Acute Effects of Dietary Carbohydrate Restriction on Glycemia, Lipemia and Appetite Regulating Hormones in Normal-Weight to Obese Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diet Compositions
The Mixed Meal Tests
2.3. Analytical Procedures
2.4. Statistical Analysis
3. Results
3.1. Subjects
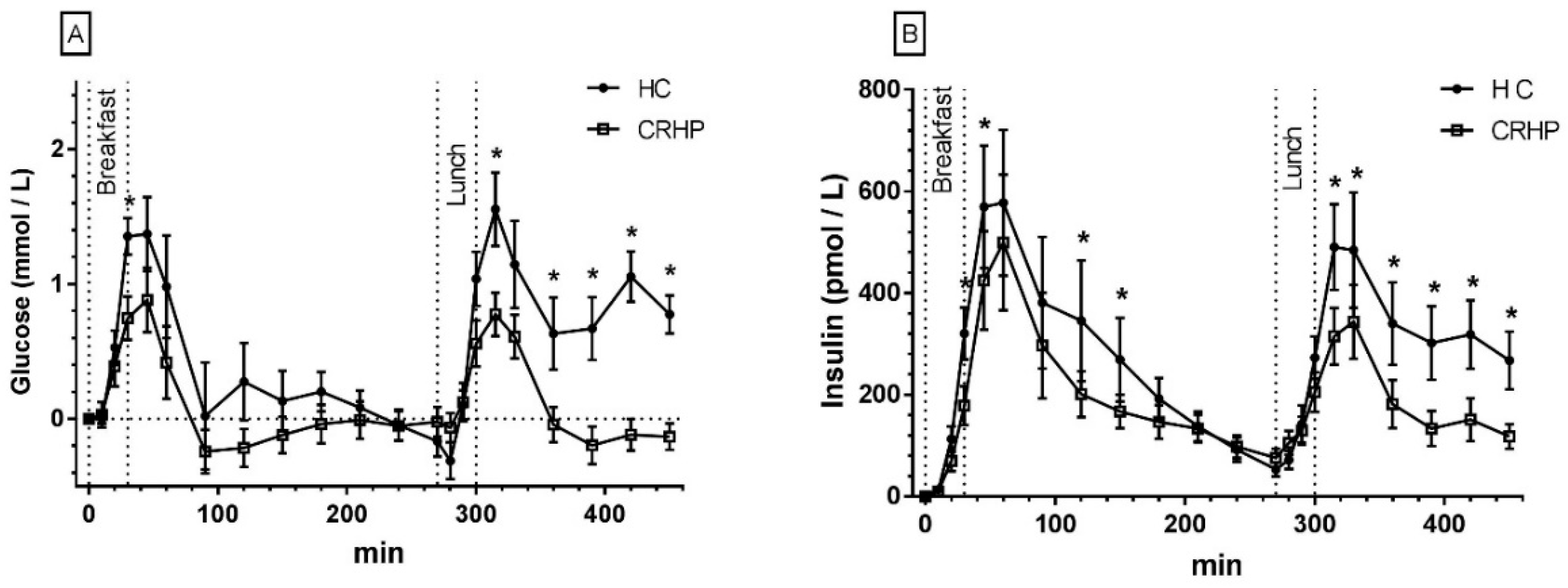
3.2. Glucose
3.3. Insulin
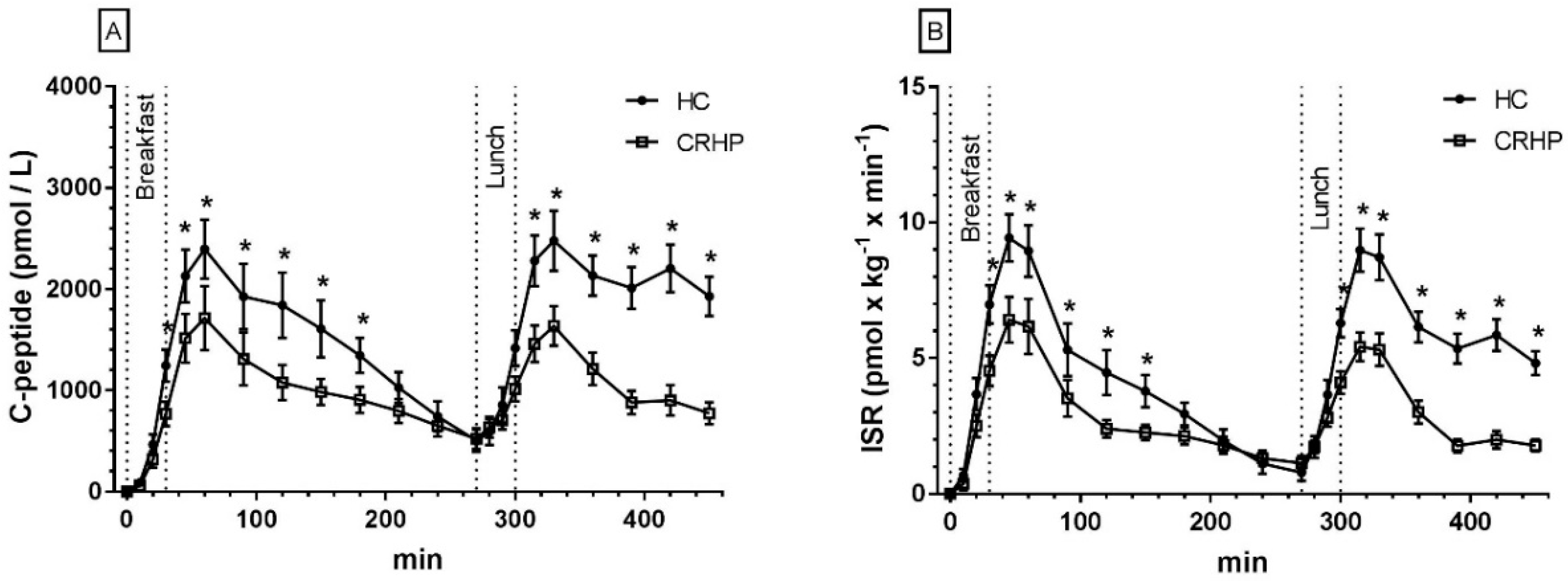
3.4. C-Peptides
3.5. Insulin Secretion Rate
3.6. β-Cell Glucose Sensitivity and Insulin Clearance
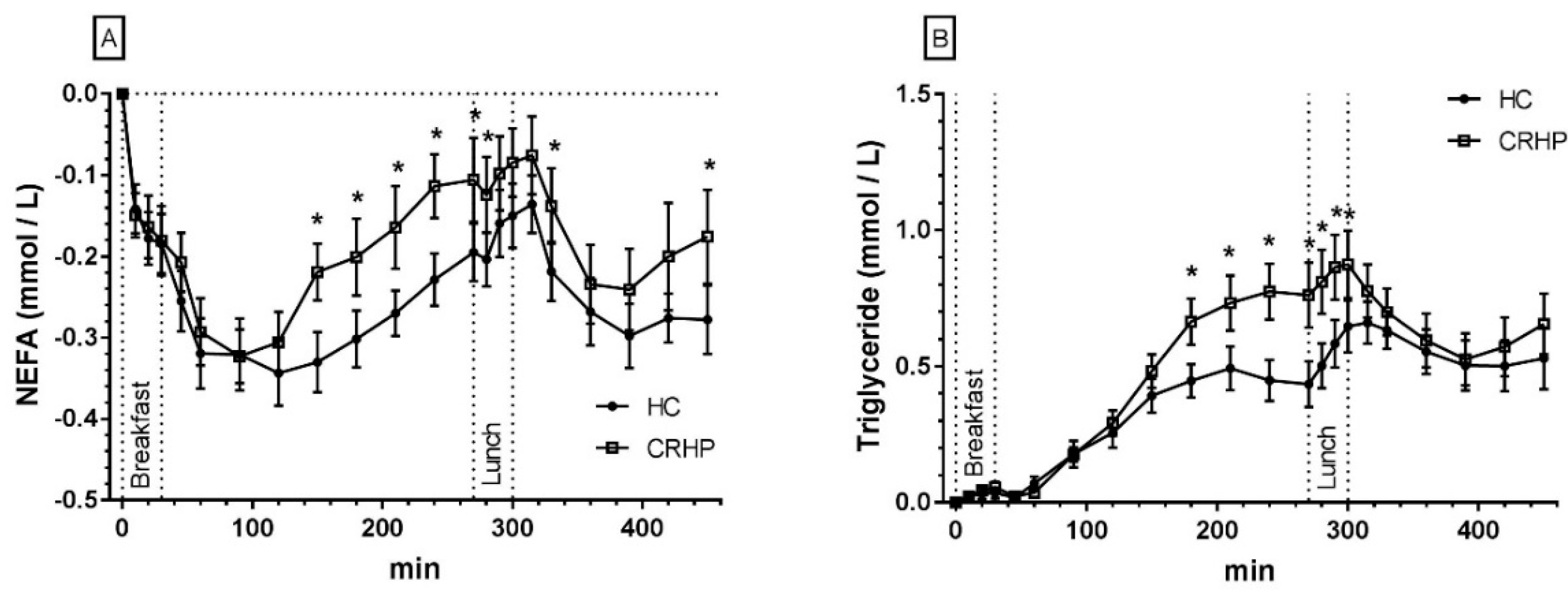
3.7. Non-Esterified Fatty Acids
3.8. Triglycerides
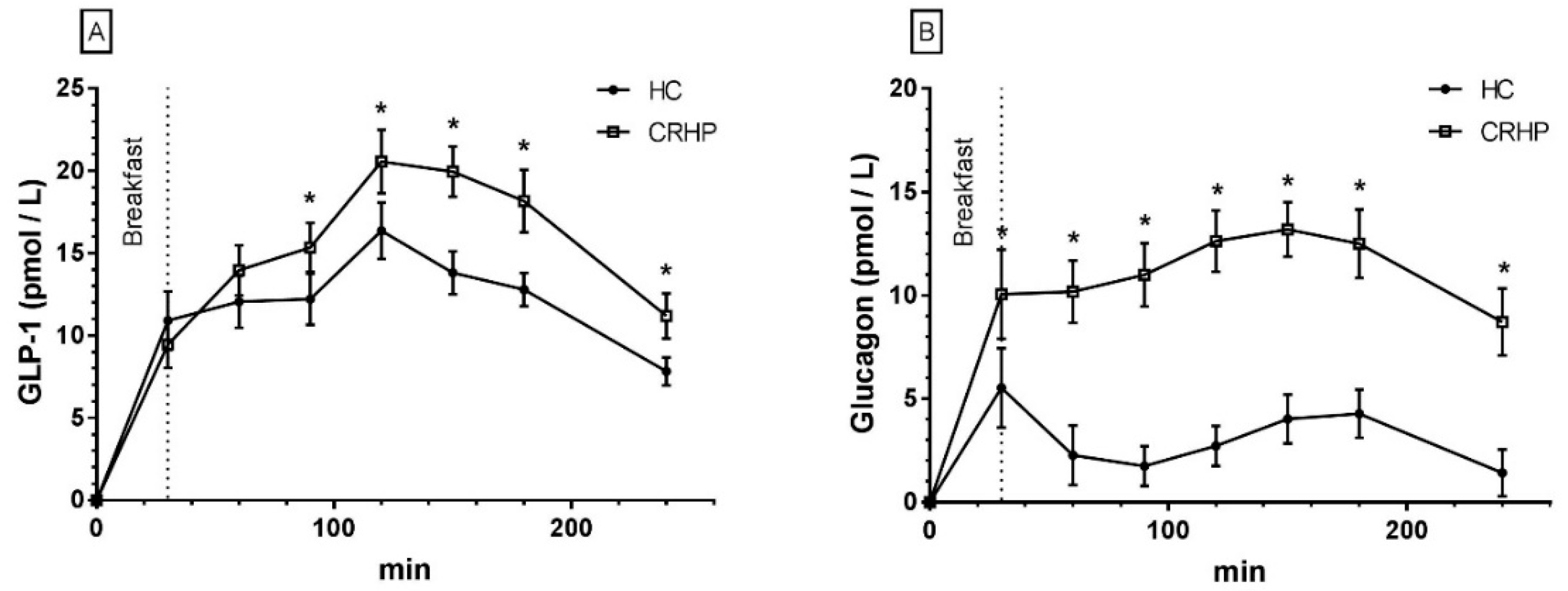
3.9. Glucagon-Like Peptide-1
3.10. Glucagon
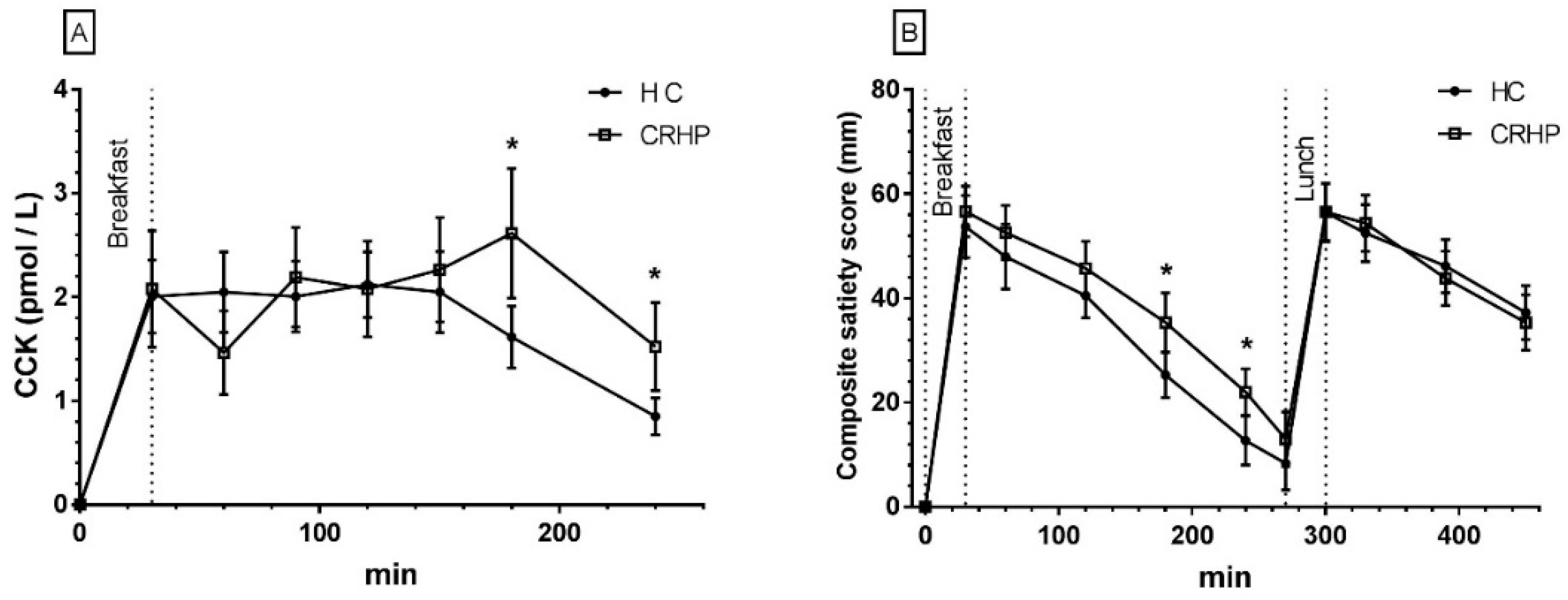
3.11. Cholecystokinin
3.12. Composite Satiety Score
3.13. Explorative Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| CRHP 1 Diet | HC 2 Diet | |
|---|---|---|
| Breakfast | ||
| Energy (kJ) | 3000 | 3000 |
| Carbohydrate (E%) | 31 | 54 |
| Protein (E%) | 29 | 16 |
| Fat (E%) | 40 | 30 |
| Fiber (g/MJ) | 2.5 | 4 |
| Ingredients (g) | ||
| Egg | 192.3 | 39.7 |
| Olive oil | 7.5 | - |
| Bread | 37.4 | 69.4 |
| Rye flour yoghurt topping | 21.4 | 49.6 |
| Tomato | 85.5 | - |
| Cheese | 16.0 | 19.8 |
| Ham | 26.7 | - |
| Skyr (icelandic yoghurt) with vanilla | 160.3 | - |
| Strawberry jam | - | 19.7 |
| Apple | - | 49.6 |
| Almond | - | 11.9 |
| Milk, acidophilus cultured | - | 198.3 |
| Lunch | ||
| Energy (kJ) | 3000 | 3000 |
| Carbohydrate (E%) | 31 | 54 |
| Protein (E%) | 29 | 16 |
| Fat (E%) | 40 | 30 |
| Fiber (g/MJ) | 3.5 | 2.7 |
| Ingredients (g) | ||
| Chicken | 137.3 | 38.1 |
| Olive oil | 14.7 | - |
| Tomato | 147.2 | 142.8 |
| Spring onion | 9.8 | 19.0 |
| Bell pepper | 29.4 | 47.6 |
| Bread | 24.5 | 47.6 |
| Milk | 245.3 | 142.8 |
| Feta cheese | 29.4 | - |
| Chick peas | 39.2 | - |
| Pasta | - | 66.6 |
| Pesto | - | 33.3 |
| Butter | - | 9.5 |
References
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, X.; Mai, W.; Li, M.; Hu, Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ 2016, 355, i5953. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Hu, F.B.; Tappy, L.; Brand-Miller, J. Dietary carbohydrates: Role of quality and quantity in chronic disease. BMJ 2018, 361, k2340. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 4. Lifestyle management: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S38–S50. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 5. Prevention or delay of type 2 diabetes: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S51–S54. [Google Scholar] [CrossRef] [PubMed]
- Belza, A.; Ritz, C.; Sorensen, M.Q.; Holst, J.J.; Rehfeld, J.F.; Astrup, A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am. J. Clin. Nutr. 2013, 97, 980–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samkani, A.; Skytte, M.J.; Kandel, D.; Kjaer, S.; Astrup, A.; Deacon, C.F.; Holst, J.J.; Madsbad, S.; Rehfeld, J.F.; Haugaard, S.B.; et al. A carbohydrate-reduced high-protein diet acutely decreases postprandial and diurnal glucose excursions in type 2 diabetes patients. Br. J. Nutr. 2018, 119, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY (3–36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after rygb. Physiol Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.L.; Noakes, M.; Keogh, J.; Clifton, P.M. Effect of carbohydrate distribution on postprandial glucose peaks with the use of continuous glucose monitoring in type 2 diabetes. Am. J. Clin. Nutr. 2008, 87, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q.; Almokayyad, R.M.; Gannon, M.C. Comparison of a carbohydrate-free diet vs. Fasting on plasma glucose, insulin and glucagon in type 2 diabetes. Metabolism 2015, 64, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmeron, J.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Spiegelman, D.; Jenkins, D.J.; Stampfer, M.J.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of niddm in men. Diabetes Care 1997, 20, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, J.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Willett, W.C.; Stampfer, M.J.; Hu, F.B.; Franz, M.; Sampson, L.; Hennekens, C.H.; Manson, J.E. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in us women. Am. J. Clin. Nutr. 2000, 71, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Manson, J.; Liu, S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am. J. Clin. Nutr. 2002, 76, 274S–280S. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Lapinski, H.; Colette, C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of HBA1c. Diabetes Care 2003, 26, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Owens, D. Postprandial and basal glucose in type 2 diabetes: Assessment and respective impacts. Diabetes Technol. Ther. 2011, 13, S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Stentz, F.B.; Brewer, A.; Wan, J.; Garber, C.; Daniels, B.; Sands, C.; Kitabchi, A.E. Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: Randomized control trial. BMJ Open Diabetes Res. Care 2016, 4, e000258. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parillo, M.; Rivellese, A.A.; Ciardullo, A.V.; Capaldo, B.; Giacco, A.; Genovese, S.; Riccardi, G. A high-monounsaturated-fat/low-carbohydrate diet improves peripheral insulin sensitivity in non-insulin-dependent diabetic patients. Metabolism 1992, 41, 1373–1378. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Schaaf, P.; Jones, C.; Zhou, M.Y.; Chen, Y.D.; Reaven, G.M. Effects of low-fat, high-carbohydrate diets on risk factors for ischemic heart disease in postmenopausal women. Am. J. Clin. Nutr. 1997, 65, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Harcombe, Z.; Baker, J.S.; DiNicolantonio, J.J.; Grace, F.; Davies, B. Evidence from randomized controlled trials does not support current dietary fat guidelines: A systematic review and meta-analysis. Open Heart 2016, 3, e000409. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, M. New nordic nutrition recommendations are here. Food Nutr. Res. 2013, 57, 22903. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.I.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlstrom, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Holst, J.J.; Knop, F.K.; Vilsboll, T.; Krarup, T.; Madsbad, S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 2011, 34, S251–S257. [Google Scholar] [CrossRef] [PubMed]
- Madsbad, S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes. Metab. 2014, 16, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Hensrud, D.D.; Romanski, S.; Levine, J.A.; Burguera, B.; Jensen, M.D. Body composition and resting energy expenditure in humans: Role of fat, fat-free mass and extracellular fluid. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Finlayson, G.; Dalton, M.; Caudwell, P.; Blundell, J.E. Metabolic phenotyping guidelines: Studying eating behavior in humans. J. Endocrinol. 2014, 222, G1–G12. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.-P.; Gilbert, J.-A.; Gregersen, N.T.; Pedersen, S.D.; Sjodin, A.M. Comparison of 150-mm versus 100-mm visual analogue scales in free living adult subjects. Appetite 2010, 54, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, V. Stability of the long chain non-esterified fatty acid pattern in plasma and blood during different storage conditions. Clin. Chim. Acta 1978, 84, 49–54. [Google Scholar] [CrossRef]
- Krebs, M.; Stingl, H.; Nowotny, P.; Weghuber, D.; Bischof, M.; Waldhausl, W.; Roden, M. Prevention of in vitro lipolysis by tetrahydrolipstatin. Clin. Chem. 2000, 46, 950–954. [Google Scholar] [PubMed]
- Owen, W.E.; Thatcher, M.L.; Crabtree, K.J.; Greer, R.W.; Strathmann, F.G.; Straseski, J.A.; Genzen, J.R. Body fluid matrix evaluation on a roche cobas 8000 system. Clin. Biochem. 2015, 48, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Orskov, C.; Rabenhoj, L.; Wettergren, A.; Kofod, H.; Holst, J.J. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 1994, 43, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; Bagger, J.I.; Wewer Albrechtsen, N.J.; Christensen, M.; Grondahl, M.; Hartmann, B.; Mathiesen, E.R.; Hansen, C.P.; Storkholm, J.H.; van Hall, G.; et al. Evidence of extrapancreatic glucagon secretion in man. Diabetes 2016, 65, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F. Accurate measurement of cholecystokinin in plasma. Clin. Chem. 1998, 44, 991–1001. [Google Scholar] [PubMed]
- Van Cauter, E.; Mestrez, F.; Sturis, J.; Polonsky, K.S. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992, 41, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Hovorka, R.; Soons, P.A.; Young, M.A. ISEC: A program to calculate insulin secretion. Comput. Methods Progr. Biomed. 1996, 50, 253–264. [Google Scholar] [CrossRef]
- Koopmans, S.J.; Ohman, L.; Haywood, J.R.; Mandarino, L.J.; DeFronzo, R.A. Seven days of euglycemic hyperinsulinemia induces insulin resistance for glucose metabolism but not hypertension, elevated catecholamine levels, or increased sodium retention in conscious normal rats. Diabetes 1997, 46, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Modan, M.; Halkin, H.; Almog, S.; Lusky, A.; Eshkol, A.; Shefi, M.; Shitrit, A.; Fuchs, Z. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J. Clin. Investig. 1985, 75, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.; Leibowitz, G.; Nesher, R. Glucotoxicity and beta-cell failure in type 2 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2003, 16, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Del Prato, S.; Leonetti, F.; Simonson, D.C.; Sheehan, P.; Matsuda, M.; DeFronzo, R.A. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia 1994, 37, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Keum, N.; Zhang, X.; Cho, E.; Giovannucci, E.L. Hyperinsulinemia, insulin resistance and colorectal adenomas: A meta-analysis. Metabolism 2015, 64, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Lakka, T.A.; Lakka, H.M.; Valkonen, V.P.; Everson, S.A.; Kaplan, G.A. Hyperinsulinemia is associated with the incidence of hypertension and dyslipidemia in middle-aged men. Diabetes 1998, 47, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, T.; Kajio, H.; Sugiyama, T. Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: A population-based observational study. Int. J. Cancer 2017, 141, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyorala, M.; Miettinen, H.; Laakso, M.; Pyorala, K. Hyperinsulinemia and the risk of stroke in healthy middle-aged men: The 22-year follow-up results of the helsinki policemen study. Stroke 1998, 29, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Pyorala, M.; Miettinen, H.; Laakso, M.; Pyorala, K. Hyperinsulinemia predicts coronary heart disease risk in healthy middle-aged men: The 22-year follow-up results of the helsinki policemen study. Circulation 1998, 98, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Chen, X.; Desantis, R.A.; Kendrick, Z. Effects of insulin on fatty acid reesterification in healthy subjects. Diabetes 1993, 42, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.J.; Carlson, M.G.; Hill, J.O.; Nurjhan, N. Regulation of free fatty acid metabolism by insulin in humans: Role of lipolysis and reesterification. Am. J. Physiol. 1992, 263, E1063–E1069. [Google Scholar] [PubMed]
- Sadur, C.N.; Eckel, R.H. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. J. Clin. Investig. 1982, 69, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.; Burdge, G.C.; Wootton, S.A.; Clark, M.L.; Frayn, K.N. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes 2002, 51, 2684–2690. [Google Scholar] [CrossRef] [PubMed]
- Pollare, T.; Vessby, B.; Lithell, H. Lipoprotein lipase activity in skeletal muscle is related to insulin sensitivity. Arterioscler. Thromb. 1991, 11, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Ruge, T.; Hodson, L.; Cheeseman, J.; Dennis, A.L.; Fielding, B.A.; Humphreys, S.M.; Frayn, K.N.; Karpe, F. Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J. Clin. Endocrinol. Metab. 2009, 94, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.M.; Nelson, R.H. Contribution of triglyceride-rich lipoproteins to plasma free fatty acids. Horm. Metab. Res. 2007, 39, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Coulston, A.M.; Zhou, M.Y.; Hollenbeck, C.B.; Reaven, G.M. Why do low-fat high-carbohydrate diets accentuate postprandial lipemia in patients with niddm? Diabetes Care 1995, 18, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.E.; Chan, I.F.; Buchi, K.N.; Horton, S.C. Postchallenge plasma lipoprotein retinoids: Chylomicron remnants in endogenous hypertriglyceridemia. Metabolism 1985, 34, 551–558. [Google Scholar] [CrossRef]
- Jeppesen, J.; Chen, Y.I.; Zhou, M.Y.; Schaaf, P.; Coulston, A.; Reaven, G.M. Postprandial triglyceride and retinyl ester responses to oral fat: Effects of fructose. Am. J. Clin. Nutr. 1995, 61, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Huntriss, R.; Campbell, M.; Bedwell, C. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Clin. Nutr. 2018, 72, 311–325. [Google Scholar] [CrossRef] [PubMed]
- van der Klaauw, A.A.; Keogh, J.M.; Henning, E.; Trowse, V.M.; Dhillo, W.S.; Ghatei, M.A.; Farooqi, I.S. High protein intake stimulates postprandial GLP1 and PYY release. Obesity 2013, 21, 1602–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, S.C.; Lutz, T.A.; Geary, N.; Langhans, W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1219–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svane, M.S.; Jorgensen, N.B.; Bojsen-Moller, K.N.; Dirksen, C.; Nielsen, S.; Kristiansen, V.B.; Torang, S.; Wewer Albrechtsen, N.J.; Rehfeld, J.F.; Hartmann, B.; et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int. J. Obes. 2016, 40, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Finlayson, G.; Caudwell, P.; Webb, D.L.; Hellstrom, P.M.; Naslund, E.; Blundell, J.E. Postprandial profiles of CCK after high fat and high carbohydrate meals and the relationship to satiety in humans. Peptides 2016, 77, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Subject (no.) | Age (years) | Gender (M/F 1) | Weight (kg) | BMI 2 (kg/m2) | HbA1c 3 (mmol/mol) | Fasting PG 4 (mmol/L) | HOMA2-IR 5 | TEE 6 (MJ/day) |
|---|---|---|---|---|---|---|---|---|
| 1 | 45 | M | 138 | 43.4 | 39 | 6.2 | 3.2 | 13.6 |
| 2 | 70 | F | 127 | 49.3 | 41 | 6.6 | 3.4 | 11.0 |
| 3 | 70 | F | 108 | 38 | 45 | 6.3 | 3.6 | 10.1 |
| 4 | 63 | F | 109 | 34.1 | 42 | 6.3 | 2.1 | 10.0 |
| 5 | 65 | F | 88 | 34.7 | 39 | 6.2 | 2.4 | 8.5 |
| 6 | 59 | F | 77 | 30.8 | 42 | 5.8 | 1.6 | 8.3 |
| 7 | 56 | M | 121 | 34.9 | 42 | 5.4 | 3 | 12.2 |
| 8 | 59 | M | 70 | 22.7 | 35 | 5.2 | 1 | 8.8 |
| 9 | 63 | F | 80 | 28.3 | 37 | 5.0 | 1.8 | 8.3 |
| 10 | 64 | F | 67 | 23.8 | 35 | 5.0 | 1.2 | 7.8 |
| 11 | 67 | M | 103 | 28.4 | 38 | 6.2 | 2.1 | 11.0 |
| 12 | 56 | M | 75 | 25.2 | 43 | 6.1 | 1.1 | 9.7 |
| 13 | 63 | F | 90 | 27.9 | 40 | 5.8 | 1.7 | 9.3 |
| 14 | 64 | F | 78 | 27.6 | 42 | 5.4 | 1 | 8.3 |
| Mean | 61.7 | 5 M/9 F | 95 | 32.1 | 40 | 5.8 | 2.1 | 9.8 |
| Range | 45–70 | 5 M/9 F | 78–138 | 22.7–49.3 | 35–45 | 5.0–6.6 | 1–3.6 | 8.3–13.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samkani, A.; Skytte, M.J.; Thomsen, M.N.; Astrup, A.; Deacon, C.F.; Holst, J.J.; Madsbad, S.; Rehfeld, J.F.; Krarup, T.; Haugaard, S.B. Acute Effects of Dietary Carbohydrate Restriction on Glycemia, Lipemia and Appetite Regulating Hormones in Normal-Weight to Obese Subjects. Nutrients 2018, 10, 1285. https://doi.org/10.3390/nu10091285
Samkani A, Skytte MJ, Thomsen MN, Astrup A, Deacon CF, Holst JJ, Madsbad S, Rehfeld JF, Krarup T, Haugaard SB. Acute Effects of Dietary Carbohydrate Restriction on Glycemia, Lipemia and Appetite Regulating Hormones in Normal-Weight to Obese Subjects. Nutrients. 2018; 10(9):1285. https://doi.org/10.3390/nu10091285
Chicago/Turabian StyleSamkani, Amirsalar, Mads J. Skytte, Mads N. Thomsen, Arne Astrup, Carolyn F. Deacon, Jens J. Holst, Sten Madsbad, Jens F. Rehfeld, Thure Krarup, and Steen B. Haugaard. 2018. "Acute Effects of Dietary Carbohydrate Restriction on Glycemia, Lipemia and Appetite Regulating Hormones in Normal-Weight to Obese Subjects" Nutrients 10, no. 9: 1285. https://doi.org/10.3390/nu10091285






