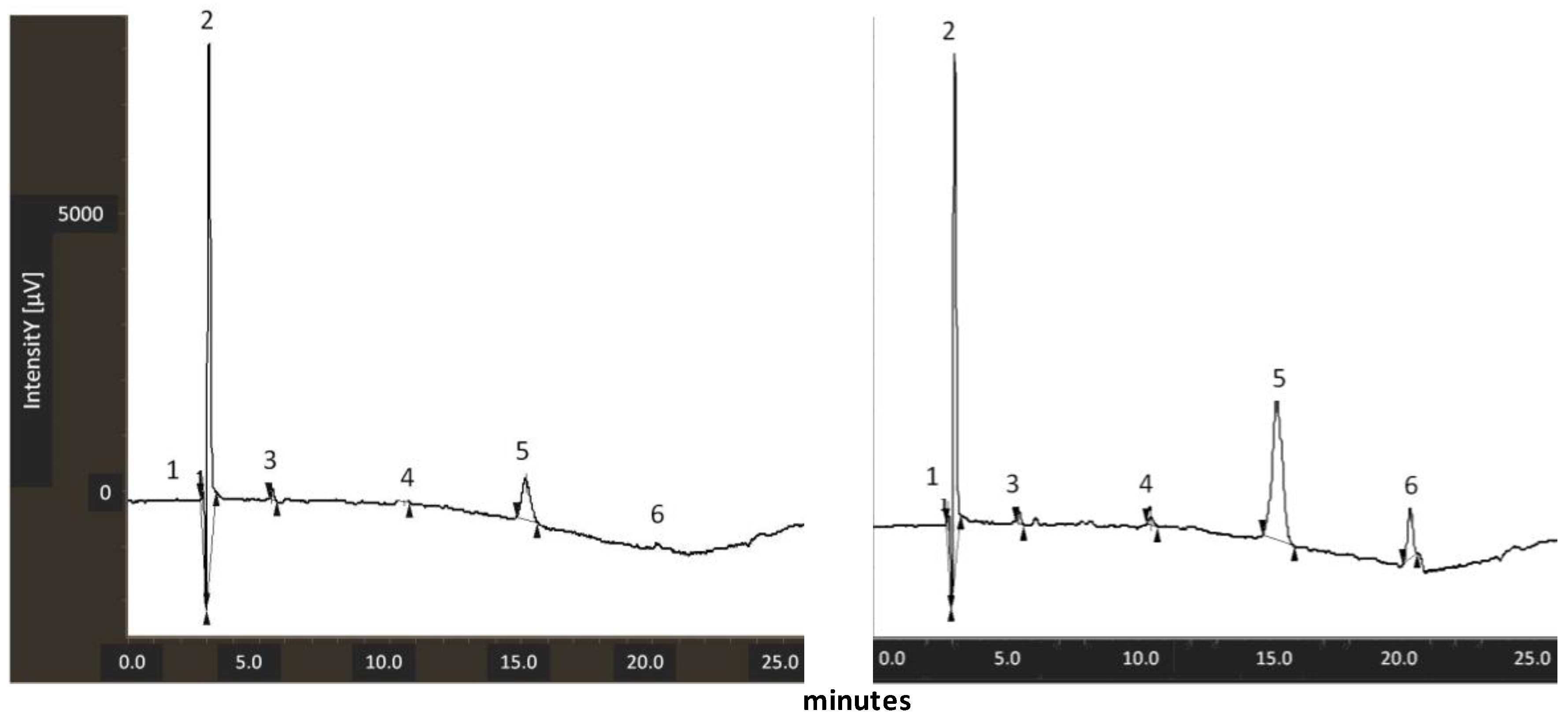
2.3. LC/MS Analysis for Lycopene Measurement
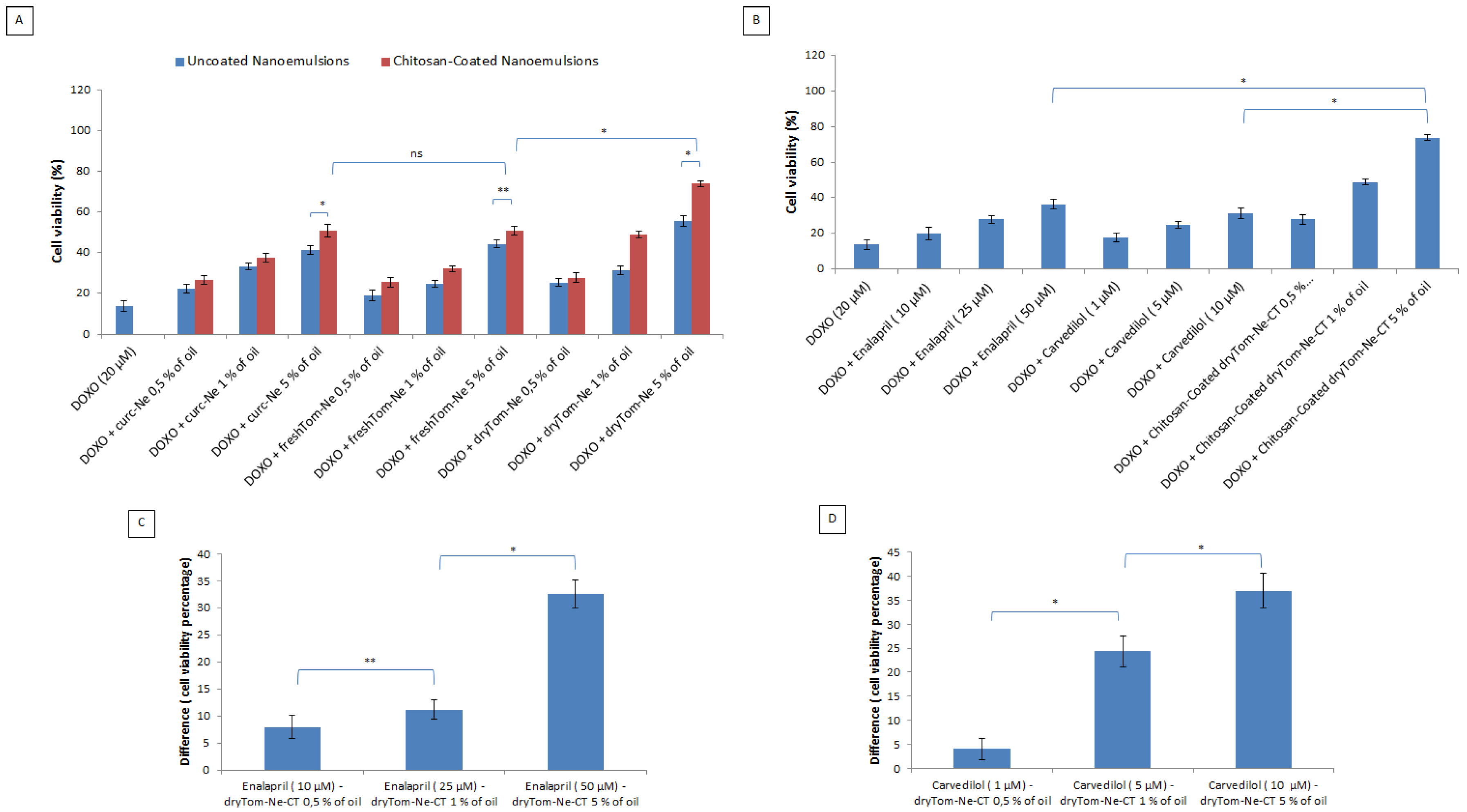
2.4. Cell Viability
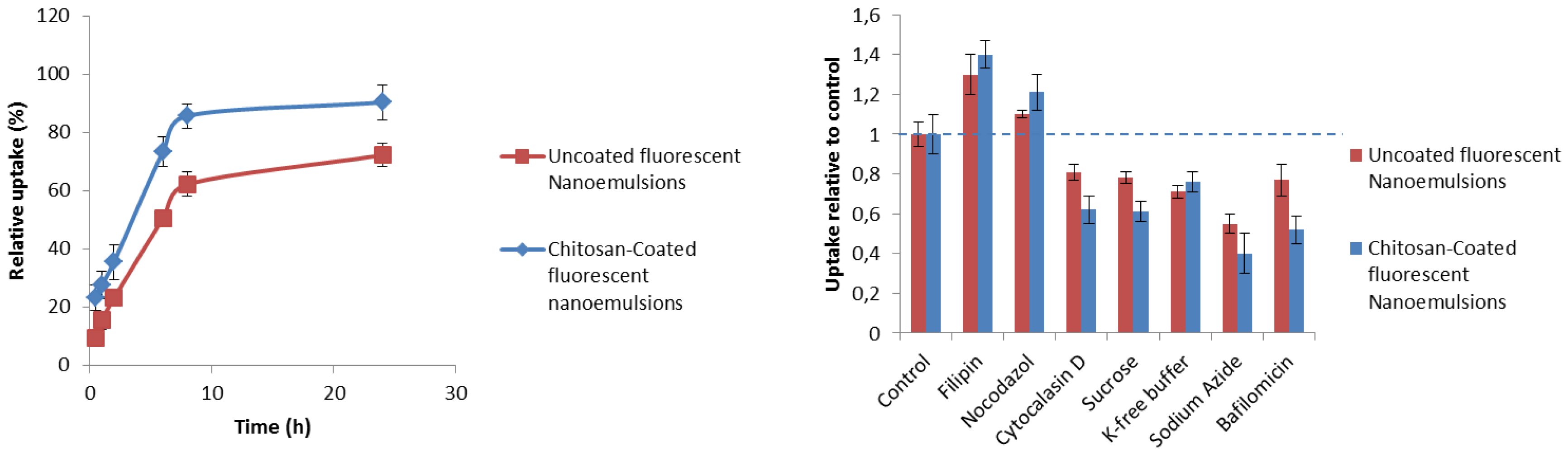
2.5. Cellular Uptake Studies
2.5.1. Uptake Quantification
2.5.2. Mechanistic Studies
2.6. Cellular Antioxidant Activity Following Oxygen Radical Generator Exposure
2.7. Detection of Intracellular Reactive Oxygen Species
2.8. Lipid Peroxidation Studies
2.9. Measurement of Nitric Oxide
2.9.1. Intracellular Calcium Level
2.9.2. Anti-Inflammatory Studies
2.9.3. Statistical Analysis
3. Results
3.1. Synthesis and Chemical-Physical Characterization of Nutraceutical-Loaded Nanocarriers
3.2. Measurement of Lycopene Content in Tomato Peel Extracts
3.3. Cell Viability
3.4. Uptake Quantification and Mechanistic Studies
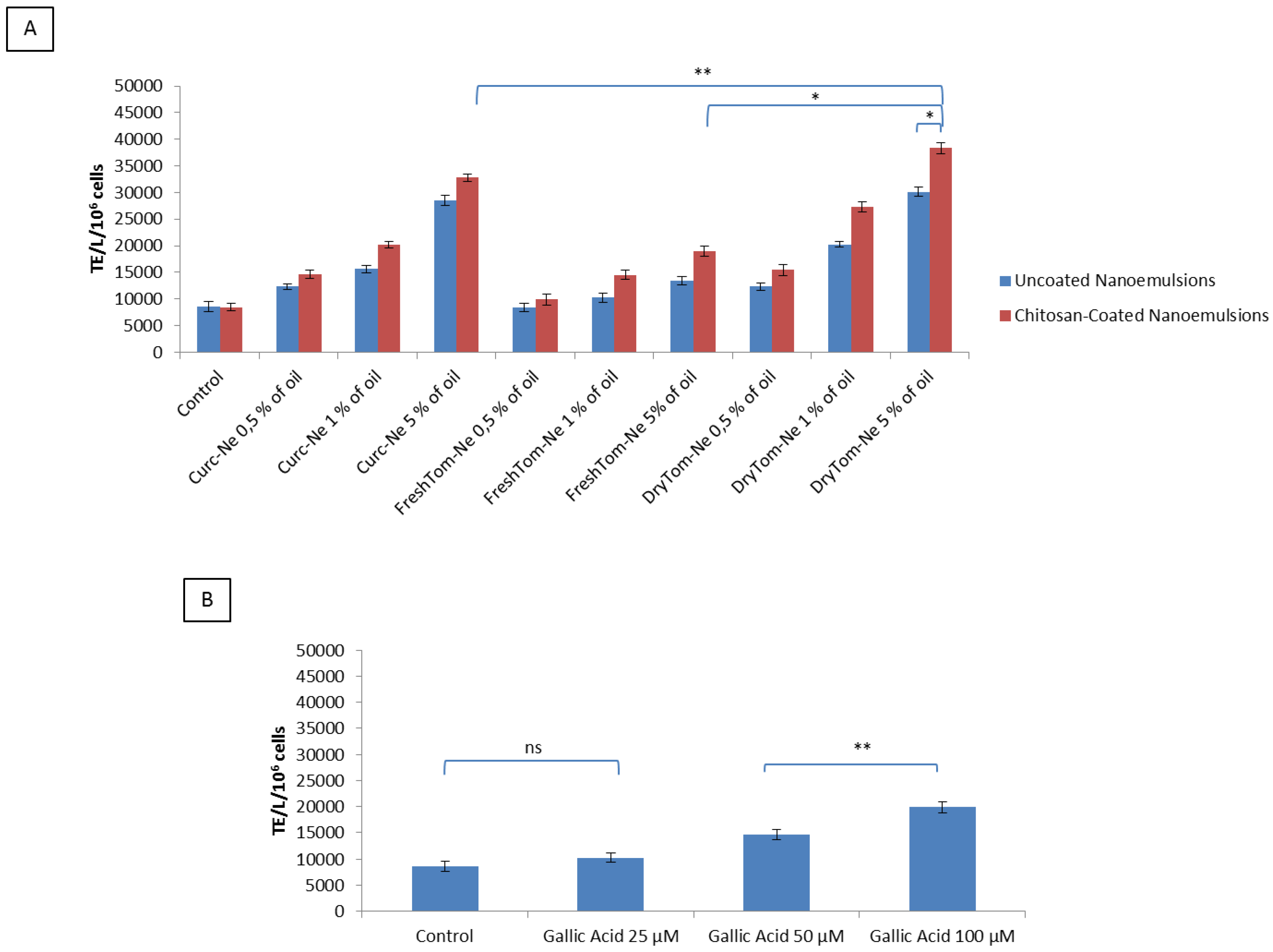
3.5. Cellular Antioxidant Activity after Oxygen Radical Generator Exposure
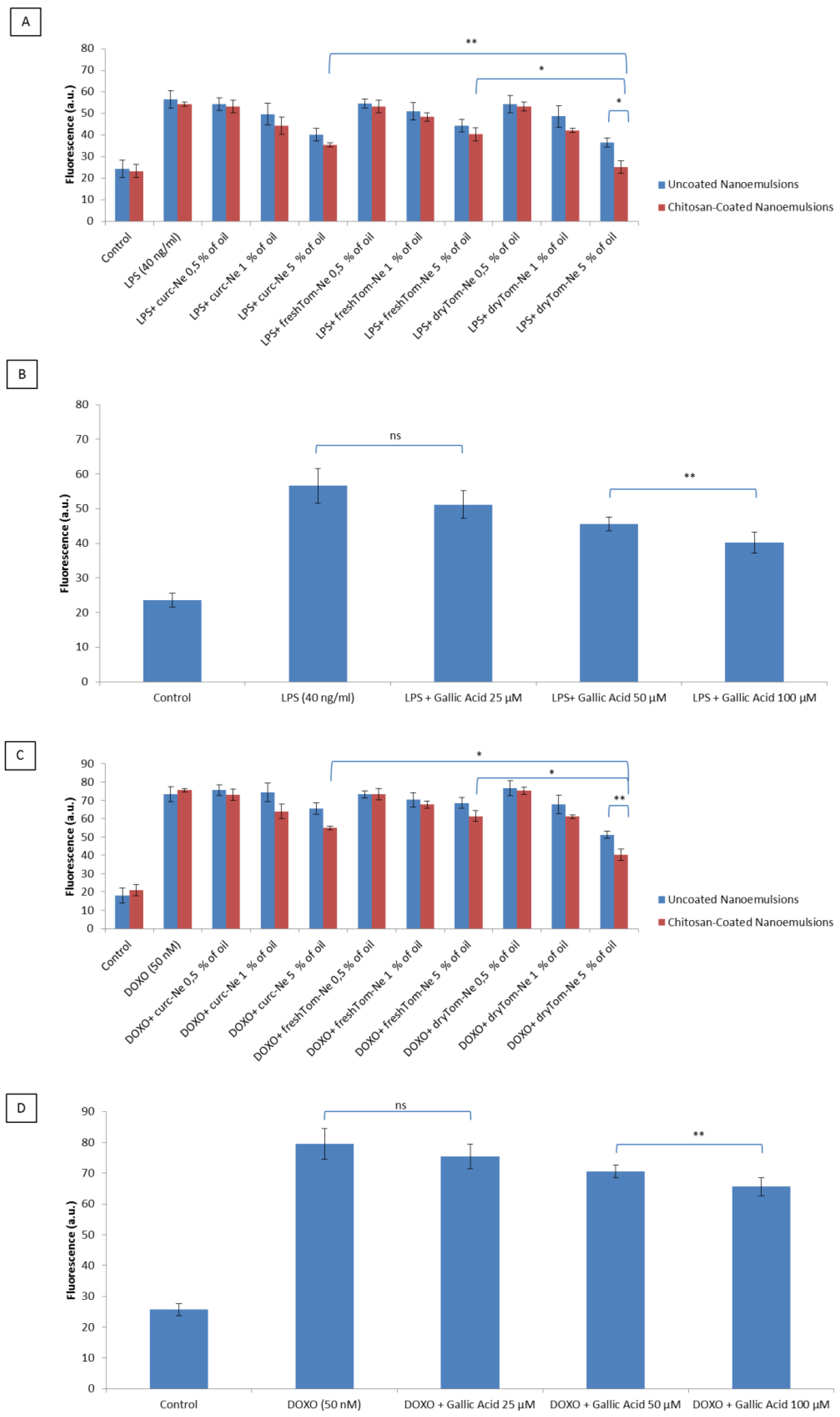
3.6. Detection of Intracellular Reactive Oxygen Species
3.7. Lipid Peroxidation Studies
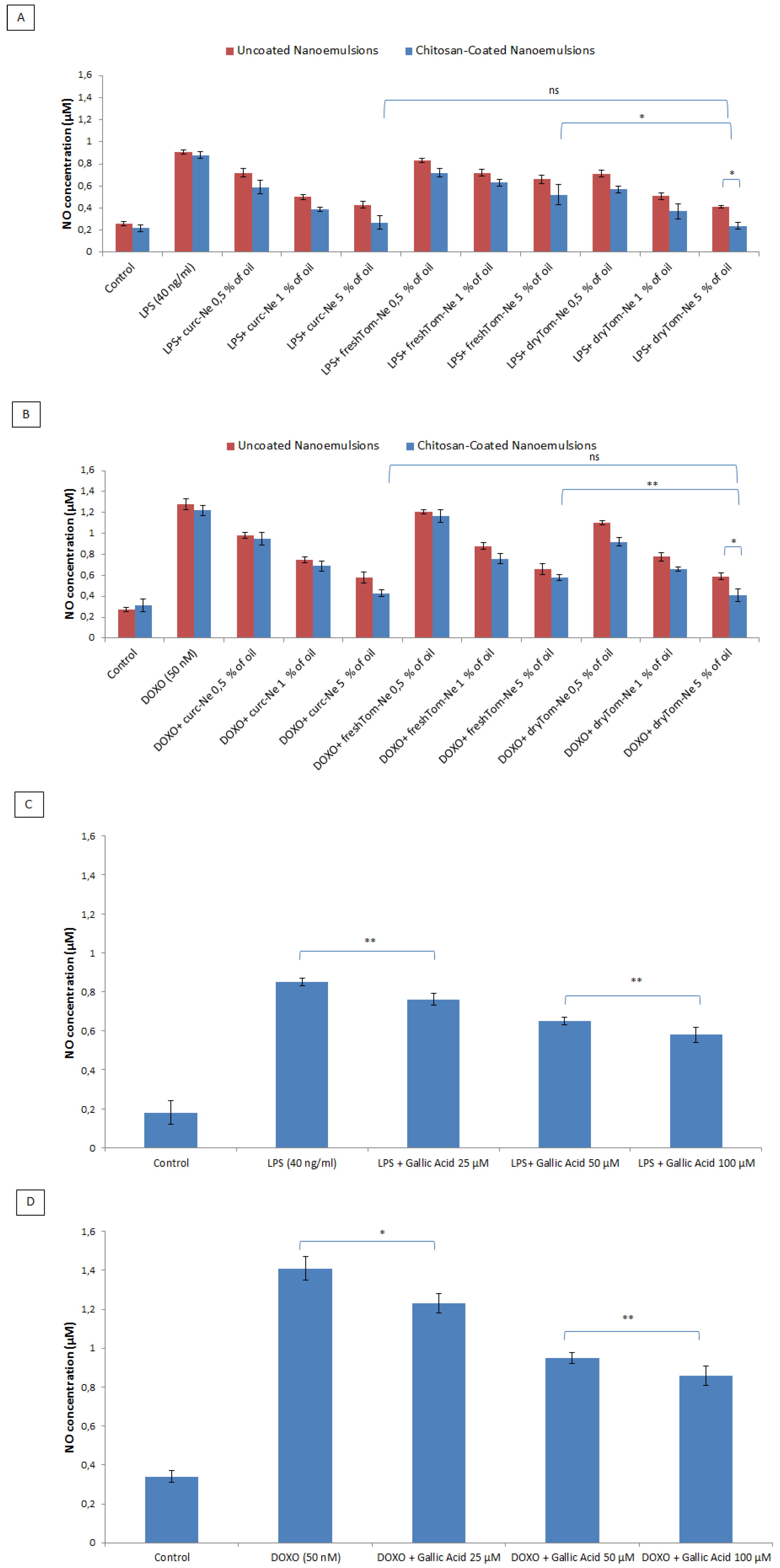
3.8. Measurement of Nitric Oxide
3.9. Calcium Levels
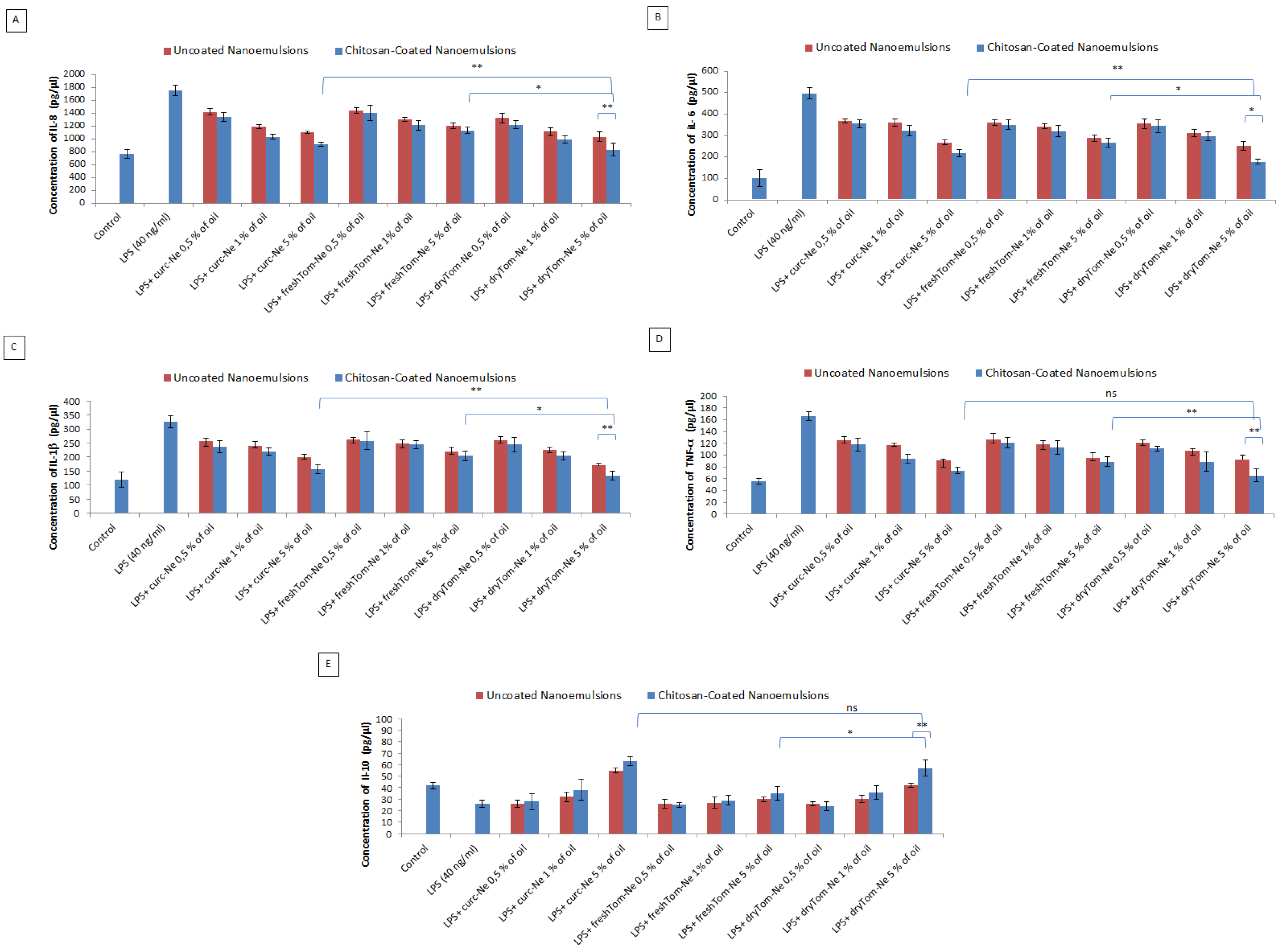
Anti-Inflammatory Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandez-Chas, M.; Curtis, M.J.; Niederer, S.A. Mechanism of doxorubicin cardiotoxicity evaluated by integrating multiple molecular effects into a biophysical model. Br. J. Pharmacol. 2018, 175, 763–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mele, D.; Tocchetti, C.G.; Pagliaro, P.; Madonna, R.; Novo, G.; Pepe, A.; Zito, C.; Maurea, N.; Spallarossa, P. Pathophysiology of anthracycline cardiotoxicity. J. Cardiovasc. Med. 2016, 17 (Suppl. 1), e3–e11. [Google Scholar] [CrossRef] [PubMed]
- Maurea, N.; Coppola, C.; Piscopo, G.; Galletta, F.; Riccio, G.; Esposito, E.; De Lorenzo, C.; De Laurentiis, M.; Spallarossa, P.; Mercuro, G. Pathophysiology of cardiotoxicity from target therapy and angiogenesis inhibitors. J. Cardiovasc. Med. 2016, 17 (Suppl. 1), e19–e26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simůnek, T.; Stérba, M.; Popelová, O.; Adamcová, M.; Hrdina, R.; Gersl, V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef]
- Wu, K.; Schwartz, S.J.; Platz, E.A. Variations in plasma lycopene and specific isomers over time in a cohort of U.S. men. J. Nutr. 2003, 133, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Del Pizzo, M.; Marzocco, S.; Sorrentino, R.; Ciccarelli, M.; Iaccarino, G.; Pinto, A.; Popolo, A. Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicol. Appl. Pharmacol. 2016, 293, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Bakhtiari, E.; Mousavi, S.H. Protective effect of Hibiscus Sabdariffa on doxorubicin-induced cytotoxicity in H9c2 Cardiomyoblast Cells. Iran. J. Pharm. Res. 2017, 16, 708–713. [Google Scholar] [PubMed]
- Capasso, I.; Esposito, E.; Maurea, N.; Montella, M.; Crispo, A.; De Laurentiis, M.; D’Aiuto, M.; Frasci, G.; Botti, G.; Grimaldi, M.; et al. Combination of inositol and alphalipoic acid in metabolicsyndrome-affectedwomen: A randomized placebo-controlled trial. Trials 2013, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Maurea, N.; Coppola, C.; Ragone, G.; Frasci, G.; Bonelli, A.; Romano, C.; Iaffaioli, R.V. Women survive breast cancer but fall victim to heart failure: The shadows and lights of targeted therapy. J. Cardiovasc. Med. 2010, 11, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Loft, S.; Lundby, C.; Olsen, N.V. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J. 2001, 15, 1181–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Fushimi, K.; Kouchi, H.; Mihara, K.; Miyazaki, M.; Ohe, T.; Namba, M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation 1998, 98, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Karimi, G.; Ramezani, M.; Abdi, A. Protective effects of lycopene and tomato extract against doxorubicin-induced cardiotoxicity. Phytother. Res. 2005, 19, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Priyadarsini, A.; Saravanan, R.; Arumugam, M. Ameliorative effects of curcumin against renal injuries mediated by inducible nitric oxide synthase and nuclear factor kappa B during gentamicin-induced toxicity in Wistar rats. Eur. J. Pharmacol. 2011, 670, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, R.; Quagliariello, V.; Calabria, D.; Calcagno, V.; De Luca, E.; Iaffaioli, R.V.; Netti, P.A. Curcumin bioavailability from oil in water nano-emulsions: In vitro and in vivo study on the dimensional, compositional and interactional dependence. J. Control. Release 2016, 233, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Yucel, C.; Quagliariello, V.; Iaffaioli, R.V.; Ferrari, G.; Donsì, F. Submicron complex lipid carriers for curcumin delivery to intestinal epithelial cells: Effect of different emulsifiers on bioaccessibility and cell uptake. Int. J. Pharm. 2015, 494, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Iaffaioli, R.V.; Armenia, E.; Clemente, O.; Barbarisi, M.; Nasti, G.; Berretta, M.; Ottaiano, A.; Barbarisi, A. Hyaluronic Acid Nanohydrogel Loaded with Quercetin Alone or in Combination to a Macrolide Derivative of Rapamycin RAD001 (Everolimus) as a New Treatment for Hormone-Responsive Human Breast Cancer. J. Cell. Physiol. 2017, 232, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Bassani, B.; Baci, D.; Dallaglio, K.; Gallazzi, M.; Corradino, P.; Bruno, A.; Noonan, D.M. Nutraceuticals and “repurposed” drugs of phytochemical origin in prevention and interception of chronic degenerative disease and cancer. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef]
- Nam, J.S.; Sharma, A.R.; Nguyen, L.T.; Chakraborty, C.; Sharma, G.; Lee, S.S. Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine. Molecules 2016, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, R.; Luciani, G.; Calcagno, V.; Jakhmola, A.; Silvestri, B.; Guarnieri, D.; Belli, V.; Costantini, A.; Netti, P.A. Multilayered silica-biopolymer nanocapsules with a hydrophobic core and a hydrophilic tunable shell thickness. Nanoscale 2016, 8, 8798–8809. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, R.; Iaccarino, G.; Bianchini, P.; Marotta, R.; D’autilia, F.; Quagliariello, V.; Diaspro, A.; Netti, P.A. Ultrastable Liquid–Liquid Interface as Viable Route for Controlled Deposition of Biodegradable Polymer Nanocapsules. Small 2016, 12, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, R.; Quagliariello, V.; Giustetto, P.; Calabria, D.; Sathya, A.; Marotta, R.; Profeta, M.; Nitti, S.; Silvestri, N.; Pellegrino, T.; et al. Oil/water nano-emulsion loaded with cobalt ferrite oxide nanocubes for photo-acoustic and magnetic resonance dual imaging in cancer: In vitro and preclinical studies. Nanomedicine 2017, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, D.; Formato, A.; Scaglione, G.; Montesano, D.; Pellegrino, A.; Villecco, F.; Gallo, M. Study of the Grape Cryo-Maceration Process at Different Temperatures. Foods 2018, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, D.; Caruso, T.; Iannece, P.; Aragòn, A.; Santini, A. Characterization of high purity lycopene from tomato wastes using a new pressurized extraction approach. J. Agric. Food Chem. 2008, 56, 6227–6231. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, R.; Ciotola, U.; Sagliano, A.; Bianchini, P.; Diaspro, A.; Netti, P.A. Tunable stability of monodisperse secondary O/W nano-emulsions. Nanoscale 2014, 6, 9300–9307. [Google Scholar] [CrossRef] [PubMed]
- Kozukue, N.; Friendman, M. Tomatine, chlorophyll, β-carotene and lycopene content in tomatoes during growth and maturation. J. Sci. Food Agric. 2003, 83, 195–200. [Google Scholar] [CrossRef]
- Meamar, R.; Dehghani, L.; Ghasemi, M.; Saadatnia, M.; Basiri, K.; Faradonbeh, N.A.; Javanmard, S.H. Enalapril protects endothelial cells against induced apoptosis in Alzheimer’s disease. J. Res. Med. Sci. 2013, 18 (Suppl. 1), S1–S5. [Google Scholar] [PubMed]
- Spallarossa, P.; Garibaldi, S.; Altieri, P.; Fabbi, P.; Manca, V.; Nasti, S.; Rossettin, P.; Ghigliotti, G.; Ballestrero, A.; Patrone, F.; et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J. Mol. Cell. Cardiol. 2004, 37, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Barbarisi, M.; Iaffaioli, R.V.; Armenia, E.; Schiavo, L.; De Sena, G.; Tafuto, S.; Barbarisi, A.; Quagliariello, V. Novel nanohydrogel of hyaluronic acid loaded with quercetin alone and in combination with temozolomide as new therapeutic tool, CD44 targeted based, of glioblastoma multiforme. J. Cell. Physiol. 2017, 233, 6550–6564. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Ma, Z.; Khor, E.; Lim, L.Y. Uptake of FITC-chitosan nanoparticles by A549 cells. Pharm. Res. 2002, 19, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Elera, G.; Garrett, A.R.; Robison, R.A.; O’Neill, K.L. The role of oxidative stress in prostate cancer. Eur. J. Cancer Prev. 2012, 21, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.K.; Bello, D.; Budhlall, B.; Rogers, E.; Milton, D.K. Screening for Oxidative Stress Elicited by Engineered Nanomaterials: Evaluation of Acellular DCFH Assay. Dose Response 2012, 10, 308–330. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, T.; Matsumoto, H.; Kanemitsu, Y.; Izuhara, K.; Tohda, Y.; Kita, H.; Horiguchi, T.; Kuwabara, K.; Tomii, K.; Otsuka, K.; et al. Integrating longitudinal information on pulmonary function and inflammation using asthma phenotypes. J Allergy ClinImmunol. 2014, 133, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Day, P.J.; Tirelli, N. HA-coated chitosan nanoparticles for CD44-mediated nucleic acid delivery. Macromol. Biosci. 2013, 13, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Y.; Yokoyama, W.; Yi, J. Endocytosis of Corn Oil-Caseinate Emulsions In Vitro: Impacts of Droplet Sizes. Nanomaterials 2017, 7, 349. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pei, C.; Yan, S.; Liu, G.; Liu, G.; Chen, W.; Cui, Y.; Liu, Y. NADPH oxidase 1-dependent ROS is crucial for TLR4 signaling to promote tumor metastasis of non-small cell lung cancer. Tumour. Biol. 2015, 36, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, E.; Kass, D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007, 49, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Feng, Z.; Cheng, W.; Xiao, Y. MicroRNA-34a mediates atrial fibrillation through regulation of Ankyrin-B expression. Mol. Med. Rep. 2018, 17, 8457–8465. [Google Scholar] [CrossRef] [PubMed]
- Bacchiega, B.C.; Bacchiega, A.B.; Usnayo, M.J.; Bedirian, R.; Singh, G.; Pinheiro, G.D. Interleukin 6 Inhibition and Coronary Artery Disease in a High-Risk Population: A Prospective Community-Based Clinical Study. J. Am. Heart Assoc. 2017, 6, e005038. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, S.; Vogiatzi, K.; Amanatidou, V.; Spandidos, D.A. Interleukin 8 and cardiovascular disease. Cardiovasc. Res. 2009, 84, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Zhang, J.; Zhang, L.; Du, R.; Xiang, D.; Wu, M.; Zhang, R.; Han, W. Interleukin-1 signaling mediates acute doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2011, 65, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.V.; Swaminathan, P.D.; Luczak, E.D.; Kutschke, W.; Weiss, R.M.; Anderson, M.E. MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J. Mol. Cell. Cardiol. 2012, 52, 1135–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, J.; Wang, Z.; Ao, L.; Zou, N.; Dinarello, C.A.; Banerjee, A.; Fullerton, D.A.; Meng, X. Cytokines link Toll-like receptor 4 signaling to cardiac dysfunction after global myocardial ischemia. Ann. Thorac. Surg. 2008, 85, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Goyal, S.; Sharma, C.; Arora, S.; Kumari, S.; Arya, D.S. Cardioprotective effect of lycopene against isoproterenol-induced myocardial infarction in rats. Hum. Exp. Toxicol. 2013, 32, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Gupta, S.K.; Ojha, S.K.; Nandave, M.; Mittal, R.; Kumari, S.; Arya, D.S. Cardioprotective effect of lycopene in the experimental model of myocardial ischemia-reperfusion injury. Mol. Cell. Biochem. 2006, 289, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Toldo, S.; Mezzaroma, E.; Abbate, A. Targeting interleukin-1 in heart disease. Circulation 2013, 128, 1910–1923. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, H.; Gu, Y.; Wang, X.; Cao, H.; Tang, Y.; Chen, H.; Huang, C. Protective effect of lycopene on cardiac function and myocardial fibrosis after acute myocardial infarction in rats via the modulation of p38 and MMP-9. J. Mol. Histol. 2014, 45, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Tocchetti, C.G.; Carpi, A.; Coppola, C.; Quintavalle, C.; Rea, D.; Campesan, M.; Arcari, A.; Piscopo, G.; Cipresso, C.; Monti, M.G.; et al. Ranolazine protects from doxorubicin-induced oxidative stress and cardiac dysfunction. Eur. J. Heart Fail. 2014, 16, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Nanoemulsions and Nutraceuticals Loaded | Acronym |
|---|---|
| Uncoated nanoemulsion loaded with fresh tomato extract | freshTom-Ne |
| Chitosan-coated nanoemulsion loaded with fresh tomato extract | freshTom Ne-CT |
| Uncoated nanoemulsion loaded with dry tomato extract | dryTom-Ne |
| Chitosan-coated nanoemulsion loaded with dry tomato extract | dryTom-Ne-CT |
| Uncoated nanoemulsion loaded with curcumin | curc-Ne |
| Chitosan-coated nanoemulsion loaded with curcumin | curc-Ne -CT |
| Uncoated fluorescent nanoemulsion loaded with FITC | FITC-Ne |
| Chitosan-coated fluorescent nanoemulsion loaded with FITC | FITC-Ne-CT |
| Nanoemulsions | Mean Hydrodynamic Size (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|
| Uncoated nanoemulsion loaded with fresh tomato extract | 97.09 (0.59) | 0.098 (0.011) | −22.9 (1.3) |
| Chitosan-coated nanoemulsion loaded with fresh tomato extract | 139.6 (0.85) | 0.076 (0.004) | 49.6 (0.5) |
| Uncoated nanoemulsion loaded with dry tomato extract | 93.23 (0.66) | 0.122 (0.020) | −23.0 (0.9) |
| Chitosan-coated nanoemulsion loaded with dry tomato extract | 128.8 (1.01) | 0.056 (0.007) | 46.1 (0.5) |
| Uncoated nanoemulsion loaded with curcumin | 105.4 (2.83) | 0.118 (0.049) | −22.0 (5.6) |
| Chitosan-coated nanoemulsion loaded with curcumin | 119.8 (0.80) | 0.079 (0.010) | 44.3 (1.7) |
| Uncoated fluorescent nanoemulsion loaded with FITC | 93.83 (0.86) | 0.090 (0.016) | −30.5 (1.5) |
| Chitosan-coated fluorescent nanoemulsion loaded with FITC | 94.50 (0.75) | 0.075 (0.010) | 23.7 (0.2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity. Nutrients 2018, 10, 1304. https://doi.org/10.3390/nu10091304
Quagliariello V, Vecchione R, Coppola C, Di Cicco C, De Capua A, Piscopo G, Paciello R, Narciso V, Formisano C, Taglialatela-Scafati O, et al. Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity. Nutrients. 2018; 10(9):1304. https://doi.org/10.3390/nu10091304
Chicago/Turabian StyleQuagliariello, Vincenzo, Raffaele Vecchione, Carmela Coppola, Chiara Di Cicco, Alberta De Capua, Giovanna Piscopo, Rolando Paciello, Viviana Narciso, Carmen Formisano, Orazio Taglialatela-Scafati, and et al. 2018. "Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity" Nutrients 10, no. 9: 1304. https://doi.org/10.3390/nu10091304